Chronic Kidney Disease (CKD)
Causes, Treatment, and Complications
Online Continuing Education Course
Course Description
This continuing education course will enhance your understanding of evidence-based care for individuals with chronic kidney disease. Key topics include the causes, risk factors, diagnosis, staging, and treatment of the disease, as well as interdisciplinary care planning and management options for end-stage renal disease, including dialysis and kidney transplantation.
Chronic Kidney Disease (CKD)
Causes, Treatment, and Complications
Copyright © 2025 Wild Iris Medical Education, Inc. All Rights Reserved.
LEARNING OUTCOME AND OBJECTIVES: Upon completion of this continuing education course, you will demonstrate increased knowledge of evidence-based guidelines for caring for persons with chronic kidney disease. Specific learning objectives to address potential knowledge gaps include:
- Describe the incidence, risk factors, and underlying causes of chronic kidney disease.
- Describe the assessment and screening criteria used to diagnose patients and stage the level of progression of chronic kidney disease.
- Review current recommendations for treating those with chronic kidney disease.
- Describe the components of an interdisciplinary plan of care.
- Review treatment options for end-stage renal disease, including medications, dialysis, and kidney transplant.
- Discuss care of patients receiving dialysis and patients undergoing kidney transplantation.
TABLE OF CONTENTS
WHAT IS CHRONIC KIDNEY DISEASE?
Chronic kidney disease (CKD) occurs when the kidneys lose their function over a period of time due to progressive damage. The nephrons and blood vessels of the kidney change in structure as a result. Often, a person with CKD does not exhibit symptoms. CKD is a medical condition that cannot be reversed (Myhre & Sifris, 2023).
ACUTE VERSUS CHRONIC KIDNEY DISEASE
Acute kidney injury (AKI) occurs suddenly (within 7 days) and is reversible. Causes of AKI include severe dehydration, damage from contrast dyes used in imaging studies, and kidney stones. In contrast, CKD occurs gradually (over months or years) and is irreversible (Myhre & Sifris, 2023).
Functions of a Healthy Kidney
A healthy kidney:
- Excretes waste products and extra water
- Concentrates urine
- Assists in the production of red blood cells
- Balances minerals
- Regulates acid-base balance
- Regulates blood pressure
- Keeps bones healthy
(NKF, 2025a; Sommers, 2023)
Pathophysiology of Chronic Kidney Disease
As the tissues in the kidneys progressively lose function, the remaining functioning tissue must work harder to keep up with regular metabolic demands. The kidneys gradually lose the ability to sustain fluid and electrolyte homeostasis; concentrate urine; or excrete excess phosphate, acid, and potassium. In the late stages of CKD, the kidneys can no longer dilute or concentrate urine. This results in fixed urine osmolality similar to plasma osmolality, leading to urine volume that does not adjust to changes in fluid intake.
As the kidneys’ glomerular filtration rate (GFR) diminishes, concentrations of creatinine and urea rise in the blood. Higher concentrations are associated with systemic manifestations called uremia (Malkina, 2023).
Incidence and Prevalence of Chronic Kidney Disease
CKD is very common in the United States. Approximately 37 million (1 in 7) adults in the United States have CKD, and most of these individuals have not been diagnosed with the condition. CKD is the ninth leading cause of death in the United States. In addition, an estimated 500,000 Americans are on dialysis (NKF, 2021).
The incidence of CKD around the world is rising, with the United States and Japan having the highest incidence. Globally, an estimated 13% of the world’s population has CKD. In resource-limited countries, few individuals who progress to the final stage of CKD (kidney failure) survive due to lack of resources for treatment. Less than half of individuals with CKD are aware of their diagnosis, and 79% of individuals are in the later stages of the disease (Sommers, 2023; Evans et al., 2021).
According to the World Health Organization, approximately 5 to 10 million people around the world die each year as a direct result of CKD. One of the contributors to the high morbidity and mortality of CKD is a lack of awareness of the disease by both patients and clinicians (Evans et al., 2021).
Risk Factors for Chronic Kidney Disease
Risk factors for CKD include:
- Diabetes
- Hypertension
- Cardiovascular disease
- Obesity
- Family history
- Over the age of 60
- History of acute kidney injury (AKI)
- Smoking and/or use of tobacco products
(NKF, 2025a; 2025b)
Causes of Chronic Kidney Disease
CKD arises mostly due to complications from diabetes and hypertension, which are the main causes in over two thirds of CKD diagnoses in the United States (Malkina, 2023). Specifically, the most common causes of CKD include:
- Diabetic nephropathy (the deterioration of kidney function due to diabetes)
- Hypertensive nephrosclerosis (poorly controlled hypertension)
- Glomerular diseases, such as glomerulonephritis
- Genetic conditions, such as polycystic kidney disease
- Autoimmune conditions, such as lupus
- Severe infections, such as sepsis
- Kidney cancer
(Malkina, 2023; NKF 2025b; Torra et al., 2021)
DIAGNOSIS AND STAGING OF CHRONIC KIDNEY DISEASE
Signs and Symptoms of CKD
People with early-stage CKD (stages 1 through 3a) do not typically present with signs or symptoms, and often individuals remain asymptomatic even as the disease progresses (NKF, 2021).
Symptoms may begin to be evident in stage 3b of CKD. In this stage, the individual may be urinating more or less often, have lethargy and trouble concentrating, experience itchy or dry skin, and have numbness and/or edema in their limbs (NKF, 2023a).
In the more advanced stages of CKD (stages 4 and 5), signs and symptoms may include:
- Anorexia/loss of appetite
- Nausea and vomiting
- Stomatitis
- Fatigue and weakness
- Nocturia/oliguria
- Water retention, especially swelling of the feet and ankles
- Seizures
- Chest pain
- Shortness of breath from fluid overload
- Uncontrollable hypertension
(Malkina, 2023; Vaidya et al., 2024)
Due to the lack of signs and symptoms in the early stages of kidney disease, blood and urine tests are essential to determine if the disease is developing or progressing (NKF, 2021).
Laboratory Tests
Two renal function tests are performed to determine whether a person has kidney disease. These are:
- Estimated glomerular filtration rate (eGFR): The eGFR is a blood test that shows the effectiveness of blood filtration in the kidney via a calculation of the flow rate of filtered fluid.
- Urine albumin-creatinine ratio (uACR): The uACR is a urine test that shows whether albumin is leaking into the urine, which is indicative of kidney damage.
It is important to use the results from both the eGFR and the uACR to determine the health of an individual’s kidney. An individual has likely sustained kidney disease if their eGFR has been <60 and/or their uACR has been >30 for more than three months (NKF, 2025a; Sommers, 2023).
Stages of Chronic Kidney Disease
There are five stages of CKD. Stages 1 through 3 are considered early stage, and stages 4 and 5 are considered advanced stage (Malkina, 2023).
| Stage | Description of Damage | eGFR (mL/min/1.73 m2) |
|---|---|---|
| (Sommers, 2023) | ||
| 1 | Kidney damage with normal or increased eGFR | >90 |
| 2 | Mild reduction in eGFR | 60–89 |
| 3 | Moderate reduction in eGFR | 30–59 |
| 4 | Severe reduction in eGFR | 15–29 |
| 5 | Kidney failure (end-stage renal disease) | <15 or on dialysis |
CKD STAGE 1
Stage 1 is the earliest stage of CKD, when evidence of ongoing kidney damage is present for longer than three months and the eGFR is >90. But because an eGFR >90 can also be in the normal range, the eGFR alone does not provide enough criteria to diagnose an individual with stage 1 CKD.
The urine albumin-creatinine ratio (uACR) test result helps determine the risk of further kidney damage and complications for stage 1 CKD:
- If the uACR is <30, the individual is at the lowest risk of further progression of the disease.
- If the uACR is 30–300, the risk for worsening of CKD is increased, along with the risk for heart disease.
- If the uACR is >300, the risk is highest for worsening of the CKD, kidney failure, and heart disease.
(NKF, 2023b)
DEFINING KIDNEY DAMAGE
While kidney damage is usually defined as the urine albumin-creatinine ratio (uACR) being >30 for longer than three months, a diagnosis of stage 1 CKD also requires evidence of ongoing kidney damage, such as:
- Frequent and/or chronic urinary tract infections (UTIs)
- Hematuria (blood in the urine)
- Kidney stones
- Kidney cysts
- Abnormal findings during ultrasound, MRI, or CT
- Abnormal urinalysis
(NKF, 2023b)
CKD STAGE 2
Stage 2 of CKD is indicated by a mild reduction in eGFR and ongoing kidney damage for at least three months. The range of eGFR for stage 2 is 60–89 mL/min/1.73 m2 (Sommers, 2023). Just as in stage 1, individuals with stage 2 CKD usually do not exhibit symptoms, and blood tests are crucial for diagnosis at this stage (NKF, 2021).
CKD STAGE 3
Stage 3a of CKD is indicated by a mild-to-moderate loss of kidney function. The range of eGFR for stage 3a is 45–59 mL/min/1.73 m2 (NKF 2025a; Sommers, 2023).
Stage 3b of CKD is indicated by a moderate-to-severe loss of kidney function. The range of eGFR for stage 3b is 30–44 mL/min/1.73 m2 (NKF 2025d; Sommers, 2023). Symptoms during stage 3b begin to be more detectable. These symptoms include:
- More- or less-frequent urination
- Itchy skin
- Lethargy
- Difficulty concentrating
- Edema of the arms, legs, ankles, or feet
- Shortness of breath
- Nausea and/or vomiting
- Loss of appetite
(NKF, 2023a)
The majority of people first receive their diagnosis of CKD during stage 3a or 3b (NKF 2025a; Sommers, 2023).
PAST THE “POINT OF NO RETURN”
Once the eGFR drops below a critical level, CKD progresses without stopping until it reaches stage 5 (end-stage renal disease). Losing a critical number of nephrons perpetuates a downward spiral of continued loss of more nephrons, and at this point the cycle cannot be reversed even if the underlying cause of CKD is treated (Shabaka et al., 2021).
For this reason, it is important to prevent further damage to the kidneys in the early stages of CKD when the disease’s progression is more likely to be slowed or stopped. However, since most individuals do not have symptoms in the early stages, they are unlikely to know they have CKD and thus do not receive treatment in time to prevent its progression.
CKD STAGE 4
Stage 4 of CKD is indicated by a severe reduction in eGFR. During this stage the individual with CKD may start to show more symptoms. The range of eGFR for stage 4 is 15–29 mL/min/1.73 m2 (Sommers, 2023). Once a patient reaches stage 4, they are referred to a nephrologist (Vaidya et al., 2024).
CKD STAGE 5
The fifth stage of CKD is end-stage renal disease (ESRD), also known as kidney failure. ESRD occurs when 85%–90% of kidney function is gone. The eGFR for stage 5 of CKD is <15 mL/min/1.73 m2 (Sommers, 2023).
Without intervention, the kidneys of individuals in kidney failure are unable to function normally, which can lead to many complications. The treatment options are dialysis, kidney transplant, or palliative care (NKF, 2025b).
Complications of Chronic Kidney Disease
Complications of CKD depend on the stage and may include:
- Cardiovascular disease, which may occur in the advanced stages of CKD due to hypertension, coronary artery disease, or dependent edema caused by fluid retention
- Hypertension, present in over 80% of individuals who have advanced CKD
- Uremia, due to build up of metabolic waste products in the blood and body tissues caused by poorly functioning kidneys
- Anemia, occurring after stage 3 CKD due to the kidneys’ inability to produce sufficient erythropoietin, a hormone that stimulates the production of red blood cells
- Metabolic acidosis, which can lead to muscle wasting and bone loss
- Mineral and bone disorders, including osteopenia and pathologic fractures, typically due to imbalanced serum calcium and serum phosphorus
- Peripheral neuropathy, which may be related to electrolyte imbalances or comorbidities such as diabetes
- Sexual dysfunction, which occurs more often as CKD worsens
- Pericarditis, often associated with uremia or dialysis
- Electrolyte imbalances, such as hyperkalemia and hyperphosphatemia
- Esophagitis and gastritis, etiology unknown
(Sommers, 2023; NKF, 2023c; Malkina, 2023; Nesheiwat & Lee, 2023)
Hypertension, proteinuria, and hyperlipidemia exacerbate CKD and cause the disease to progress to later stages. For this reason, these complications should be treated aggressively in order to prevent CKD from worsening (Vaidya et al., 2024).
TREATMENT OPTIONS AND LIFESTYLE INTERVENTIONS
Because CKD cannot be cured, first-line treatment consists of preventing the progression of the disease, minimizing complications, and changing modifiable risk factors through lifestyle interventions (Evans et al., 2021).
Goals of Treatment
The goals of treatment for CKD include:
- Controlling underlying conditions and treating contributing comorbidities
- Restricting protein, phosphate, and potassium, as needed
- Supplementing vitamin D, as needed
- Treating anemia, as needed
- Adjusting medications to appropriate renal dosing, as needed
- Maintaining the sodium bicarbonate level between 23 and 29 mmol/L
- Avoiding potential nephrotoxins
- Reducing cardiovascular risk
(Malkina, 2023; Shabaka et al., 2021)
MANAGING UNDERLYING CONDITIONS
Controlling both hypertension and hyperglycemia is crucial in order to prevent the progression of CKD to further stages and to support kidney function.
All individuals with CKD require strict blood pressure control (Sommers, 2023). The American Heart Association recommends maintaining blood pressure below 130/80 (Malkina, 2023). Patients with CKD are educated about the importance of measuring their blood pressure every day and recording the measurements into a log that can be reviewed by the treating clinicians. Diuretics also help with hypertension (Vaidya et al., 2024).
Diabetes is a significant risk factor for the development of CKD because hyperglycemia is a factor in both the development and progression of CKD. The relationship between diabetes and CKD is bidirectional, since CKD can also lead to the development of diabetes. Therefore, proper management of diabetes for an individual who also has CKD involves effectively controlling blood glucose levels in order to prevent the progression of CKD. For individuals with both conditions, effective glycemic control can be complicated by the need to preserve renal function. For example, metformin, a commonly prescribed antidiabetic medication, is processed by the kidneys. Metformin can build up in the bloodstream in individuals with CKD and lead to lactic acidosis (Kumar et al., 2023).
SLOWING CKD PROGRESSION
Hypertension, proteinuria, and hyperlipidemia should be treated aggressively with medications in order to prevent CKD from worsening (Vaidya et al., 2024).
An individual with CKD can also make healthier lifestyle choices to try to halt the progression of the disease, such as:
- Quitting smoking or the use of tobacco products
- Exercising regularly
- Getting an adequate amount of sleep
- Losing weight by improving diet and increasing physical activity
- Reducing and managing stress
(NKF, 2023b)
Medications
Medications are an integral part of the treatment plan for a patient with CKD. The renin-angiotensin-aldosterone (RAAS) blockade, sodium-glucose cotransporter 2 (SGLT2) inhibitors, and statins are commonly prescribed medications.
ACEIs/ARBs
The renin-angiotensin-aldosterone (RAAS) blockade refers to treatment with angiotensin-converting enzyme inhibitors (ACEIs) such as lisinopril or angiotensin receptor blockers (ARBs) such as losartan. This treatment is considered the gold standard for patients with CKD. ACEIs and ARBs reduce proteinuria, slow the progression of CKD, and lower cardiovascular risk. The SMART study of 269 patients found that patients on the maximum dosage of an ACEI had 33% greater reduction in proteinuria than patients on lower doses (Shabaka et al., 2021).
SGLT2 INHIBITORS
Sodium-glucose cotransporter 2 (SGLT2) inhibitors work in the proximal convoluted tubule of the kidneys to reduce the loss of glomerular filtration rate (GFR) over time. The most common SGLT2 inhibitors are Jardiance (empagliflozin) and Farxiga (dapagliflozin), which are both currently only available as brand-name medications.
Just like the RAAS blockade, SGLT2 inhibitors slow the progression of CKD. While originally developed to control blood glucose in patients with diabetes, SGLT2 inhibitors have been found to provide renal and cardiovascular protection in patients with CKD. This renal protection is an added effect of the medication and not just an effect of improved glucose levels. Preliminary results from the DAPA-CKD trial, studying the effect of dapagliflozin on patients with CKD, showed that the medication reduced the risk of 50% decline in GFR, end-stage kidney disease, or death by 39% compared to the placebo (Shabaka et al., 2021).
STATINS
Statins are a type of oral medication that lowers LDL cholesterol levels. They are frequently prescribed for patients with CKD due to their increased cardiovascular risk, especially if the patient also has albuminuria. Statins are recommended for patients with CKD who are over the age of 50 and who are not on dialysis. Individuals ages 18 to 49 who are not on dialysis may be prescribed a statin if they have a history of myocardial infarction, stroke, angina, diabetes, or peripheral artery disease.
Studies show conflicting results regarding the benefit of taking statins while on dialysis. The general recommendation is to continue statins if they were being taken prior to the start of dialysis, but not to start taking them for the first time if already on dialysis (NKF, 2025c).
In addition to lowering LDL cholesterol levels, statins provide the following benefits:
- Reduced plaque buildup and atherosclerosis
- Reduced inflammation in the body
- Enhanced blood flow throughout the body
- Reduced risk of blood clots in the heart and brain
- Antioxidant effects by preventing damage to body cells
The generic names of statin medications end in -statin. Some of the most common statins are atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin (NKF, 2025c).
EPOETIN ALFA
Recombinant human erythropoietin, also known as epoetin alfa, is used to treat anemia in individuals with advanced CKD. It is important to use only the lowest effective dose of epoetin in order to maintain the hemoglobin at 10–11 g/dL due to the risk of cardiovascular complications inherent with this medication (Malkina, 2023). Patients with CKD may be educated on taking epoetin alfa at home, if appropriate (Vaidya et al., 2024).
Nutrition Therapy
Good nutrition is an important aspect of therapy for individuals with CKD. A well-balanced diet with appropriate amounts of protein, sodium, and electrolytes can help prevent CKD from progressing to later stages. A well-balanced diet for CKD also helps prevent infection, provide energy to complete activities of daily living (ADLs), maintain muscle mass, and maintain weight.
Diets for individuals with CKD are highly individualized, based on their personal eating preferences as well as their stage of CKD. Over time, the plan may need to be adjusted and certain foods removed or added (NKF, 2024a).
REDUCING SODIUM
The kidneys regulate sodium levels in the body, and kidneys of individuals with CKD are not able to process sodium as efficiently. Excess sodium can lead to volume overload, swelling, hypertension, and strain on the heart (NKF, 2024a). Individuals with CKD whose eGFR is <60 mL/min/1.73 m2 and who have hypertension, volume overload, or proteinuria should restrict their sodium intake to <2 g (2,000 mg) per day (Malkina, 2023).
Individuals with CKD should be educated about how to reduce their sodium intake. Recommendations may include:
- Use herbs and spices while cooking instead of salt to add flavor to meals.
- Always read food labels and select foods that are low in sodium. (In particular, cans of soup, cured meats like bacon and lunchmeat, processed foods, and frozen foods are typically very high in sodium.)
- Request food to be prepared without additional salt when eating out, and ask for sauces on the side, as these are usually high in sodium.
(NKF, 2025d)
REDUCING POTASSIUM, PHOSPHORUS, AND CALCIUM
Individuals with CKD should reduce the amount of potassium, phosphorus, and calcium in their diet.
Potassium Restriction
The restriction of potassium depends on the progression of the individual’s CKD as well as their blood level, dietary customs, and use of potassium-increasing medications. If the eGFR is >30 mL/min/1.73 m2, potassium is not usually restricted.
Hyperkalemia can increase an individual’s risk for cardiac arrhythmias, which can damage vital organs and lead to stroke, heart failure, or myocardial infarction. If the individual has a serum potassium level >5.1 mmol/L, interventions to reduce hyperkalemia include:
- Dietary restrictions, such as avoiding salt substitutes (which are high in potassium) and foods high in potassium (such as bananas, avocados, beef, and many fruits)
- Correcting metabolic acidosis using sodium bicarbonate or an alkaline-ash diet that consists mostly of fruits and vegetables
- Using potassium-lowering diuretics (such as hydrochlorothiazide, bumetanide, and furosemide) and gastrointestinal cation exchangers (such as Kayexalate)
(Malkina, 2023)
MEDICATIONS THAT MAY INCREASE POTASSIUM
For patients in the early stages of CKD, certain medications may increase the potassium level of the blood, and clinicians prescribe these with caution. These medications include:
- Potassium-sparing diuretics
- Angiotensin-converting enzyme inhibitors
- Beta blockers
- Nonsteroidal anti-inflammatory drugs (NSAIDs)
- Cyclosporine
- Tacrolimus
- Sulfamethoxazole/trimethoprim
- Pentamidine
- Angiotensin II receptor blockers
(Malkina, 2023)
When in doubt, contact a pharmacist for clarification on whether a patient with any stage of CKD should be taking a specific medication, as certain medications may need to be avoided or renally dosed to prevent hyperkalemia.
Phosphorus Restriction
Excessive phosphorus in the body can pull calcium from the bones and lead to osteoporosis. This can lead to hypercalcemia levels in the blood, which can cause dangerous calcium deposits to build up in the bloodstream, lungs, eyes, and heart. With time, these deposits increase the likelihood of the individual having a cardiovascular event, which can lead to death.
Phosphorus is found naturally in protein-rich foods like beef, pork, poultry, fish, nuts, beans, and dairy. Phosphorus from animal products is absorbed better in the bloodstream than phosphorus from plant-based products. Phosphorus is also used as a preservative and can be found in fast foods and most processed foods. Individuals with CKD may be prescribed a phosphate-binding medication, such as sevelamer, to take with each meal to reduce the amount of phosphate absorbed from food (NKF, 2025d).
Calcium Restriction
Calcium intake may need to be reduced for individuals with CKD. Foods high in calcium are typically also high in phosphorus, which should be minimized in a CKD diet. When the kidneys of an individual with CKD do not produce enough vitamin D, secondary hyperparathyroidism may result. Secondary hyperparathyroidism leads to a buildup of calcium in the heart and blood vessels. In this situation, the patient may be prescribed vitamin D or cinacalcet, a medication that lowers blood parathyroid hormone levels (NKF, 2025e).
LIMITING PROTEIN
While not every patient with CKD may need to restrict protein in their diet, protein restriction may be beneficial for individuals with an eGFR <60 mL/min/1.73 m2 who do not have nephrotic syndrome. Protein waste is something the kidneys must work hard to remove, and limiting protein can slow the progression of CKD. A goal of 0.6 g/kg/day or less of protein may reduce uremic symptoms and slow progression of CKD for these individuals. A consultation with a registered dietitian can help patients make appropriate protein choices for their specific lifestyle.
However, if a patient reaches kidney failure and starts dialysis, protein levels in the diet should be increased to maintain blood protein levels (Malkina, 2023; NKF, 2025d).
Avoiding Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
NSAIDs like ibuprofen and naproxen can cause renal function to decline, worsen hypertension, and exacerbate electrolyte disturbances (Malkina, 2023). Patients with CKD should avoid taking NSAIDs and may need to be educated to choose an alternative like acetaminophen for pain management, as appropriate. In some healthcare organizations, NSAIDs are included on the allergy list in the medical record as a reminder that these medications cannot be tolerated by a patient with CKD.
Increasing Physical Activity
There are no restrictions on activity for an individual with CKD, and activity is encouraged as a way to manage blood glucose levels, maintain weight, and reduce cardiovascular risk. However, it is important to be aware that fatigue and a lack of interest in increased activity may impact the individual’s ability to exercise (Malkina, 2023).
Other barriers to increased physical activity for individuals with kidney disease include:
- Restrictions on diet
- Alterations to their muscular structure
- Alterations in blood supply (e.g., anemia)
- Weight gain
(Physiopedia, 2025)
Quitting Smoking
Smoking increases the risk of nephrosclerosis, which leads to scarring of the kidney tissues. Studies show that quitting smoking can slow the progression of CKD (Vaidya et al., 2024).
KIDNEY REGENERATION
Currently, reversing kidney damage in patients with CKD is not possible. However, strong evidence from studies on animals has shown that interstitial fibrosis may be reversible. Kidney regeneration is an emerging concept in CKD that would have large, beneficial therapeutic implications if it can be achieved. Kidney regeneration may be achievable through the use of growth factors or multipotent cells directed to aid in kidney regeneration. Stem cells are also being investigated for potential use in preventing further kidney damage and regenerative beneficial effect in patients with CKD (Shabaka et al., 2021).
INTERDISCIPLINARY CARE OF THE PATIENT
The care of a patient with CKD is complex and requires a team approach for effective management. The management team for patients with CKD may include:
- Primary care provider
- Nephrologist
- Nurses who specialize in nephrology
- Registered dietitian
- Physical therapist
- Occupational therapist
- Social worker
- Case manager
- Vascular access surgeon
There are also CKD clinics staffed with physicians, nurses, dietitians, pharmacists, and others who focus primarily on kidney care, evaluation and treatment of complications, patient education, and assistance with lifestyle modifications (Vaidya et al., 2024).
Role of Nursing
Patients with CKD may require nursing care in a hospital, clinic, or dialysis center. Roles for nurses who specialize in nephrology are various and include:
- Staff nurse, such as in a hospital or outpatient setting
- Home dialysis therapies nurse
- Hemodialysis/peritoneal dialysis nurse
- Vascular access coordinator/manager
- Transplant coordinator/manager
- Organ recovery coordinator
- Nurse educator
(ANNA, 2025)
For all of these roles, the nursing process is used to assess the health needs of patients and prevent disease. The care of patients with CKD can be complex since these patients may also have other conditions that affect the care of their kidneys, and vice versa.
Nursing diagnoses for individuals with CKD may include:
- High blood pressure
- Edema
- Dyspnea
- Chest discomfort
- Confusion
- Oliguria
- Anxiety
(Vaidya et al., 2024)
Specific nursing management for patients who have CKD includes:
- Strictly monitoring “ins and outs” of patients in the hospital, including charting how much the patient drinks and urinates at least once per shift
- Being aware of medications that can be nephrotoxic, including statins, aminoglycosides, and NSAIDs; for patients scheduled to receive these medications, checking with the pharmacist or prescribing clinician prior to administering them
- Assessing for edema, especially in the lower extremities; monitoring lung sounds; and administering prescribed diuretics
- Monitoring the patient’s mental status and reporting any abnormal changes in mental status to the clinician
- Monitoring potassium levels, blood urea nitrogen (BUN), and creatinine levels.
- Assessing for changes on telemetry and EKG, which could relate to high or low levels of electrolytes
- Ensuring the patient is eating a low-protein, low-sodium diet and educating the patient about making healthy dietary choices at home
- Educating the patient about CKD stages and progression and emphasizing the importance of taking medications as prescribed to prevent further kidney damage
(Vaidya et al., 2024)
Role of Physical Therapy
An individual with CKD may experience malnutrition, osteoporosis, and sarcopenia (loss of muscle tissue) due to protein-energy wasting (PEW) syndrome as a result of their condition, even in the early stages of the disease. CKD adversely affects body composition and overall musculoskeletal health, resulting in a reduction in muscle performance and physical functioning. These comorbidities can further lead to limitations with mobility and an increased risk for falls.
Individuals with CKD are twice as likely as those without CKD to experience “low-energy” fractures due to disordered bone mineralization, turnover, and growth caused by changes in how the body absorbs vitamin D, serum calcium, phosphate, and parathyroid hormone.
Physical therapy is an important resource to patients with CKD to assess risk for falls, reduce the risk of bone fracture, and determine physical limitations (Hernandez et al., 2018).
Advanced studies show that exercise has many benefits for patients with CKD, including:
- Improved aerobic capacity
- Enhanced muscular functioning
- Improved cardiovascular health
- Better walking ability
- Improved health-related quality of life
- Better survival rates
- Lower mortality
(Gerosideris et al., 2024)
Physical therapy assessments for patients with CKD may include clinical evaluation of muscle mass, body composition, muscle strength, and functional mobility status. Hand grip dynamometry and standardized physical performance-based testing may also be used to assess the extent to which sarcopenia has affected the patient with CKD. Additional resources for assessment of sarcopenia in patients with CKD include:
- Timed up and go (TUG) test
- Falls Efficacy Scale
- Short Physical Performance Battery
- Sarcopenia and Quality of Life questionnaire (SarQoL)
- SARC-F questionnaire
(Hernandez et al., 2018)
Sarcopenia can be most effectively managed through progressive strength training, vitamin D supplementation, and appropriate protein intake. Modifications to physical therapy programming may be necessary based on the patient’s exercise-related blood pressure and nutritional status as well as their specific needs and abilities.
RENAL REHABILITATION PROGRAMS
Renal rehabilitation programs are designed to address the barriers to physical activity that individuals with kidney disease may face and to improve their quality of life. Renal rehabilitation programs are usually 6–12 weeks long and range from 1 to 2 hours per class.
The goals of a renal rehabilitation program are to:
- Enhance muscle strength, mobility, and fitness
- Improve the quality of everyday life
- Lower blood pressure
- Enhance blood glucose control
- Improve cardiovascular and pulmonary function
- Control weight and enhance self-esteem
- Reduce or neutralize side effects of steroid treatment commonly used after kidney transplant, which include muscle wasting, bone thinning, and weight gain
(Physiopedia, 2025)
Renal rehabilitation programs typically include five components:
- Exercise training
- Diet and fluid management
- Medication and medical surveillance
- Education
- Psychological and vocational counseling
(Physiopedia, 2025)
The amount of physical activity and the intensity of exercise are both important aspects of a renal rehabilitation program. Anaerobic exercise can reduce the rate of muscle loss and strength decline. Progressive resistance exercise has been found to improve physical functioning in patients with CKD (Gerosideris et al., 2024; Hernandez et al., 2018).
Some renal rehabilitation programs include the option for the patient to cycle during hemodialysis with an upright or recumbent stationary bike. Research shows that exercising during hemodialysis can help the blood to be cleaned more effectively and result in blood pressure improvement and enhancement of cardiac function (Physiopedia, 2025).
Role of Occupational Therapy
CKD greatly affects the quality of life of individuals with the condition. CKD limits their energy and may also decrease range of motion, causing patients to become extremely debilitated.
CKD may affect a patient’s ability to sleep and wake up, groom, clean, cook, and mobilize while at home. Approximately one fifth of individuals with CKD require help performing basic ADLs, and four fifths of individuals with CKD require assistance with shopping, cooking, and managing medications (London OT, 2025; Gerosideris et al., 2024).
Patients may also have more difficulty engaging in social activities or participating in leisure activities. They may feel depressed, anxious, or stressed because of their diagnosis of CKD.
Once a patient reaches kidney failure and starts hemodialysis, they experience a substantial decrease in their abilities to perform ADLs independently. Because of this, they are at high risk to be either partially or entirely dependent on a caregiver.
Modern advancements in medical care of CKD means patients with CKD are living longer, which highlights the importance of improving the patient’s overall well-being as well as their physical, psychological, and social functioning (Gerosideris et al., 2024).
Occupational therapists help to empower patients with CKD to manage their ADLs. Occupational therapists provide support and modifications based on their professional assessments, which may include:
- Cognitive assessment
- Functional assessment
- ADLs assessment
- Aids and adaptions assessment
- Risk assessment
- Workplace assessment
During assessments, the occupational therapist gets to know the patient and finds out what ADLs are the most important to them in order to individualize treatment. Treatment focuses on modification or adaption of the ADLs that the assessment has isolated.
Goals for patients with CKD receiving occupational therapy may include:
- Improved functional independence
- Increased social interaction
- Increased safety
- Improved self-care
- Ability to manage moods
- Improved mobility
(London OT, 2025)
Role of Pharmacy
Pharmacists play an important role in renally dosing prescribed medications for patients with CKD. Renal dosing means ensuring the dose is appropriate for the patient’s specific level of kidney function based on the stage of their kidney disease. Patients with CKD are not able to excrete medications the same as patients with healthy kidneys, which makes renal dosing important to prevent further damage to the kidneys.
Medications that commonly require renal dosing include:
- Penicillins
- Cephalosporins, such as cefazolin and cephalexin
- Aminoglycosides, such as gentamicin
- Fluoroquinolones, such as ciprofloxacin
- Vancomycin
- Digoxin
- Pain medication
(Malkina, 2023)
Role of the Registered Dietitian
A registered dietitian is an important member of the team for a patient who has CKD plus uncontrolled diabetes and/or hypertension. Registered dietitians help the individual with CKD choose appropriate foods according to the individual’s lifestyle, food preferences, and lab results.
By helping the patient choose foods that are more conducive to their stage of CKD, a registered dietitian can assist with controlling comorbidities and preventing CKD from progressing to later stages (NKF, 2024a). A registered dietician is recommended for patients with CKD who are restricted to less than 0.8 g/kg/day of protein (Malkina, 2023).
CASE
Shusila, a nurse at a primary care provider’s office, is offering patient education to Mrs. Jackson, a 53-year-old female who has just been diagnosed with stage 2 CKD.
Mrs. Jackson says, “My mother died of kidney failure before she turned 60, and now that I’ve been diagnosed with kidney disease, I am very afraid I will end up dying young like my mom did.”
Shusila responds, “It can be scary receiving a diagnosis of chronic kidney disease, especially since your mother died of kidney failure. The good news is that because you receive regular checkups, you were diagnosed in an early stage of the disease. With proper management there are ways to help prevent the disease from progressing to kidney failure. Let’s talk a little about your lifestyle and manageable changes you can make to keep your kidneys as healthy as possible.”
As Shusila reviews Mrs. Jackson’s diet with her, the patient says she eats a banana and yogurt every morning. Shusila replies, “A banana is often a healthy food choice, but it is also high in potassium. I see that your potassium level is 4.9, which is toward the high end of a healthy level. Perhaps you could cut back to half a banana with yogurt in the morning, and we will recheck your potassium levels next month.”
Mrs. Jackson agrees, and after they finish discussing her dietary habits, Shusila lets the patient know that she will be referring her to a registered dietitian as well. They then talk about Mrs. Jackson’s activity level. “I like to go on walks, but I have a hard time finding time due to my busy schedule,” Mrs. Jackson explains.
Shusila responds, “A 10-minute walk a few times a day adds up to the recommended 30 minutes of exercise per day.” Mrs. Jackson determines that she could walk for 10 minutes during her two breaks at work and still have plenty of time to eat her lunch. “Then I could walk around the block when I get home,” she says.
“That’s a great plan,” Shusila replies. “The most important thing is to be consistent and to do some type of activity that you enjoy every day. I will also arrange for physical therapy and occupational therapy consults so that those providers can evaluate both your physical mobility and any needs you may have related to activities of daily living.”
Shusila then educates Mrs. Jackson about her medications, which include an ACE inhibitor, a SGLT2 inhibitor, and a statin. Shusila emphasizes the importance of taking the medication each day without skipping doses. She helps Mrs. Jackson set an alarm on her phone for the appropriate times that are most convenient for Mrs. Jackson to take the medication. This way the alarm will remind Mrs. Jackson to take her medications, and she won’t have to rely on her memory alone.
Shusila continues, “We want to keep your blood pressure under 130/80 to prevent your chronic kidney disease from getting worse. The lisinopril that the doctor has prescribed will help lower your blood pressure. We will recheck your blood pressure at your visit next month in case the dose needs to be adjusted.”
Shusila finishes by asking Mrs. Jackson if she has any questions and makes sure to give her handouts for the lifestyle changes and medications they discussed during her visit.
MEDICAL TREATMENT OPTIONS FOR KIDNEY FAILURE
Stage 5 of CKD is called kidney failure or end-stage renal disease (ESRD). Treatment options at this stage include:
- Dialysis
- Kidney transplant
- Palliative care
(NKF, 2025b)
Dialysis
Dialysis is a process that removes waste products from the blood of a patient who is in kidney failure. The first human dialysis was performed in Germany in 1924. The procedure utilized an “artificial kidney” created from celloidin tubing and crushed leech heads for anticoagulation. In the 1970s, large surface-area dialyzers became available, and the first internal arteriovenous fistula was developed (Arslanian et al., 2020).
There are two types of dialysis for patients with kidney failure: peritoneal dialysis and hemodialysis.
PERITONEAL DIALYSIS
Peritoneal dialysis can be self-administered by the patient overnight in their own home while they sleep. Peritoneal dialysis may be a more convenient option for a patient who does not want to go to a dialysis center three days a week for hours of hemodialysis.
During peritoneal dialysis, one to two liters of dialysate fluid are placed into a surgically inserted catheter in the abdomen. The dialysate fluid contains glucose, magnesium, calcium, chloride, and lactate. The peritoneal membrane is semipermeable and allows solutes and water to be exchanged between the peritoneal space and the dialysate fluid via osmosis and diffusion (Baxter, 2020). In the morning, the patient drains the fluid from their abdomen using the peritoneal dialysis catheter.
HEMODIALYSIS
Hemodialysis provides intermittent filtration that substitutes the natural kidney’s slow, continuous filtration function. Hemodialysis is accomplished through a process that removes the blood in the body through a vascular access and filters it through a dialyzer machine. There are two separate areas on the dialyzer machine, for the dialysate liquid and the patient’s blood. The areas are separated by a semipermeable membrane. Fluid and waste products are able to flow through the semipermeable membrane via diffusion since the pressure on the side with dialysate is lower than on the side that contains the blood. After the blood is filtered, it is returned to the patient until all the blood has been filtered (Sommers, 2023).
However, hemodialysis does not provide the hormonal functions of a kidney, such as blood pressure control, mineral and bone metabolism, and red blood cell production. Medications are used to supplement these hormonal functions when necessary (Arslanian et al., 2020).
Hemodialysis is typically performed at a hemodialysis treatment center by trained nurses and other staff, usually three times per week per patient at a prescheduled appointment. Select patients may be able to self-perform their hemodialysis at home, which provides more flexibility in their daily schedule. The training to perform hemodialysis at home is extensive and can take several weeks to a few months. In addition, the patient will need specific supplies and a hemodialysis machine that their health insurance will likely need to approve (NKF, 2025g).
PAYING FOR DIALYSIS
Prior to the 1970s, a patient who was diagnosed with end-stage renal disease had few options for financial assistance with dialysis treatment, since it was not typically covered by insurance and kidney transplantation was only being introduced as new practice. In 1973, legislation was passed in the United States that supported payment for patients with ESRD. This allowed patients who may have otherwise died to be able to receive life-continuing treatments to manage their condition.
In 2008, the Centers for Medicare and Medicaid Services (CMS) revised the Conditions for Coverage for ESRD that had initially been established in the 1970s. In 2011, the payment for dialysis changed to a “bundled payment” that covered costs for dialysis, medications, labs, and a quality incentive program instead of reimbursing items using a fee-for-service model (Arslanian et al., 2020).
CASE
Malik is a hemodialysis nurse at a hemodialysis treatment center. It is 6 a.m., and he has just arrived to the center to begin his shift. Before the patients arrive, he ensures that the required safety checks for the water treatment, dialysis machines, and other necessary equipment have been conducted by the certified hemodialysis technicians.
As the first-shift patients arrive for their hemodialysis sessions, Malik assesses and weighs each patient. He then calculates the goal for the amount of fluid to be removed during hemodialysis according to the dialysis prescription ordered by their nephrologist. Malik then accesses the patient’s arteriovenous fistula and initiates the hemodialysis treatment.
Together with the certified hemodialysis technician, he obtains and documents each patient’s vital signs every half hour for the next few hours, monitoring for intradialytic hypotension. After the treatment is complete, Malik discontinues the treatment and removes the needle from the patient’s fistula according to policy and procedure. He ensures that the patients’ vital signs are stable and that each patient has been weighed again after treatment.
Malik then cleans and disinfects each station so that everything is ready for the next round of patients (Daily Nurse, 2025).
Kidney Transplant
In 2023, 27,332 individuals received a kidney transplant in the United States. Kidney transplants may originate from a living donor or from the kidney transplant waiting list with kidneys donated from deceased registered donors. In 2023, approximately 30% of individuals who received a kidney transplant obtained it from a living donor, and the remainder received the kidney from a deceased donor. The survival rate one year after a kidney transplant is 90%–95%. A transplanted kidney from a living donor lasts approximately 15 to 20 years and from a deceased donor 8 to 12 years (HRSA, 2024; NKF, 2024d).
Kidney transplantation is considered the most effective long-term treatment option for individuals with kidney failure. Individuals who receive a kidney transplant live longer on average than individuals who receive dialysis to treat their kidney failure, and they also experience a drastically improved quality of life (Kumar et al., 2023; NKF, 2024d).
During kidney transplantation, the new kidney is placed in the iliac fossa. The original kidney usually remains unless there is a need (e.g., infection) to remove it (Sommers, 2023).
TRANSPLANT FROM A LIVING DONOR
Living donation of a kidney involves one person donating one of their kidneys to another person. Most of the time, this donation is from a family member, such as a parent, child, or sibling of the person in need of a kidney. The donor can also be a friend of the recipient or a stranger.
The benefits of kidney transplantation from a living donor include:
- Shorter wait times compared to the kidney transplant waiting list
- Potentially avoiding dialysis, depending on how quickly a living donor can be found
- Reduced risk of rejection
- Longer-lasting kidney compared to kidney from a deceased donor
- Surgery planned at a convenient time for both the donor and recipient
(NKF, 2024b)
To become a living kidney donor in the United States, the donor must be at least 18 years old and have no contraindications for transplant (such as hypertension, cancer, HIV, or hepatitis). The ultimate decision for whether someone can be a donor is made by the transplant center, and the donor must be a compatible match for the recipient (NKF, 2024b).
TRANSPLANT FROM THE KIDNEY TRANSPLANT WAITING LIST
The average wait time for a kidney transplant is three to five years. To transplant a kidney, the blood group and antibody levels of the donor and recipient must match. There were 89,792 Americans on the Health Resources and Services Administration’s kidney transplant waiting list in September of 2024, and 27,332 kidney transplants were performed in 2023 (HRSA, 2024).
Palliative Care
In patients with life-limiting illness such as end-stage renal disease, palliative care is used as an added layer of support for their physical, emotional, and psychological needs. Palliative care may be used to provide comfort, reduce hospital stays, and reduce the use of healthcare resources. Palliative care is provided in a number of settings such as physician practices, hospitals, cancer centers, dialysis units, homes, hospices, and long-term care facilities (NHPCO, 2025).
Palliative care is a recognized subspecialty by the American Board of Medical Specialties. The word palliative comes from the Latin word palliare, which means “to cloak” or “to disguise.” The purpose of palliative care is to reduce the symptoms of a medical condition in order to provide the patient with comfort (Merriam-Webster Dictionary, 2025).
Palliative care provides support to patients with CKD by helping them:
- Manage their symptoms
- Cope with and reduce emotional stress
- Improve their quality of life
(NKF, 2025f)
The National Kidney Foundation recommends that every patient with end-stage renal disease receive a referral for palliative care as soon as possible following diagnosis to better prepare and support the patient throughout the course of the disease (NKF, 2025f). Palliative care combined with conservative management may be a decision chosen by an individual who does not want to pursue renal replacement therapy (kidney transplantation or dialysis). This decision may be ideal for very frail individuals who have poor functional status and many comorbidities (Vaidya et al., 2024).
(See also the course “End-of-Life, Palliative, and Hospice Care” from Wild Iris Medical Education.)
CARE OF THE PATIENT IN KIDNEY FAILURE
Care of the Patient Receiving Dialysis
The patient receiving dialysis may have access sites that require care specific to their type and location. These include:
- Peritoneal catheter in the abdomen
- Surgically inserted vascular access, such as a shunt
- Arteriovenous fistula (arterialized vein)
- Vascular access through a large artery, usually in the neck
(Sommers, 2023)
CARE OF THE PERITONEAL CATHETER
The peritoneal catheter site should be covered with a sterile dressing at all times when the site is not being accessed (Sommers, 2023). To prevent infection and peritonitis when accessing the peritoneal catheter, it is important for healthcare providers to:
- Adhere to aseptic technique
- Wear a mask and ensure others in the room are wearing a mask
- Use sterile gloves
(Baxter, 2020)
Patient education includes:
- Dressing the site appropriately and which materials to use
- Recognizing signs of access site infection, including swelling, redness, drainage, and foul smell
- Wearing loose clothing that is not restrictive around the waist
- Preventing external abdominal pressure
(Sommers, 2023)
Complications can occur initially following surgical insertion of the peritoneal catheter, including:
- Pain (may indicate perforation of another organ when the catheter was placed)
- Bleeding (may indicate perforation of another organ when the catheter was placed)
- Obstruction of the catheter (could be due to a blockage or kink in the catheter)
- Infection (may indicate perforation of the intestines)
- Leaks (may indicate the need for adjustment of catheter placement)
(Baxter, 2020)
Potential complications of the peritoneal catheter site that can occur over time may include:
- Pain
- Perforations
- Peritonitis
- Obstruction of the catheter
- Herniation at the insertion site (as evidenced by pain, distended abdomen, and cloudy dialysate fluid)
(Baxter, 2020)
Placement complications may require surgical intervention, and infections may require antibiotics. Antibiotics may be added directly to the dialysate fluid or may be given parenterally or enterally. Individuals with peritonitis that does not respond to initial antibiotic therapy should receive hemodialysis until the peritonitis resolves and a new catheter can be placed (Baxter, 2020).
CARE OF THE DIALYSIS CATHETER
A dialysis catheter is a soft tube that is usually placed in the subclavian artery. It is typically intended as a temporary access but can sometimes be permanent (NKF, 2025h).
When the dialysis catheter is not being directly accessed, some type of anticoagulant (like heparin) will reside in the catheter to prevent the access from clotting. The anticoagulant must be removed prior to accessing the catheter because pushing the anticoagulant into the patient’s primary circulation could cause bleeding complications by changing their coagulation status (Baxter, 2020).
A dialysis catheter is a central venous catheter, which has an inherently high risk of infection. For this reason, patient teaching centers around preventing infection of the catheter. Patient teaching for patients who have a dialysis catheter also includes:
- Ensuring the dressing on the site (usually on the neck) stays clean and dry
- Changing the dressing at home, if needed, between treatments
- Ensuring the dressing is in place and the catheter is not open to air
(NKF, 2025h)
CARE OF THE SHUNT
An external arteriovenous dialysis access, also called a shunt or graft, is surgically placed, commonly on the nondominant arm of the patient undergoing hemodialysis. One end of a soft tube is placed directly into an artery to join to a vein (NKF, 2025h). A shunt is the second choice for a patient whose blood vessels are not able to tolerate the amount of pressure from a fistula (discussed below). The shunt site is considered sterile and must always be covered with a sterile dressing when it is not being accessed (Sommers, 2023).
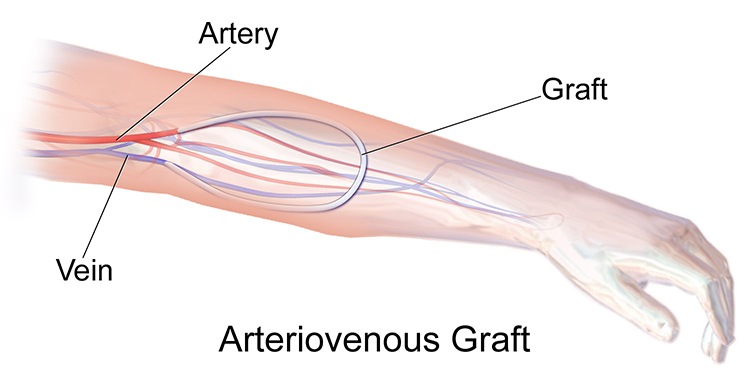
(Source: Blausen.com staff, 2014. CC BY 3.0.)
Complications of a shunt can include:
- Instant hemorrhaging if the shunt accidentally becomes dislodged from the artery
- Clotting, as evidenced by darker blood inside the shunt
(Sommers, 2023)
Patient teaching for patients who have a shunt includes:
- Carrying a clamp and how to use it if the shunt is accidentally dislodged
- Verifying presence of the “thrill” of blood (a buzzing vibration that can be felt while lightly touching the shunt with fingertips) as it moves through the shunt
- Notifying a clinician for signs and symptoms of clotting in the shunt
- Avoiding any direct pressure on the limb with the shunt, including taking blood pressure, carrying heavy items, wearing tight clothing, or sleeping on the side where the shunt is located
- Protecting the site during bathing to keep it sterile
- Not using creams or lotions on or near the shunt
(Sommers, 2023)
CARE OF THE ARTERIOVENOUS FISTULA
An arteriovenous fistula is surgically placed by attaching an artery and vein in the arm (NKF, 2025h). A fistula is the preferred vascular access for a patient on hemodialysis (Vaidya et al., 2024). Unlike a shunt, there is no tube connecting the blood vessels. The connection causes a large, very visible vessel that has enough pressure for hemodialysis to be conducted. After the placement surgery, the patient may require strengthening exercises such as ball grips to help the arterialized vein grow larger (Sommers, 2023).
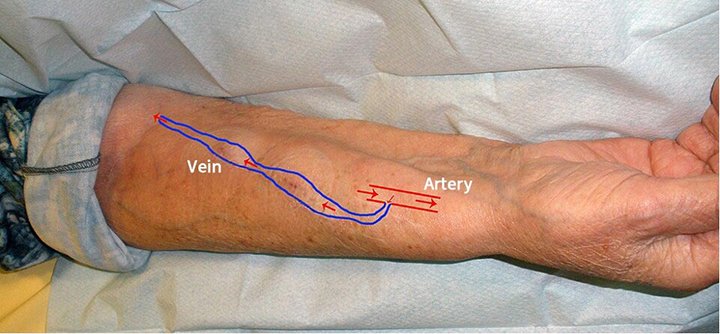
Schema of an arteriovenous fistula used for hemodialysis. (Source: Pravdaz. CC Attribution-Share Alike 3.0.)
In general, a fistula lasts longer than a shunt and usually has fewer complications (NKF, 2025h). Unlike the shunt, the access site does not need to remain sterile and does not need to be protected during bathing.
Pressure dressings applied to shunts and fistulas should not be too tight, as this could cause clotting of the access site. Also, tape or wrap should not be applied circumferentially, as this could lead to clotting as well (Baxter, 2020).
Patient teaching for patients who have a fistula includes:
- Verifying presence of the “thrill” of blood on a daily basis as it moves across the fistula
- Avoiding blood pressure readings or phlebotomy on the arm with the fistula
- Notifying the dialysis team of significant bleeding from the fistula after a hemodialysis treatment
(Sommers, 2023)
Bleeding from a fistula or graft after hemodialysis has been completed should be controlled with direct pressure for 5 to 10 minutes, with care not to occlude the access site while applying pressure. If the direct pressure is not sufficient to control the bleeding, then a topical hemostatic agent may be necessary. Topical hemostatic agents include:
- Gelfoam pads (which create a structure that helps improve clot formation)
- Chitosan (which encourages adhesion)
- Thrombin powder
(Baxter, 2020)
HEMODIALYSIS SAFETY
There are important basic safety considerations for healthcare personnel to keep in mind when a patient is undergoing dialysis, whether the patient is receiving hemodialysis at an outpatient care center or in the hospital. These include:
- Alarms on hemodialysis machines exist for patient safety. Always assess the patient and equipment and be positive about the cause and need for correction of an alarm before clearing the alarm.
- Vascular access and the patient’s face must be visible at all times while the patient is undergoing hemodialysis treatment.
- Bedside nurses caring for patients who receive hemodialysis while in the hospital must follow any instructions from the dialysis nurse while the patient is receiving treatment.
- Blood pressure must be monitored at least every 30 minutes during treatment.
- Eating during hemodialysis is not advised due to the risk of choking and hypotension; if a patient has to eat, provide a small but sufficient quantity and avoid high-carbohydrate foods.
- All staff who participate in patient care must be familiar with their facility’s emergency procedures and their respective role.
- Personnel must understand and follow facility practices to service and maintain equipment, including how to report a machine that may need repairs. (Facility practice must be based on manufacturer’s guidelines.)
- Adhering to the CMS-established limit of ultrafiltration of less than 13 mL/kg/hour will improve patient safety and reduce mortality risk during hemodialysis.
(Arslanian et al., 2020)
TIMING OF MEDICATIONS
Prior to hemodialysis, reducing systolic blood pressure (SPB) can potentially lead to hypotension during hemodialysis (called intradialytic hypotension), increased risk for a major cardiovascular event, and thrombosis of the vascular access site (Kim et al., 2023). Therefore, nurses caring for a patient who will receive dialysis in the next 12 hours should follow their facility’s policy regarding holding antihypertensive medications preceding dialysis.
For concerns about whether a patient should take their antihypertensive medication immediately preceding dialysis, it is important to contact the dialysis nurse, nephrologist, or assigned clinician, as appropriate, to determine whether the antihypertensive medication should be given or held. It is some hospital systems’ policy to hold antihypertensive medications prior to dialysis.
NUTRITIONAL CONSIDERATIONS
Patients in kidney failure are not able to excrete electrolytes and waste products effectively, if at all, without dialysis. Nutritional considerations for patients on dialysis include increasing protein and calories while limiting certain electrolytes and fluid (NKF, 2025i).
Increase Protein and Calories
Unlike patients with earlier stages of CKD, patients on dialysis should increase the level of protein in their diet in order to build muscle, repair wounds, and keep their immune system healthy. Protein can come from including eggs, meat, poultry, fish, and certain vegetables and grains in the diet. Albumin blood levels can indicate whether a patient is not receiving enough protein or calories. Patients on dialysis are advised to increase their calorie intake, but they may not feel hungry. Nutritional supplements such as shakes, liquid drinks, and energy bars are a way for these patients to obtain the nutrition they need (NKF, 2025i).
Limit Electrolytes and Fluids
Patients with kidney failure should restrict their sodium, potassium, phosphorus, and calcium intake. Fluid restriction may be necessary if a patient on dialysis has a rapid increase in weight, swelling of the eyes or extremities, shortness of breath, or an increase in blood pressure. If a patient is gaining too much fluid weight, the dialysis team will let the patient know during dialysis (NKF, 2025i).
OTHER COMPLICATIONS
Common complications of dialysis include:
- Intradialytic hypotension
- Exsanguination (losing >40% of blood volume)
- Dialysis disequilibrium syndrome
- Clotted vascular access site
- Infection of the vascular access site
- Hypoxemia
- Muscle cramps
- Nausea and vomiting
- Itching
- Allergic reactions
- Fever and chills (the third most common reaction)
- Cardiac problems (chest pain and angina, dysrhythmia, cardiac stunning, cardiac arrest)
(Arslanian et al., 2020)
Intradialytic Hypotension
Intradialytic hypotension (IDH) is the most common complication during dialysis, occurring in 20%–30% of dialysis sessions. IDH has been shown to be associated with adverse events such as lower quality of life, inadequate dialysis, and death (Chang et al., 2021). The most common cause of IDH is a reduction in peripheral vascular resistance combined with the rapid reduction in blood volume that occurs during dialysis. Removing an estimated 10% of the patient’s total blood volume does not affect cardiac output, but removing 15% or more will cause cardiac output to drop significantly (Arslanian et al., 2020).
| Loss | Probable result |
|---|---|
| (Arslanian et al., 2020) | |
| 5%–10% |
|
| 12%–20% |
|
| 20%–30% |
|
| 30%–40% |
|
| 40%–50% |
|
| 50% or more |
|
During dialysis, blood pressure is typically checked every 30 minutes or as needed based on the patient’s symptoms in order to evaluate for hypotension. For a patient undergoing hemodialysis, hypotension may be considered a systolic blood pressure <90 mmHg, a drop in systolic BP >20–30 mmHg, or a patient-specific percentage drop of systolic BP.
When hypotension occurs, treatment includes:
- Reducing or stopping the ultrafiltration rate, as appropriate
- Placing the patient in modified Trendelenburg position with their legs elevated and head down
- Providing supplemental oxygen, as appropriate
- Replacing fluids via 100–200 mL bolus of normal saline, as appropriate (taking care not to replace too rapidly, as this could cause fluid overload)
After the hypotension resolves, hemodialysis may be continued if the provider decides that it is appropriate and safe (Arslanian et al., 2020).
Exsanguination
Exsanguination (losing >40% of blood volume) is the point at which hemorrhagic shock occurs. Exsanguination may occur in the following situations:
- Blood lines are accidentally and traumatically separated
- The access needle becomes dislodged
- The vascular access ruptures
- Internal bleeding, such as a gastrointestinal bleed or cardiac tamponade
- Anticoagulant overdose
(Arslanian et al., 2020)
Initial treatment for exsanguination during dialysis entails immediately stopping the dialysis blood pump, clamping the separated blood lines and catheter, and returning the patient’s blood back to them, if possible. In cases where the access needle is dislodged or the access ruptures, direct pressure is applied to the access site to stop the bleeding (Arslanian et al., 2020).
Additional treatment steps include:
- Applying oxygen
- Administering fluids for hypotension
- Monitoring the patient’s vitals
- Contacting the clinician for further orders
The patient may require a blood transfusion and/or emergency services if they do not respond to initial treatment (Arslanian et al., 2020).
Dialysis Disequilibrium Syndrome
Dialysis disequilibrium syndrome is a collection of neurologic symptoms that occurs during or after dialysis. Symptoms of dialysis disequilibrium syndrome can include:
- Headache
- Nausea
- Blurred vision
- Restlessness
- Confusion
- Coma (severe and rare)
- Seizure (severe and rare)
(Bhandari & Komanduri, 2023)
A patient is more likely to experience dialysis disequilibrium syndrome during or after their first hemodialysis treatment, if they are a child or of advanced age, if their blood urea nitrogen (BUN) level is >175 mg/dL prior to the start of dialysis, or if they have preexisting neurologic diseases or conditions such as stroke or cerebral edema.
To treat dialysis disequilibrium syndrome, no matter the severity, the dialysis prescription must be modified by changing the sodium concentration in dialysis fluid (called sodium remodeling). To do this, the sodium dialysate liquid is changed or the dialysis machine is programmed with the modified prescription. The symptoms of dialysis disequilibrium may subside within as few as 30 minutes after the dialysis prescription is modified. In more severe cases that do not respond to sodium remodeling, it may be necessary to reduce the intracerebral pressure with hypertonic saline or intravenous mannitol (Bhandari & Komanduri, 2023).
Clotted Vascular Access Site
A clotted fistula or graft should be emergently declotted. Options for declotting include surgery or locally instilled fibrinolytics such as alteplase (Baxter, 2020).
Infection of the Vascular Access Site
Dialysis sites can become infected and lead to sepsis if not treated. Symptoms of infection at the access site are redness, drainage, or swelling. A complete blood count in addition to blood cultures is performed if signs or symptoms of infection are noted, to assess for sepsis. Depending on the level of infection, the access may need to be removed or not used until the infection has resolved. Temporary dialysis access (lasting only 2–3 days) via the subclavian or femoral artery may need to be utilized while the infection is being treated (Baxter, 2020).
CASE
Ryan is a medical-surgical nurse who is caring for Miguel, a 68-year-old male with end-stage renal disease. At the start of his shift, Ryan gets a report that Miguel will be undergoing hemodialysis at 1100 hours. Per established facility protocols, the dialysis nurse comes to the bedside and remains with the patient while the patient is undergoing dialysis.
Regularly scheduled blood pressure medications (hydralazine and lisinopril, both by mouth) and anticoagulant medications (heparin subcutaneous) are to be administered to Miguel at 0900 hours. Ryan contacts the dialysis nurse, Carolina, who states to give the heparin but give the blood pressure medications only if the patient’s systolic blood pressure is >160. Ryan checks Miguel’s blood pressure, and it is 147/82. Ryan holds the blood pressure medication and communicates this to the primary clinician.
At 1100, Carolina arrives to the bedside and starts the patient’s hemodialysis treatment. First, she verifies that there is a consent for the treatment uploaded to the computer. Carolina explains to Ryan that during the treatment it is very important to always be able to see the access site, which is a fistula on Miguel’s arm.
The treatment lasts for four hours and is complete by 1500. Carolina lets Ryan know that during the treatment 3 liters of fluid was removed from Miguel. Carolina gathers the equipment and leaves. Ryan assesses Miguel, who reports a new-onset headache with a pain level of 8/10. Ryan contacts the primary clinician, who orders a dose of prn acetaminophen. Ryan administers the acetaminophen at 1530 and goes to check on Miguel at 1615. Miguel reports his headache is now mild, indicating the acetaminophen was effective.
However, Ryan spots some blood on Miguel’s sheets. Ryan checks Miguel’s fistula and sees that the dressing placed by the dialysis nurse has come off the fistula. There is a scant amount of blood oozing from the fistula. After performing hand hygiene, Ryan puts on clean gloves. He applies direct pressure for five minutes while taking care not to apply the pressure so hard as to occlude the fistula. After five minutes, there is no more bleeding from the site. Ryan places a new gauze pad to the fistula and tapes it in place around the edges. Ryan knows he should not wrap tape or self-adherent wrap circumferentially around the fistula because that could lead to clotting. Ryan assesses the dressing after 30 more minutes, and it is clean, dry, and intact.
Care of the Patient Receiving a Kidney Transplant
Preop and postop care of the patient receiving a kidney transplant is the same as care provided to an individual having any other abdominal surgery.
The greatest postoperative issue is rejection of the transplanted kidney. Immediate postop care of the individual who received a kidney transplant includes strict monitoring of urine output, since a decrease in urine output could indicate that the kidney is being rejected.
The nurse also watches for these additional signs of rejection/infection during the immediate postop period:
- Sudden weight gain
- Edema
- Fever
- Pain over the surgical site
- Hypertension
- Increased white blood cell (WBC) count
Individuals who receive a kidney transplant must take antirejection medications (such as tacrolimus and mycophenolate) to prevent the kidney from being rejected. These medications suppress the immune system, putting the individual at higher risk for infection (Sommers, 2023).
Nursing Interventions
Patients who receive a kidney transplant receive extensive discharge teaching prior to leaving the hospital. The nurse educates the patients and family about:
- Antirejection (immunosuppressant) medications, including information on dosage, potential side effects, interactions with other medications, and importance of taking medications as prescribed without missing doses as the best way to protect the transplanted kidney
- Risk for infection and how to decrease that risk, such as avoiding others who are sick
- Practicing sun safety, since immunosuppressants increase the risk of skin cancer
- Signs of rejection, such as fever greater than 101 ˚F (38.3 ˚C), decreased urine output, and pain or tenderness over the new kidney
- Activity restrictions, including contact sports and heavy lifting (due to the placement of the transplanted kidney)
- Contacting the physician immediately if signs of infection, rejection, or skin changes are noticed
- Avoiding certain foods, such as raw or undercooked meat, fish, and seafood; raw or undercooked eggs; unpasteurized dairy products; and grapefruit and unwashed produce (because an individual who received a kidney transplant is more likely to contract a food-borne illness due to taking immunosuppressants)
(Sommers, 2023; NKF, 2024c)
Exercise Rehabilitation Interventions
After kidney transplantation, physical activity is an important aspect of post-transplant management to improve overall health and reduce the likelihood of complications. Patients who receive a kidney transplant commonly gain weight and struggle with management of diabetes, hypertension, and dyslipidemia. Increasing physical activity and exercise can help with managing these conditions and lowering cardiovascular risk for these patients.
Kidney transplant recipients may have lower exercise tolerance because of factors including:
- Physical limitations
- Comorbidities
- Muscle atrophy
- Depression
- Fatigue
- Fear of injury
- Problems with motivation levels
Fewer than one third of kidney transplant recipients are able to maintain physical activity levels recommended for optimal maintenance of health. Post-transplant exercise rehabilitation has been demonstrated to provide several benefits, including improved exercise capacity, renal function, and heart rate. However, significant changes to body mass or blood pressure due to post-transplant exercise rehabilitation have not been demonstrated (Wilkinson et al., 2021).
CONCLUSION
Chronic kidney disease is an irreversible condition in which the kidneys gradually lose their ability to function properly. The disease is divided into five stages. Stage 1 CKD represents the most minimal damage to the kidneys, while stage 5 CKD equates to complete kidney failure. Treatment during stages 1 through 4 is targeted toward stopping the disease from progressing to later stages, while treatment during kidney failure involves replaces the functions of the kidney through kidney transplant or dialysis. Education for patients in early stages of CKD focuses on helping the patient make changes to prevent the disease from progressing. Care for patients in kidney failure focuses on helping patients cope with their diagnosis while supporting decisions they make related to their care.
Interventions for patients with CKD include education on the disease, medications, and lifestyle changes. Physical therapy and occupational therapy providers are an important resource for helping patients to maintain or regain their mobility and ability to complete activities of daily living. Basic nursing care for patients who receive dialysis includes safety regarding fistula, shunt, and dialysis catheter care.
Individuals diagnosed with CKD are living longer due to advancements in medications, dialysis, and kidney transplantation. A team-based approach is needed in order to provide appropriate support and improve overall quality of life.
RESOURCES
American Association of Kidney Patients (AAKP)
CKD kidney dietitian directory (National Kidney Foundation)
Kidney CARES Program (NYU Langone Health)
Life Options Rehabilitation Program
REFERENCES
NOTE: Complete URLs for references retrieved from online sources are provided in the PDF of this course.
American Nephrology Nurses Association (ANNA). (2025). The nephrology nursing specialty. https://www.annanurse.org
Arslanian J, Burwell K, Dewald G, et al. (2020). Chapter 22: Hemodialysis. In CS Counts (Ed.), Core curriculum for nephrology nursing (7th ed.). American Nephrology Nurses’ Association.
Baxter CS. (2020). Renal and genitourinary emergencies. In V Sweet & A Foley (Eds.), Sheehy’s emergency nursing: Principles and practice (7th ed.). Elsevier.
Bhandari B & Komanduri S. (2023). Dialysis disequilibrium syndrome. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov
Blausen.com staff. (2014). Medical gallery of Blausen Medical 2014. WikiJournal of Medicine, 1(2). https://doi.org/10.15347/wjm/2014.010
Chang TI, Tatoian ET, Montez-Rath ME, & Chertow GM. (2021). Timing of antihypertensive medications on key outcomes in hemodialysis: A cluster randomized trial. Kidney 360, 2, 1752–60. https://doi.org/10.34067/KID.0001922021
Daily Nurse. (2025). A day in the life of a nephrology nurse. https://www.dailynurse.com
Evans M, Lewis RD, Morgan AR, Whyte MB, Hanif W, Bain SC, Davies S, et al. (2021). A narrative review of chronic kidney disease in clinical practice: Current challenges and future perspectives. Advances in Therapy, 39(1), 33–43. https://doi.org/10.1007/s12325-021-01927-z
Gerosideris N, Daskalou SD, Ouzouni C, Vlotinou P, & Katsouri IG. (2024). Occupational therapy for individuals with chronic kidney disease undergoing renal rehabilitation: A literature review. Brazilian Journal of Science, 3(10), 16–27. https://doi.org/10.14295/bjs.v3i10.643
Health Resources and Services Administration (HRSA). (2024). Organ donation statistics. https://www.organdonor.gov
Hernandez H, Obamwonyi G, & Harris-Love M. (2018). Physical therapy considerations for chronic kidney disease and secondary sarcopenia. Journal of Functional Morphology and Kinesiology, 3(1), 5. https://doi.org/10.3390/jfmk3010005
Kim IS, Kim S, Yoo TH, & Kim JK. (2023). Diagnosis and treatment of hypertension in dialysis patients: A systematic review. Clinical Hypertension, 29(1), Article 24. https://doi.org/10.1186/s40885-023-00240-x
Kumar M, Dev S, Khalid MU, Siddenthi SM, Noman M, John C, Akubuiro C, et al. (2023). The bidirectional link between diabetes and kidney disease: Mechanisms and management. Cureus, 15(9), e45615. https://doi.org/10.7759/cureus.45615
London OT. (2025). Chronic kidney disease. https://www.londonot.co.uk
Malkina A. (2023). Chronic kidney disease—genitourinary disorders. Merck Manuals Professional Edition. https://www.merckmanuals.com
Merriam-Webster Dictionary. (2025). Palliative. http://www.merriam-webster.com
Myhre J & Sifris D. (2023). What is kidney disease? Verywell Health. https://www.verywellhealth.com
National Hospice and Palliative Care Organization (NHPCO). (2025). Explanation of palliative care. https://www.nhpco.org
National Kidney Foundation (NKF). (2025a). Stages of chronic kidney disease (CKD). https://www.kidney.org
National Kidney Foundation (NKF). (2025b). Treatment and support. https://www.kidney.org
National Kidney Foundation (NKF). (2025c). Statins. https://www.kidney.org
National Kidney Foundation (NKF). (2025d). NKF nutrition coach. https://www.kidney.org
National Kidney Foundation (NKF). (2025e). Secondary hyperparathyroidism. https://www.kidney.org
National Kidney Foundation (NKF). (2025f). How does palliative care improve the quality of life for kidney patients? https://www.kidney.org
National Kidney Foundation (NKF). (2025g). Home hemodialysis. https://www.kidney.org
National Kidney Foundation (NKF). (2025h). Hemodialysis access. https://www.kidney.org
National Kidney Foundation (NKF). (2025i). Nutrition and kidney failure. https://www.kidney.org
National Kidney Foundation (NKF). (2024a). Nutrition and kidney disease, stages 1-5 (not on dialysis). https://www.kidney.org
National Kidney Foundation (NKF). (2024b). Getting a living donor kidney transplant. https://www.kidney.org
National Kidney Foundation (NKF). (2024c). Life with a kidney transplant. https://www.kidney.org
National Kidney Foundation (NKF). (2024d). Kidney transplant. https://www.kidney.org
National Kidney Foundation (NKF). (2023a). Stage 3b chronic kidney disease (CKD). https://www.kidney.org
National Kidney Foundation (NKF). (2023b). Stage 1 chronic kidney disease (CKD). https://www.kidney.org
National Kidney Foundation (NKF). (2023c). Chronic kidney disease (CKD). https://www.kidney.org
National Kidney Foundation (NKF). (2021). The basics: Kidneys and chronic kidney disease. https://www.kidney.org
Nesheiwat Z & Lee JJ. (2023). Uremic pericarditis. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov
Physiopedia. (2025). Renal rehabilitation. https://www.physio-pedia.com
Shabaka A, Cases-Corona C, & Fernandez-Juarez G. (2021). Therapeutic insights in chronic kidney disease progression. Frontiers in Medicine, 8, 1–12. https://doi.org/10.3389/fmed.2021.645187
Sommers MS. (2023). Chronic kidney disease. In Davis’s diseases and disorders: A nursing therapeutics manual (7th ed.), F. A. Davis Company.
Torra R, Furlano M, Ortiz A, & Ars E. (2021). Genetic kidney diseases as an underrecognized cause of chronic kidney disease: The key role of International Registry Reports. Clinical Kidney Journal, 14(8), 1879–85. https://doi.org/10.1093/ckj/sfab056
Vaidya SR, Aeddula NR, & Doerr C. (2024). Chronic kidney disease (nursing). StatPearls [Internet]. https://www.ncbi.nlm.nih.gov
Wilkinson TJ, Bishop NC, Billany RE, Lightfoot CJ, Castle EM, Smith AC, & Greenwood SA. (2022). The effect of exercise training interventions in adult kidney transplant recipients: a systematic review and meta-analysis of randomized control trials. Physical Therapy Reviews, 27(2), 114–34. https://doi.org/10.1080/10833196.2021.2002641