Cardiac Patient Care: Coronary Artery Disease (CAD)
Online Continuing Education Course
Course Description
Coronary Artery Disease (CAD) CEU course on caring for patients with or at risk. Cardiac patient care continuing education covers risk factors for heart disease and heart attack, prevention measures, assessment and diagnosis, complications and comorbidities, emergency treatment, and management.
Course Price: $49.00
Contact Hours: 9
Pharmacotherapeutic Hours: 0.75
Course updated on
December 3, 2024
"This is one of the best overall CEU courses I've taken online. This course was easy to understand and worded at an appropriate level. I recommend this course." - Jane, RN in Florida
"Very detailed regarding nursing and medical management. Also very thorough regarding background information and interventions. Helpful and easy to implement into practice." - Annelise, PT in California
"Always love your courses. Well written and informative!" - Michelle, OT in Louisiana
"The course was evidence based. I was able to understand the complications and comorbidities associated with CAD. Excellent information to utilize in my clinical practice." - Donna, PT in North Carolina
Cardiac Patient Care: Coronary Artery Disease (CAD)
Copyright © 2024 Wild Iris Medical Education, Inc. All Rights Reserved.
LEARNING OUTCOME AND OBJECTIVES: Upon completion of this course, you will be better prepared to plan, deliver, and evaluate evidence-based preventative and therapeutic care for patients with or at risk for coronary artery disease. Specific learning objectives to address potential knowledge gaps include:
- Describe the anatomy and normal blood circulation of the heart.
- Review the pathophysiology of CAD.
- Differentiate between the major clinical presentations of CAD.
- Discuss nonpreventable and preventable risk factors.
- Identify the signs, symptoms, and clinical test outcome criteria used to screen and diagnose coronary artery disease.
- Analyze actions taken to manage acute disease.
- Explain the components of a comprehensive plan of care and monitoring for patients with chronic CAD.
TABLE OF CONTENTS
INTRODUCTION
Coronary artery disease (CAD) is caused by atherosclerosis of the coronary arteries that leads to a restriction of blood flow to the heart. Atherosclerosis (or arteriosclerosis) is a word that comes from the Greek athere, meaning “fatty mush,” and skleros, meaning “hard.” Thus, it is commonly referred to as hardening of the arteries.
Atherosclerosis is a process that develops slowly over time. Typically, atherosclerosis begins in a person’s teenage years or earlier, and the disease worsens quietly for decades, based primarily on diet, lifestyle, and genetic traits. As people age, their atherosclerosis becomes more likely to involve the arteries of the heart and to become coronary artery disease.
Atherosclerosis is a chronic condition that narrows arteries by building lipid bulges in the arterial walls. These bulges are called atherosclerotic plaques, or simply plaques. These plaques can cause a narrowing of small blood vessels such as the coronary arteries, restricting the blood flow to the myocardium. Injury to the endothelium (the lining of the blood vessel wall) occurs, causing inflammation. In some people, the plaques become covered by collagen, narrowing the blood vessel lumen and restricting blood flow to distal tissue. When the blood vessels in question are the coronary arteries, the myocardium receives an insufficient amount of blood and, therefore, oxygen, resulting in ischemia and pain (Harding et al., 2022).
The myocardium is constantly active, and it requires a continuous blood supply. When a coronary artery is sufficiently narrowed or blocked, the heart muscle it supplies works less efficiently. If ischemia continues unrelieved, the inadequate supply of oxygen to the heart tissue causes the cells to infarct or die. Dead tissue is referred to as necrotic.
A reduced blood supply will reduce the oxygen supply to heart muscle, as oxygen is carried on the hemoglobin molecule. An oxygen-starved heart muscle responds with a characteristic feeling of pain or discomfort called angina. Angina is caused by either a decreased supply of oxygen to the myocardium, an increased oxygen demand, or a combination of both. An estimated 9.8 million people in the United States are believed to have angina of some form or another, and over 500,000 new cases are diagnosed each year (Dorwart, 2022).
When its arteries are narrowed by atherosclerosis, a heart may still get enough oxygen to pump blood at rest. But exercise increases the work of the heart, and narrowed arteries cannot always deliver the excess oxygen required by an exercising heart. A person with narrowed coronary arteries will develop angina when exercising. One of the first symptoms of coronary artery disease is the appearance of angina when a person is working strenuously. (Angina is discussed in detail later in this course.)
Preventative Measures
The progression of atherosclerosis can be slowed or even stopped by a few preventive measures. These include stopping smoking, maintaining a healthy weight for one’s height and age, exercising regularly, and eating a low-fat, balanced diet. This includes foods with a low glycemic index and the right sort of fats. To control atherosclerosis, it is also important to keep blood pressure low, reduce low-density cholesterol levels, increase high-density cholesterol levels, and treat diabetes by maintaining fasting glucose levels at 70–100 mg/dL.
People who develop symptomatic CAD should begin or continue these anti-atherosclerotic programs. They should take aspirin daily to prevent platelets aggregating or clumping together, and they should take other medications (typically, beta blockers) to reduce the workload of the heart. Nitroglycerin tablets can be used to alleviate or prevent anginal pain, and interventional procedures are available to widen narrowed arteries and maintain their newly expanded diameter.
Incidence and Impact
According to 2021 data, cardiovascular diseases were the underlying causes of 931,578 deaths in the United States and claim more lives each year than all forms of cancer and chronic lower respiratory diseases combined. CAD was the leading cause (40.3%) of deaths attributable to cardiovascular diseases, followed by stroke (17.5%), high blood pressure (13.4%), heart failure (9.1%), and diseases of the arteries (2.6%). It is estimated that 13% of deaths in the United States, or 375,476 people per year, are due to heart disease alone. In addition, heart disease is the primary cause of death in women, taking more lives than all cancers combined. It is estimated that nearly one half of all middle-aged men and one third of middle-aged women in the United States will develop some form of the disease (AHA, 2024a).
However, the U.S. annual death rate due to coronary heart disease has declined 15% between 2011 and 2021. This is believed to be influenced by improved public education, earlier initiation of treatment, and improved treatment modalities.
Coronary artery disease is not just a problem in the United States. Throughout the world, coronary artery disease causes nearly 20 million deaths annually and is responsible for more than 13% of healthcare-related costs, higher than any other single illness (AHA, 2024a).
TERMS RELATED TO CAD
Coronary artery disease is the result of atherosclerosis of the coronary arteries of the heart. Other names for CAD include:
- Cardiovascular heart disease
- Coronary heart disease (CHD)
- Ischemic heart disease (IHD)
- Atherosclerotic heart disease
- Coronary atherosclerotic disease
The main forms of CAD are:
- Chronic stable angina
- Acute coronary syndromes
The three main acute coronary syndromes are:
- Unstable angina
- Myocardial infarction (MI)
- Sudden cardiac death
ANSWERING PATIENT QUESTIONS
Q: I’ve heard that women get different heart disease than men. Is this true?
A: As far as we know, women and men get the same disease, called coronary artery disease or coronary heart disease. This disease is caused by the same atherosclerosis in both men and women, and it affects the arteries of the heart the same way in everyone.
Just as with men, CAD is the number one killer of women in the United States. For both men and women, the likelihood of getting heart disease increases as a person gets older. The same factors also increase the chances of getting the disease for both men and women: smoking, a fat-filled diet, being overweight, having high cholesterol, doing little or no physical exercise, having diabetes, having high blood pressure, and coming from a family that tends to have heart disease.
Nonetheless, there are some differences in how the disease affects men and women. Before menopause, women are less likely to get heart disease than men of the same age. After menopause, a woman’s risk increases to levels similar to a man’s, but this risk can be reduced earlier in a woman’s premenopausal years by improving her lifestyle (stopping smoking, maintaining a moderate weight, eating nutritiously, exercising regularly, keeping her blood pressure and low density cholesterol low, and treating diabetes).
CIRCULATION OF THE HEART
The heart is made up almost entirely of muscle. Cardiac muscle, which differs from the skeletal and smooth muscle of the rest of the body, is dependent on aerobic metabolism. This means that the heart cannot function without a constant supply of oxygen.
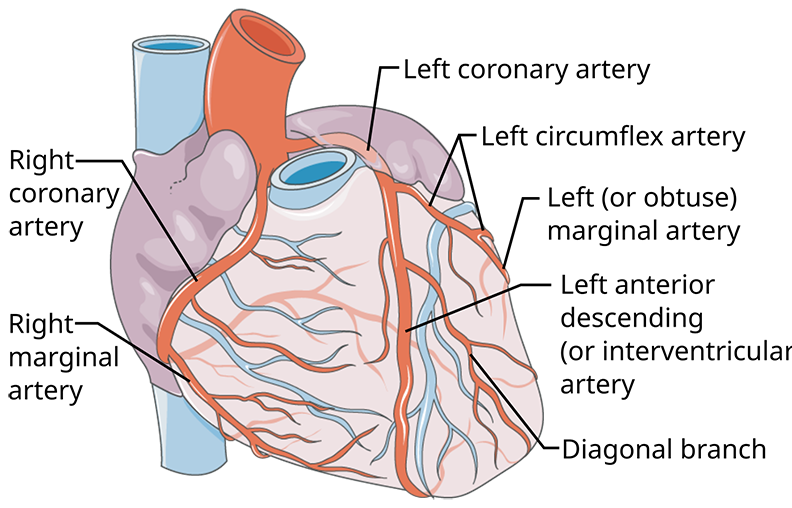
The coronary arteries and their main branches are large, and they run along the outer surface of the heart. The smaller arteries, which directly feed the heart muscle, dive deep into the walls of the heart. (Source: Servier Medical Art. Annotations by Michael Haggstrom, MD. Creative Commons Attribution 3.0.)
Coronary Arteries
Just beyond the aortic valve—the outflow valve of the left ventricle of the heart—the right and left coronary arteries are the first branches of the aorta. The two coronary arteries and their main branches run in grooves along the outside of the heart; these grooves separate the left and right ventricles, and they also separate the atria from the ventricles. The coronary arteries and their main branches are called epicardial arteries because they run on the outer surface of the heart.
From the coronary arteries and their major branches, many small arteries run into the muscular walls of the heart, and these small arteries give rise to rich capillary networks that bathe the cardiac muscle cells with blood and oxygen during diastole. All arteries inside the heart walls are fed by branches of either the right or left main coronary arteries.
People may vary in the way the blood supply to the heart is divided between the right and left coronary arteries due to anatomical differences. In most people, the left coronary artery supplies most of the blood used by the left ventricle, the interventricular septum, and part of the right ventricle. The right coronary artery supplies most of the blood used by the walls of the right ventricle and part of the posterior wall of the left ventricle. In 90% of people, the right coronary also supplies the atrioventricular (AV) node and the bundle of His, causing serious dysrhythmias in the presence of blockage.
There is not much overlap between the territories of the major branches of the coronary arteries. Therefore, if one of the major branches suddenly becomes blocked, there is no other blood supply to the territory served by that branch, and muscle in that territory will be deprived of oxygen (Harding et al., 2022).
A common finding in coronary artery disease is collateral circulation, the development of additional arteries that form a natural bypass from one side of a blocked artery to the other. Research suggests that coronary collateral circulation may help to prevent angina, reduce ischemia, preserve ventricular contractile function, and improve prognosis in patients with coronary artery disease. Collateral circulation may be increased by physical exercise (Harding et al., 2022).
LEFT CORONARY ARTERY
The left coronary artery splits into two main branches, the left anterior descending (LAD) coronary artery and the left circumflex coronary artery. The LAD coronary artery runs down the front of the heart along the groove between the left and right ventricles. In most people, the LAD supplies blood to the front wall of the left ventricle and to the interventricular septum. Loss of blood flow to the left ventricle causes infarcted tissue that will compromise the ventricle’s ability to pump blood to the rest of the body. Between 40% and 50% percent of MIs are caused by an obstruction of LAD coronary artery.
The left circumflex coronary artery runs to the left (at a right angle to the LAD) along the groove between the left atrium and the left ventricle. The left circumflex coronary artery supplies blood to the side or lateral wall of the left ventricle. Fifteen to 20% of MIs are caused by an obstruction of the left circumflex coronary artery (Harding et al., 2022).
RIGHT CORONARY ARTERY
The right coronary artery (RCA) runs to the right, along the groove between the right atrium and the right ventricle. The RCA branches behind the heart and gives rise to the posterior descending coronary artery, which parallels the LAD in front. The RCA supplies the apex and the posterior of the heart. In most people, it supplies blood to the right ventricle and to the sinus and AV nodes of the heart’s electrical conduction system. Between 30% and 40% of myocardial infarctions (MI) are caused by an obstruction of the RCA (Harding et al., 2022).
Normal Blood Flow to the Heart
The blood flow through the heart usually keeps up with the body’s demand. The demand is increased by exercise and strong emotions, both of which make the heart pump more quickly and more forcefully, causing the heart to use more oxygen. When the heart beats twice as fast, it needs twice as much oxygen. Increased cardiac workload leads to increased oxygen demand. Normally, the extra oxygen needed during exercise is supplied by a faster and more voluminous blood flow through the coronary arteries.
HEART RATE
Faster blood flow is a direct result of a faster heart rate. Blood flow to the heart automatically speeds up as the heart beats more quickly because the coronary arteries are fed directly by the outflow of the aorta (Harding et al., 2022).
ARTERIAL WALL TENSION
Throughout the body, the volume of blood flow is regulated by the size of the arteries. Arteries have an innate tension in their walls. This tension keeps arterial volume at a particular level, and the tension also creates a resistance to blood flow. When the arterial wall tension is reduced, the artery stretches more easily and can carry a larger volume of blood.
The natural state of coronary arteries and their main branches is relatively wide open, and in general, these arteries do not limit the volume of blood getting to the muscle cells inside the heart. Instead, it is the small arteries inside the walls of the heart that widen and narrow, thus controlling the volume of blood flow to the muscle cells.
The control of the arterial wall tension (the force that widens and narrows the arteries) is local. As muscle cells work harder, they change the concentration of molecules (e.g., oxygen) surrounding them. Most molecular changes resulting from hard work relax the arteries in the vicinity. In addition, during exercise or stress, sympathetic nerves reduce the tension in the walls of arteries. Together, these factors relax the walls of the arteries and increase the local blood flow.
In older adults, the arteries become progressively stiffer with aging. This causes displacement of the arterial wall, especially when exacerbated by hypertension or other comorbidities, which can put the person at higher risk for atherosclerosis (Harding et al., 2022).
Myocardial Ischemia
Myocardial ischemia occurs when blood flow and blood volume are insufficient to supply all the oxygen needed by the heart muscle.
BLOOD LOSS TO MUSCLE CELLS
What happens to heart muscle cells when they become ischemic? As soon as the blood flow to an area of heart muscle is stopped, the cells begin to lose their energy stores, and within a few minutes the muscle cells are no longer able to contract. Any region of the heart that loses all its blood flow will stop working almost immediately.
Although muscle cells stop working, they do not begin to die until 20–30 minutes after losing their blood supply. This is because the tissues remain at least partially oxygenated for a brief period until the lack of new or insufficient blood supply causes irreversible tissue necrosis. If blood flow is restored within a half hour, most muscle cells will eventually recover; however, the recovery can take from 10 minutes to several days. During that time, the heart acts “stunned” and may not contract well unless stimulated by inotropic drugs (Ecgwaves, 2024).
Another effect of sudden ischemia of the heart is electrical irregularity. Before muscle cells begin to die, they become electrically unstable. After the blockage of a major coronary artery, the electrical instability of some people’s hearts may lead to dysrhythmias, which can be potentially fatal.
SYMPTOMS OF ISCHEMIA
Cardiac ischemia usually produces symptoms, and the classic symptom of reduced oxygen supply to the myocardium is a type of chest pain called angina pectoris, or simply angina. Angina, from the Latin word that means “squeezing,” typically feels like crushing or squeezing, although sometimes it is described as burning or pressure. The sensation is usually felt inside the chest behind or just to the left of the sternum. The feeling can also radiate to the lower part of the neck, jaw, shoulder, back, or down the ulnar side (inside) of the left arm. The feeling can radiate to either or both arms.
The sensation of angina can vary from mild to diffuse unbearable pain. It is transient and does not cause cell damage but may be a precursor to the tissue death that will occur if the ischemia that causes angina is not treated and progresses to an MI. Other symptoms that may accompany the chest pain include nausea, dyspnea, fatigue, and dyspepsia (Morata & Flynn, 2024).
Although women tend to visit their physicians more often than men and therefore report more symptoms, including chest pain, their angina symptoms usually present in the form of upper abdominal discomfort, neck or jaw pain, or shortness of breath as opposed to crushing or squeezing chest pain. Women are also more likely than men to associate their angina with emotional or mental stress. Both the American Heart Association and the Centers for Disease Control and Prevention recommend that women be educated on gender-specific symptomology to ensure that diagnostic procedures and treatment start within one hour of cardiac-based symptoms (CDC, 2024a).
Dysrhythmias
Dysrhythmias (changes in the heart’s rhythm) are another significant result of sudden ischemia and can be serious. The dysrhythmias that sometimes result from cardiac ischemia (notably, ventricular fibrillation or ventricular tachycardia) are the most common causes of sudden cardiac deaths after an acute myocardial infarction. When frequent premature ventricular contractions (PVCs), runs of ventricular tachycardia (VT), or brief runs of ventricular fibrillation (VF) are noted on the monitor, the patient’s care provider must be immediately notified to determine the possible causative factor and to expedite treatment to prevent cardiac arrest.
PATHOPHYSIOLOGY
Coronary artery disease is the umbrella term for various syndromes of heart ischemia that are caused by atherosclerotic obstruction of the coronary arteries. The atherosclerotic damage ranges from gradual narrowing of the coronary arteries (due to bulging patches of fibrous plaque) to the obstruction of a coronary artery that can eventually lead to an MI because of the gradual narrowing of the interior diameter of the coronary arteries or the more sudden blockage of the artery(ies) by the rupture of a plaque.
Imaging technology, such as a cardiac angiogram or a coronary artery CT scan, allow for the identification and quantification of the presence of atherosclerotic plaques in the coronary arteries (Harding et al., 2022).
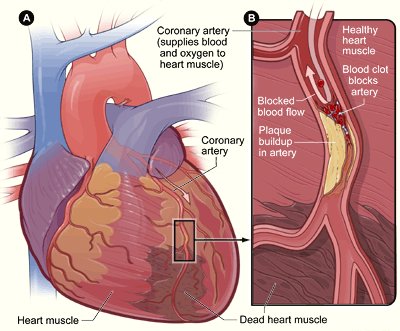
The heart damage in coronary artery disease ranges from narrowing of a coronary artery to complete blockage of a coronary artery. (Source: National Heart, Lung, and Blood Institute.)
Atherosclerosis
Atherosclerosis is the disorder that underlies coronary artery disease. Atherosclerosis thickens the walls of medium and large arteries. The atherosclerotic thickenings occur as bulges, called plaques, in the arterial walls. Plaques contain lipids, white cells, smooth muscle cells, and connective tissue in a poorly organized mass that lies just under the endothelial lining of the artery wall.
The atherosclerotic plaques combine with inflammation and scar tissue to exacerbate partial or complete blockage of the coronary arteries, causing myocardial ischemia or infarction. When atherosclerosis affects the coronary arteries, the problem is usually systemic as well. Occlusions may occur in large or small arteries, compromising circulation (Harding et al., 2022).
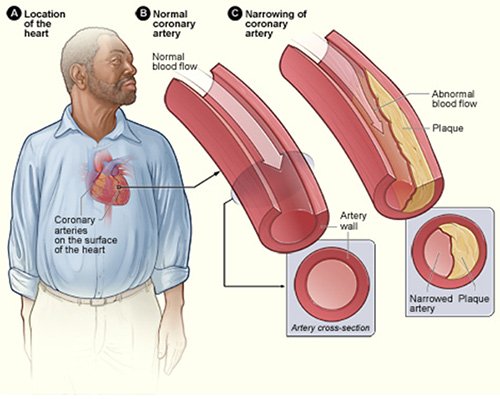
In atherosclerosis, fat and cells collect in bulges just below the surface of the lining of arteries. These bulges are called plaques. Over time, the plaques thicken and reduce the inner diameter of the arteries, allowing less blood to get to tissues beyond the plaques. (Source: National Heart, Lung, and Blood Institute.)
In the United States, atherosclerosis usually begins in childhood or adolescence and then gradually worsens over many decades. Children as young as 2 years have been found to have atherosclerotic plaques throughout their arteries. Childhood obesity and diabetes contribute to this.
Any medium or large artery in the body can be affected. Most atherosclerosis causes no clinical problems. Many people have atherosclerosis throughout their bodies but develop no serious medical symptoms, and the disease is only discovered at autopsy. This is referred to as subclinical atherosclerosis.
When atherosclerosis causes the coronary arteries to become very narrow or when plaques rupture and send clots into the arteries of the heart, a person is said to have CAD.
ATHEROSCLEROTIC PLAQUE FORMATION
Atherosclerosis is characterized by the formation of atherosclerotic plaques formed primarily of lipid deposits, which develop slowly over many years and in three stages.
Stage One: Fatty Streaks Appear
As atherosclerosis begins, the first detectable changes are the appearance of fatty streaks along artery walls, typically observed via CT scan or cardiac catheterization. These streaks are places where excess fat is accumulating.
Most of the fat (lipids) in the blood is carried by proteins in molecular complexes called lipoproteins. The surface of a lipoprotein is made of the more water-soluble lipids (cholesterol and phospholipids). The least soluble lipids (cholesteryl esters and triglycerides) are carried in the centers of the lipoproteins.
Lipoproteins are found in five sizes. From the largest to the smallest, these are chylomicrons, VLDL, IDL, LDL, and HDL. Each size lipoprotein has its own characteristic balance of lipids. The largest lipoproteins (chylomicrons and VLDL) are especially rich in triglycerides, while 70% of all blood cholesterol is contained in the LDL lipoproteins. Treatment that lowers LDL cholesterol levels may reverse the process that causes fatty streak formation.
When there is an excess of lipoproteins in the blood, as happens in hypercholesterolemia, more lipoproteins than normal get through the endothelial cells and into the artery walls. These excess lipoproteins stick to extracellular molecules, and eventually enough excess fat becomes stuck just below the endothelial cells to form visible yellowish (fatty) streaks along the arterial walls. These may develop as early as age 20 and involve progressively greater areas of the tunica intima of the interior of arteries as people age.
Stage Two: Fibrous Plaque
With injury to the epithelial lining of the arterial wall, smooth muscle cells move into the fatty plaques and cause arterial thickening. Collagen covers the fatty streak and forms a fibrous plaque that is greyish white. Lipoproteins move cholesterol and other lipids into the tunica intima of the arterial wall. White blood cells are also attracted to the lipids, causing the plaque to grow larger. The result is further narrowing of the opening or lumen of the artery, reducing and slowing the flow of blood. Progressive changes in the artery wall can begin as early as age 30.
Stage Three: Complicated Lesion
As the fibrous plaque continues to grow, inflammation can occur. Inflammation is the proliferation of white blood cells (WBCs) that respond to fight what the body perceives as an invader. Any interruption in the smooth inner wall of the artery is seen as an invader. This may cause the plaque to become unstable, causing it to rupture or leading to an ulceration or lesion.
When atherosclerotic plaque formation triggers an inflammation response, WBCs (particularly lymphocytes and macrophages) collect under the epithelial cells in the arterial wall and release inflammatory molecules, cytokines, and proteolytic enzymes. As they continue to evolve, some plaques also accumulate calcium, which can sometimes be seen in X-rays.
In time, the endothelial cells covering the bulge begin to rip, letting blood come in contact with the underlying collagen and other extracellular molecules. Extracellular molecules are stimulants of blood clotting. Therefore, small blood clots and clumps of platelets form along the rips in the endothelial lining of the artery. Disrupted plaques create blood clots, and if the clots break loose, they are carried into the smaller arteries downstream. The result can be a blocked artery (Harding et al., 2022; Morata & Flynn, 2024).
CLOTS AND VASOSPASMS
The rupture of a plaque can also cause the walls of the artery to constrict in that region. The resulting vasospasm narrows the artery suddenly and causes ischemia downstream. Alone and together, clots and vasospasms can cause emergency medical conditions, including MIs and sudden death. A coronary artery vasospasm can occur at rest. It is treated with nitrates and calcium channel blockers, and the precursors can be detected in diagnostic tests such as a chest X-ray, electrocardiogram, and serum troponin levels (Harding et al., 2022; Morata & Flynn, 2024).
The rupture of an atherosclerotic plaque can happen quickly. It can be set off by a sudden spurt of output from the sympathetic nervous system. Such spurts can occur when people are waking in the morning or when people are subjected to strong emotional stress. External stresses, however, do not disrupt stable plaques. External stresses only rupture those plaques that have already become weakened and destabilized by inflammation or other internal changes.
Besides slowly narrowing the coronary arteries, atherosclerosis can cause a sudden medical crisis. The degeneration of a plaque can seed clots into the bloodstream and can also trigger local vasospasm. These lead to a marked reduction of blood flow, and the resulting damage can range from temporary to permanent and from mild to fatal.
ATHEROSCLEROSIS OF THE CORONARY ARTERIES
Rather than uniformly thickening arterial walls, atherosclerosis is patchy and unevenly distributed. The specific coronary arteries affected by atherosclerosis vary from person to person, but there is a common feature: within a coronary artery, plaques are found most often at branch points, places where the blood flow naturally becomes turbulent.
The narrowing of coronary arteries usually occurs slowly, and in response, new small collateral arteries have time to grow into the fields of the atherosclerotic arteries to help bolster the local oxygen supply. These collateral arteries will sometimes provide enough extra blood flow to keep the heart muscle working comfortably at a resting rate. The collateral arteries are small, however, and they do not have the capacity to keep up with the oxygen demands of the heart muscle during exercise.
Even with the growth of small collateral arteries, the continual narrowing of the coronary arteries by atherosclerosis can eventually produce ischemia and anginal pain. Initially, these symptoms occur only when the patient is exercising; later, the symptoms begin to occur even when the patient is at rest.
CAUSES AND CONTRIBUTORS
There are several causative factors that contribute to the formation of atherosclerosis in the arteries. Some people have a genetic propensity for developing atherosclerosis, but it appears that the disease can occur in almost everyone. Contributing factors can increase the extent of atherosclerosis and the possibility that the condition will be symptomatic.
- There is a prevalence of atherosclerotic formation in families with higher episodes of cardiac incidents.
- Nutritional intake of trans fats and lipids contributes to atherosclerosis.
- The use of tobacco in any form causes vasoconstriction that will increase arterial wall tension.
- Physical inactivity prevents a person from obtaining the benefits of exercise, such as weight and blood pressure reduction and the development of collateral circulation.
- Obesity is associated with subclinical atherosclerosis, including coronary artery calcification and carotid intima–media thickness.
- High blood levels of LDL and lipids contribute to the production and size of arterial wall plaques.
- The development of atherosclerotic plaques are progressive and worsen as a person ages.
- Diabetes mellitus types 1 and 2 cause an increase in LDL and triglyceride levels.
(Harding et al., 2022; Morata & Flynn, 2024)
CLINICAL FORMS OF CAD
Many people who have atherosclerosis of the coronary arteries live their lives symptom-free. Other people develop symptoms and heart damage from atherosclerosis. The ischemic heart problems of atherosclerotic coronary artery disease fall into two general classes: chronic and acute.
Chronic Coronary Syndromes
- Stable angina
- Stable ischemic heart disease
Acute Coronary Syndromes
- Sudden cardiac death
- Myocardial infarction (MI)
- Unstable angina
Chronic Coronary Syndromes
Coronary artery disease is a chronic, progressive disease that is punctuated by sudden medical emergencies, the acute coronary syndromes. The long, chronic phases of the disease have two forms: stable angina and stable ischemic heart disease. When oxygen demand exceeds the ability of the coronary arteries to supply a sufficient amount of blood flow, myocardial ischemia is the result (Harding et al., 2022).
STABLE ANGINA
Insufficient blood flow to the myocardium through coronary arteries whose internal diameter is narrowed or blocked causes chest pain. This may be brought about by exertion or stress. Such pain that occurs in a recognizable pattern and ceases upon rest or after anti-anginal medication is taken is known as stable angina (NHLBI, 2023).
The occurrence of angina is influenced by the general tone of the sympathetic nervous system (which tends to be higher in the mornings) and by the demands of blood flow by the gastrointestinal tract after a meal. Although the symptoms of chronic stable angina are predictable, the amount of exercise or stress that will produce these symptoms varies during the course of a day.
The chest pain of chronic stable angina can also be brought on by any medical condition that increases the work of the heart, such as hypertension, aortic stenosis, systemic infections, or thyrotoxicosis. Likewise, conditions that reduce the oxygenation of the blood, such as COPD, anemia, or intolerance to high altitudes, can also cause angina. The complaint usually only lasts a few minutes. The pain is usually relieved by resting or taking a nitrate such as sublingual (SL) nitroglycerin (NTG). This form of angina may progress to unstable angina.
STABLE ISCHEMIC HEART DISEASE
A second chronic syndrome is stable ischemic heart disease (or ischemic cardiomyopathy), in which years of damage from ischemia have weakened the heart muscle or myocardium sufficiently that it gradually fails. Stable ischemic heart disease is a major cause of heart failure in older adults.
Most patients with this condition have had acute myocardial infarctions in the past, although not all infarctions may have been symptomatic. In people who have had “silent” myocardial infarctions, heart failure from stable ischemic heart disease can be the first evidence of their coronary artery disease.
PROGNOSIS
A patient with any form of coronary artery disease has a higher chance of dying when the left ventricle of the heart has been weakened. Signs of a failing left ventricle include an enlarged heart, pulmonary edema, leg and ankle edema, jugular venous distension, or a third heart sound (S3). Previous myocardial infarctions weaken the heart, so a history of past MIs also worsens a patient’s prognosis. Frequently, an echocardiogram is ordered to evaluate the pumping action of the heart, to determine the prognosis for the patient, and to guide treatment.
The CHA2DS2-VASc score stands for:
- Congestive heart failure
- Hypertension
- Age (>65 years = 1 point, >75 years = 2 points)
- Diabetes
- Previous Stroke/transient ischemic attack (2 points)
- VASc (vascular disease, including peripheral arterial disease, previous myocardial infarction, or aortic atheroma)
Sex is also included in this scoring system. It was initially used for the assessment of the risk of thromboembolic events in patients with atrial fibrillation. Now it can be used to predict adverse outcomes in various cardiovascular diseases. When used to predict the occurrence of mortality in patients who have chronic stable angina with no history of myocardial infarction, the score has predicted a significant increase in the possibility in deaths specifically with diabetes, hypertension, and cardiac dysrhythmias (Healio.com, 2024).
Acute Coronary Syndromes (ACS)
The term acute coronary syndromes refers to unpredictable episodes of severe heart ischemia. The ischemia is prolonged and not immediately reversible. These syndromes include sudden cardiac death, myocardial infarction, and unstable angina. ACS results from a disruption of a formerly stable plaque that then causes ischemia severe enough to injure or kill muscle cells in the heart, infarction, or necrosis. This transpires when the ruptured plaque causes platelet aggregation (clumping) and thrombus (blood clot) formation, leading to partial or complete blockage of a blood vessel, possibly one of the coronary arteries. This condition is exacerbated by inflammation in the arteries (Harding et al., 2022; Morata & Flynn, 2024).
Symptoms of ACS usually occur suddenly and may include:
- Chest pain or discomfort, often described as aching, pressure, tightness, or burning (also called angina)
- Pain that starts in the chest and spreads to other parts of the body, including the shoulders, arms, upper belly area, back, neck, or jaw
- Nausea or vomiting
- Indigestion
- Shortness of breath (also called dyspnea)
- Sudden, heavy sweating
- Racing heartbeat
- Feeling lightheaded or dizzy
- Fainting
- Unusual fatigue
(Mayo Clinic, 2023a)
An acute coronary syndrome needs immediate treatment in a prepared emergency room. People with the highest risk of developing an acute coronary syndrome are those who already have serious cardiovascular disease or diabetes.
Similar to other types of heart disease, risk factors for acute coronary syndromes include:
- Older age (>45 for men and >55 for women)
- High blood pressure
- High blood cholesterol
- Cigarette smoking
- Physical inactivity
- Unhealthy diet
- Obesity or overweight
- Diabetes
- Family history of chest pain, heart disease, or stroke
- For women, a history of high blood pressure, preeclampsia, or diabetes during pregnancy
(See also “Risk Factors and Prevention Measures” below.)
SUDDEN CARDIAC DEATH
The most catastrophic of the acute coronary syndromes is sudden cardiac death (SCD), an unexpected death from cardiac causes that happens quickly, usually within an hour of the first symptoms. In adults SCD is usually associated with coronary artery disease (in 80% of all cases). The cause may also be due to such diverse diseases such as cardiac dysrhythmias, congenital coronary artery anomalies, hypertrophic cardiomyopathy, arrhythmogenic right ventricular dysplasia, dilated cardiomyopathy, and aortic valve stenosis.
One possible etiology involves considerable stimulation of the sympathetic nervous system’s stress response, leading to elevation of circulating catecholamines. This is a theoretical foundation for the SCD of young athletes in the absence of abnormal cardiac physiology or drug use (Yow et al., 2024).
The direct cause of these deaths is often a fatal dysrhythmia, such as ventricular tachycardia or ventricular fibrillation. The dysrhythmias develop in cardiac cells that have been made overly excitable because of sudden ischemia from a blood clot or a vasospasm.
UNSTABLE ANGINA
Unstable angina (UA) is another common acute coronary syndrome. An episode of unstable angina includes symptoms of heart ischemia that resolve after more than 10 minutes of rest or the use of nitrates, including sublingual nitroglycerin. Unstable angina occurs without a recognizable pattern and may also follow exercise or exertion (NHLBI, 2023). In unstable angina, the level of heart damage is much less than occurs in a myocardial infarction, but unstable angina often foreshadows a subsequent MI. Chronic stable angina may progress to UA (Harding et al., 2022).
MICROVASCULAR ANGINA
In this type of angina, the myocardial ischemia is caused by atherosclerosis or spasm of the distal branches of the coronary artery branches and microcirculation, rather than the actual coronary arteries. This is also known as coronary microvascular disease (MVD) or cardiac syndrome X. It is more common in postmenopausal women, may be very prolonged, and is often caused by physical exertion. Angina caused by arteriospasm is often fleeting, and the diagnosis is made by ruling out any other anatomical cause. The response to nitrates is intermittent. Supplemental oxygen may help relieve the pain if administered while hypoxia is occurring (Johns Hopkins, 2024).
PRINZMETAL’S ANGINA
This is a rare type of angina that often occurs at rest. It is more common in those with a history of migraine headaches, Reynaud’s disease, alcohol consumption, cocaine usage, or heavy smoking. The most common cause of this angina is not necessarily related to CAD but is usually caused by the spasm of a coronary artery resulting in a temporary loss of oxygenated blood to the related area of the myocardium. The pain may be accompanied by a transient episode of ST segment elevation, indicating hypoxia, and occurs in short bursts at approximately the same time each day. The spasm occurs during a period of increased oxygen demand such as rapid eye movement (REM) sleep or exposure to cold. The pain may subside in response to moderate exercise or sublingual NTG. Supplemental oxygen may help relieve the pain if administered while hypoxia is occurring.
TAKOTSUBO CARDIOMYOPATHY
This form of angina is also known as stress cardiomyopathy or “broken heart syndrome.” Chest pain is accompanied by ST segment elevation on ECG (which can mimic a large MI), mild cardiac biomarker (e.g., serum troponin) elevation, but no coronary artery narrowing. In the presence of stress hormones (as in the case of a “broken heart”), the cardiac muscle will temporarily bulge at the apex, resembling the shape of an octopus trap. (Takotsubo is the name of a Japanese trap used to capture an octopus.) The pain is caused by a temporary decrease in blood flow through the coronary arteries, as in CAD. The pain may not be severe enough to require supplemental oxygen, but it is unusual for there to be any permanent myocardial damage as a result (Harvard Health Publishing, 2023).
MYOCARDIAL INFARCTION
Myocardial infarctions are a type of acute coronary syndrome. MIs are caused by ruptured plaques, blood clots dislodged from atherosclerotic plaques, blunt trauma, or vasospasms. These cause an imbalance between oxygen demand and oxygen supply.
Myocardial infarctions occur when the plaque, blood clot, vasospasm, or some combination of these partially or completely obstruct a coronary artery or one of its major branches. If the obstruction persists for more than 20 minutes, some of the cell injury will be permanent. Contractility of the injured (infarcted) tissue becomes impaired, resulting in weakness of the cardiac pump; eventually, poor cardiac contractility becomes pump failure.
The area of infarction determines the portion of the cardiac pump that fails. The most damaging area of infarction is the left ventricle. The left ventricle is responsible for supplying the body with reoxygenated blood. An infarction in the myocardium of this ventricle is the most likely to cause pump or cardiac failure.
A myocardial infarction produces distinctive ECG changes. On a 12-lead ECG, an elevated ST segment indicates the corresponding coronary artery is completely obstructed, causing an MI. The ST segment elevation occurs only in the leads facing the area of infarction. This is referred to as an ST segment elevated MI (STEMI). An MI caused by an incompletely blocked coronary artery does not cause the ST segment to be significantly elevated on the ECG. This is referred to as a non–ST segment elevated MI (NSTEMI).
The area of infarction is electrically unstable, causing dysrhythmias particular to that area. Infarcted ventricular tissue will cause ventricular dysrhythmias, which may be the most life-threatening. The area surrounding the infarcted tissue may still be ischemic. This ischemic tissue post infarction is referred to as the corona (crown). If the blocked coronary artery that caused the infarction continues to supply an inadequate amount of oxygenated blood to the area, the ischemic corona will quickly become infarcted. This can be prevented by using percutaneous coronary intervention (PCI) to reopen the blocked artery within 90 minutes of infarctions (see “Management of Acute CAD” later in this course). The larger the area of infarction, the more likely there will be dysrhythmias and ST segment changes (Ecgwaves, 2024; Harding et al., 2022; Morata & Flynn, 2024).
The symptoms of an MI may be different for women and therefore not as easy to assess. The American Heart Association stresses the importance of clinicians recognizing the difference in symptoms between the genders and the necessity of teaching these differences to patients and families.
MYOCARDIAL INFARCTION SIGNS IN WOMEN
As with men, women’s most common MI symptom is chest pain or discomfort. But women are somewhat more likely than men to experience some of the other common symptoms, including:
- Pain or discomfort in one or both arms, back, neck, jaw, or abdomen
- Shortness of breath with or without chest discomfort
- Nausea and/or indigestion
- Other signs, such as breaking out in a cold sweat or lightheadedness
(AHA, 2024b)
ANSWERING PATIENT QUESTIONS
Q: What does a heart attack feel like?
A: Most people get a very uncomfortable pressure, squeezing, or pain in the center of their chest. This chest pain lasts for more than a few minutes; sometimes it goes away briefly but soon comes back. Some people feel the pain in their arms, shoulders, back, jaw, or stomach. There can also be a feeling of breathlessness, lightheadedness, cold sweat, or nausea.
PROGNOSIS
MIs are the cause of most deaths from coronary artery disease. The most common causes of mortality in the first 30 days are cardiogenic shock, sudden cardiac death, heart failure, mechanical cardiac complications, or another MI event.
In patients who survive to be admitted to the hospital, mortality rates have decreased from 5.3% to 3.8% due to recent developments in reperfusion techniques. Interventions such as reperfusion thrombolytic therapy, immediate use of aspirin, PCI, statins, ACE inhibitors, and beta blockers account for the improvement in survival rates. Thirty-day post-MI mortality rates are 13% with medical therapy alone, including lifestyle changes such as smoking cessation, weight management, dietary changes, stress management, decreased alcohol consumption, exercise, and medications. The postmortality rates are 6%–7% with fibrinolytic therapy and 3%–5% with primary percutaneous coronary intervention within two hours of hospitalization.
The importance of reperfusion is to limit permanent myocardial damage, necrosis, and scar tissue formation, which would all cause later cardiac diseases. The use of early invasive reperfusion techniques such as primary percutaneous coronary intervention (PCI) in NSTEMI patients likewise decreases MI recurrence, rehospitalization, and mortality (ACLS.com, 2024).
One method of predicting post-MI mortality is the thrombolysis in myocardial infarction (TIMI) score (see boxes below).
TIMI SCORE FOR STEMI
- Diabetes mellitus history, hypertension, or history of chest pain (1 point)
- Systolic blood pressure <100 mmHg (3 points)
- Heart rate >100 bpm (2 points)
- Killip class II–IV (2 points)
- Body weight <150 pounds (1 point)
- Age ≥75 years (3 points)
- Age 65–74 years (2 points)
- Age <65 years (0 points)
| Points | Risk |
|---|---|
| (ACLS.com, 2024) | |
| 0–1 | 3%–5% |
| 2 | 3%–8% |
| 3 | 5%–13% |
| 4 | 7%–20% |
| 5 | 12%–26% |
| 6–7 | 19%–41% |
TIMI SCORE FOR NSTEMI
- Age ≥65 years (1 point)
- Three or more CAD risk factors (1 point)
- Known CAD with more than 50% stenosis (1 point)
- Aspirin use in the past seven days (1 point)
- Severe angina in the preceding 24 hours (1 point)
- Elevated cardiac markers (1 point)
- ST deviation greater than 0.5 mm (1 point)
| Points | Risk |
|---|---|
| (ACLS.com, 2024) | |
| 0–1 | 3%–5% |
| 2 | 3%–8% |
| 3 | 5%–13% |
| 4 | 7%–20% |
| 5 | 12%–26% |
| 6–7 | 19%–41% |
Over the past 20 years the 10-year rate of mortality and rehospitalization due to a recurrence of an MI has been steadily decreasing. Improvement is considered due to more timely treatments and the widespread use of interventions such as thrombolytics and especially more invasive measures such as PCI. Statistics from the past 25 years show an annual 1.5% reduction in post-MI mortality and a 2.7% reduction in recurrence of the infarction with rehospitalization. For patients who died within 10 years after an MI, 30% died within the first year (Wang et al., 2022).
ANSWERING PATIENT QUESTION
Q: Will I die if I have a heart attack?
A: Most people who have heart attacks survive, and the survival rates have been improving as new medicines and new medical procedures are developed. If you have a heart attack, your chances of doing well go way up if you get to an emergency department quickly. If you think you are having a heart attack, don’t take chances. Call 911 if your symptoms don’t go away in a few minutes.
RISK FACTORS AND PREVENTION MEASURES
Certain risk factors are universally recognized as potentially causing CAD. Some may be preventable and worth including in a cardiac education program to improve health. These include diet, physical activity, stress tolerance, tobacco use, alcohol consumption, type 2 diabetes, and hypertension, along with weight loss under certain circumstances and cholesterol level to a certain extent. Other risk factors can’t be altered by lifestyle changes, including age, gender, ethnicity, genetics, and type 1 diabetes.
Nonpreventable Risk Factors
AGE
Age is the strongest risk factor for coronary artery disease. Most cases occur in patients ages 40 years or older, although mortality and morbidity are higher in the elderly. Men are more likely to have an MI after age 45. Women are more likely to have an MI after age 55 (AHA, 2024a).
GENDER
Men are at slightly higher risk than women to have MIs and have them at an earlier age than women. It is thought that the higher estrogen levels in premenopausal women protect them from some of the heart damage done by atherosclerosis, but this protection disappears after menopause. Women who experience menopause at a higher age have a lower risk of cardiovascular disease and death as a result (Williamson, 2023). Elderly women who have MIs are more likely to die from them within a few weeks, since they typically experience MIs at an older age than men. They are also less likely to recognize their cardiac symptoms and seek treatment.
ETHNICITY
Disparities in CAD due to ethnicity may be due to a complex set of issues, including diet, obesity, lack of access to care, mistrust of healthcare workers, fewer ethnic minority healthcare professionals to relate to, and more.
African Americans have a higher death rate (22.6%) from CAD than White Americans (18%). In part, the difference results from the higher incidence of hypertension, obesity, and metabolic syndrome among African Americans. This racial disparity may also be due to the fact that African Americans, on average, seek treatment later than Whites for a variety of reasons regarding access, past and present. Data indicates that when compared to White patients, African American patients have longer emergency department wait times, longer lengths of stay, lower acuity levels, a 10% lower likelihood of admission, and 1.26 times higher odds of mortality. African Americans are also less likely to receive invasive treatment. This effect on access to healthcare and resultant outcomes also extends to other areas of healthcare such as cardiology (Macias-Konstantopoulos, 2023; Yow et al., 2024).
Heart disease death rate is also higher among Native Hawaiians or other Pacific Islanders (18.3%) and some Asian Americans (18.6%) than among White people. This may be due in part to higher rates of obesity and diabetes in these populations (CDC, 2024b).
GENETICS/FAMILY HISTORY
Children of parents with heart disease are more likely to develop it themselves. Most people with a strong family history of heart disease have one or more other contributing risk factors.
First-degree relatives who are biologically related (parents, children, and siblings) share approximately 50% of their genetic material with each other. For this reason, members of the same family tend to inherit the same diseases and traits because of gene mutations in the DNA. It is sometimes difficult to determine if genetics is the basis for CAD in families or if environmental risk behaviors such as smoking or obesity contribute to the appearance of the same diseases in families (Jellis, 2023).
A genetic disorder can change the way that a protein works so that the body processes cholesterol differently. This may increase the occurrence of atherosclerosis. Genetic differences are inherited in the DNA of the ovum and sperm. The parents’ genetic codes are copied into every cell of the offspring.
Preventable Risk Factors and Evidenced-Based Prevention Measures
SMOKING/TOBACCO USE
In the United States, smoking has decreased from 20.9% of adults in 2005 to 11.5% in 2021, but it remains a serious health risk. People who smoke have a risk of developing CAD or lung disease that is 30% higher than that of nonsmokers. Nicotine causes the sympathetic nervous system to constrict arteries and raises blood pressure, causing arterial wall damage. The damage encourages the formation of atherosclerotic plaque. Smoking can raise total cholesterol, lower HDL, increase clotting and clot formation, and cause arterial wall thickening. These outcomes can contribute to CAD over time.
Cigarette smoking is also an important independent risk factor for sudden cardiac death in patients with CAD. Cigarette smoking adds a cumulative effect when other risk factors are present to greatly increase the risk for CAD. People who smoke cigars, pipes, e-cigarettes, or vape seem to have a higher risk of death from CAD as well. The mortality rate for current smokers is three times that of people who have never smoked. Exposure to secondhand smoke also increases the risk of heart disease for nonsmokers (CDC, 2024c).
Patients who smoke should be strongly encouraged to quit smoking. An important factor is to educate patients on the risks of smoking and offer assistance in developing an action plan to help the patient stop smoking. The best smoking cessation programs include a combination of the following components:
- Behavioral modification therapies
- Medications such as antidepressants
- Nicotine replacement strategies such as patches or gum
- Counseling to make a plan to quit smoking
- Smoking cessation “quit lines” (such as 1-800-NOBUTTS)
- Free texting programs (e.g., SmokefreeTXT)
HIGH CHOLESTEROL
As cholesterol rises in the blood, so does the risk of CAD. When other risk factors (e.g., hypertension and smoking) are present, this risk increases even more. Low high-density lipoprotein (HDL) cholesterol is also a risk factor for heart disease. Likewise, a high triglyceride level combined with low HDL cholesterol or high LDL cholesterol is associated with atherosclerosis, which increases a person’s risk for CAD (CDC, 2024d).
Cholesterol level is affected by:
- Age
- Gender (women have higher prevalence of high total cholesterol than males)
- Heredity
- Ethnicity (higher cholesterol predominantly in Black and Hispanic individuals)
- Diet
Genetic factors, type 2 diabetes, and certain drugs, such as beta blockers and anabolic steroids, also lower HDL cholesterol levels. Smoking, being overweight, and being sedentary can all result in lower HDL cholesterol (CDC, 2024e).
| LEVEL (mg/dL) | CLASSIFICATION |
| (Mayo Clinic, 2024a) | |
| LDL Cholesterol (Primary target of therapy) |
|
|---|---|
| <100 | Optimal |
| 100–129 | Near optimal/above optimal |
| 130–159 | Borderline high |
| 160–189 | High |
| ≥190 | Very high |
| HDL Cholesterol | |
| <40 | Low |
| ≥60 | High | Total Cholesterol |
| <200 | Desirable |
| 200–239 | Borderline high |
| ≥240 | High |
HYPERTENSION
Hypertension (HTN) causes inflammation, which can damage the lining of arteries and increase fatty deposits, contributing to the development of atherosclerosis and CAD. For people at increased risk for CAD, blood pressure control is an important factor. A diagnosis of HTN is confirmed when two or more elevated blood pressure readings are obtained on separate visits.
| Category | Systolic | Diastolic |
|---|---|---|
| (AHA, 2024c) | ||
| Normal | <120 | and <80 |
| Elevated | 120–129 | and <80 |
| Hypertension stage 1 | 130–139 | or 80–89 |
| Hypertension stage 2 | ≥140 | or ≥90 |
| Hypertensive crisis | >180 | and/or >120 |
HTN is a comorbidity in many other diseases and conditions, including diabetes, CAD, heart failure, obesity, and renal failure.
There are distinct differences in the prevalence of HTN among different ethnicities. Black men and women in the United States have the highest occurrence of hypertension in the world. Black Americans experience HTN much earlier than Whites and measure much higher blood pressures. HTN incidence by race/ethnicity is:
- 56%, non-Hispanic Blacks
- 48%, Hispanic Whites
- 46%, non-Hispanic Asians
- 39%, Hispanics
(Sekkarie et al., 2024)
Treating hypertension is an important factor in preventing CAD and includes the following strategies:
- Lifestyle modifications, such as smoking cessation, exercise, weight loss, and dietary changes
- Medications to control blood pressure, such as beta blockers, calcium channel blockers, angiotensin receptor blockers, and thiazide diuretics
During 2017–2021, the age-standardized prevalence of antihypertensive medication use among persons with hypertension increased from 59.8% to 62.9%. In 2021, the use of antihypertensive medications was higher among women (68.5%) than men (59.4%), among all adults age ≥65 years (92.5%), and among Black adults (71.3%) than White adults (62%) (Sekkarie et al., 2024).
PHYSICAL INACTIVITY
A sedentary lifestyle is a risk factor for CAD. Patients with a sedentary lifestyle are also more likely to be overweight, obese, or hypertensive, which contributes to the risk of developing CAD.
The benefits of physical activity are well-established in many diverse studies. Even low levels of exercise (up to 75 minutes of brisk walking per week) were associated with a reduced risk of mortality in patients with CAD. Time in sedentary behavior is associated with a higher risk of mortality regardless of the underlying pathophysiology (Harding et al., 2022).
Patient goals for physical activity should begin with 10–15 minutes per day and gradually work up to a goal of 30 minutes per day of moderate to vigorous exercise. The more vigorous the activity, the greater the benefits. The level of activity is based on the patient’s baseline condition and other comorbid diseases. Patients should always work with their healthcare provider prior to starting an exercise program.
The cardiovascular benefits of exercise include a positive impact on:
- Lipid metabolism, by increasing HDL
- Blood pressure
- Insulin sensitivity, causing a reduction in blood sugar
- Reduced risk for metabolic syndrome
- Calories burned
- Strengthened bones and muscles
- Improved memory
- Improved mood
- Promoting sleep
- Reduced risk for some cancers
(CDC, 2024h)
Although a program of regular exercise does not typically reduce LDL cholesterol levels to a significant degree, it will reduce insulin resistance and blood levels of triglycerides, and it will also increase blood levels of HDL cholesterol.
For patients who are just beginning an exercise program, it is important to start slowly and consult a professional, such as an exercise physiologist, for assistance in developing a plan that will work for them. For high-risk patients with comorbidities who are deconditioned or have had recent cardiac events, careful supervision of physical rehabilitation is recommended. Referral to a physical therapist to evaluate, plan, and monitor the patient’s progress with his or her exercise program is an important consideration.
OBESITY
The incidence of most cardiovascular diseases, including CAD, are increased in the setting of obesity. Obesity increases the risk for heart disease by causing the heart to work harder. This increases the resistance against which the left ventricle must pump blood, leading to hypertension. With obesity, high blood cholesterol and triglyceride levels also increase, while HDL levels decrease.
Obesity is defined as a body mass index (BMI) (weight in kg divided by height in meters squared) of ≥30 kg/m2 and occurs in 42% of adults in the United States according to 2022 data. Overweight indicates a BMI >25 kg/m2 and occurs in 72.4% of the U.S. adult population. Patients who have a larger waist measurement than hip measurement are at increased risk for CAD. Patients who are obese are also at increased risk for developing some cancers, osteoarthritis, metabolic syndrome, and diabetes (Mayo Clinic, 2023b; WHO, 2024a; WHO, 2024b).
Obesity usually results as an imbalance between caloric intake and expenditure. Diets of obese people usually include an increase in energy-dense foods that are high in fat and carbohydrates. There is also usually an increase in physical inactivity due to the sedentary nature of many forms of work, changing modes of transportation to the more passive, and increasing urbanization. Referral to a dietitian may be indicated to assist patients with meal planning and monitoring.
Treatment for obesity should include:
- Limiting energy intake from total fats and carbohydrates
- Increasing the amounts of fruits, vegetables, legumes, whole grains, lean proteins, and nuts
- Engaging in regular physical activity (60 minutes a day for children and 150 minutes spread through the week for adults)
(WHO, 2024c)
Even a modest weight loss makes a difference. Patients who are overweight should be encouraged to follow a comprehensive weight-loss plan. A goal of achieving a 10% weight loss will lower a person’s risk for CAD. A small but consistent weight loss of one half to two pounds per week is the safest way to accomplish this.
DIABETES MELLITUS (DM)
Diabetes is a strong risk factor for developing CAD; the two diseases often coexist. Even when glucose levels are under good control, diabetes increases the risk of heart disease and stroke. The risks are even greater if blood sugar is not well controlled.
In 2021, an estimated 38.1 (14.7%) million U.S. adults had a diagnosis of diabetes mellitus (DM), 8.7 million (3.4%, and including 22.8% of all those with diabetes) had undiagnosed DM, and 97.6 million (38%) had prediabetes. (A blood hemoglobin A1C ≥6.5% is the threshold used to diagnose DM.) With DM, age-adjusted cardiovascular disease prevalence was higher among males than among females.
Patients with type 2 DM may have an increased risk of CAD because of shared risk factors such as age and gender; anthropometric (measurement and proportion), metabolic, socioeconomic, and lifestyle variables; psychosocial stress; environmental pollutant exposure; and disturbances in protein and fat metabolism, which may lead to weight problems. As a result, most patients with type 2 diabetes are overweight or obese. Maintaining a normal weight with diet and exercise as well as taking prescribed medications is important to maintain adequate blood sugar control (CDC, 2024i).
Physical exercise significantly improves glucose tolerance and insulin resistance. The benefits of exercise show that higher fitness is associated with a lower risk of incident DM regardless of demographic characteristics and baseline risk factors.
ANSWERING PATIENT QUESTIONS
Q: How can I tell whether I am a person who is likely to have a heart attack?
A: One good way is to ask your primary care provider. You can also get an idea by counting how many of the following nine characteristics apply to you:
- You have a father, mother, brother, or sister who had heart disease in middle age or earlier.
- You are older than 45 years if you are a man or older than 55 years if you are a woman.
- You have high blood cholesterol.
- You have high blood pressure.
- You have already had a heart attack, chest pain, heart surgery, stroke, or blocked arteries.
- You are overweight.
- You get little or no physical exercise.
- You smoke cigarettes.
- You have diabetes.
These characteristics increase your risk of a heart attack. The more that apply to you, the greater your chances of heart trouble. Most items on the list can be fixed or controlled; each item that you fix will reduce your risk of a heart attack.
METABOLIC SYNDROME
According to the International Diabetes Federation, the NHLBI, and the AHA, metabolic syndrome is diagnosed when a patient exhibits three of the following risk factors:
- Fasting plasma glucose ≥100 mg/dL or those undergoing drug treatment for elevated glucose.
- HDL-C <40 mg/dL in males or <50 mg/dL in females or those undergoing drug treatment for reduced HDL-C.
- Triglycerides ≥150 mg/dL or those undergoing drug treatment for elevated triglycerides.
- Waist circumference >102 cm in males or >88 cm in females for people of most ancestries living in the United States; ethnicity and country-specific thresholds can be used for diagnosis in some groups, particularly Asians and individuals of non-European ancestry who have predominantly resided outside the United States.
- BP ≥130 mmHg systolic or ≥85 mmHg diastolic or undergoing drug treatment for hypertension, or antihypertensive drug treatment in a patient with a history of hypertension.
Metabolic syndrome is linked to several related disorders, including nonalcoholic fatty liver, sexual and reproductive dysfunction (erectile dysfunction in men and polycystic ovarian syndrome in women), obstructive sleep apnea, certain cancers, and osteoarthritis, as well as general proinflammatory and prothrombotic tendencies (AHA, 2021).
OTHER RISK FACTORS
CAD is a multifaceted disease with more than 250 recognized psychosocial, nutritional, genetic, and metabolic risk factors.
Stress may be a contributing factor for developing CAD. For example, stress may cause people to overeat, start smoking, or smoke more than they otherwise would. Psychosocial stress causes inflammation due to an increase in stress hormones that promotes the production of atherosclerosis. Certain types of adversity or trauma are linked to increased occurrence and worse CAD. Some examples of these are childhood trauma, sexual or physical abuse, type A and D personalities, job stress including overtime, depression, and anxiety (Elendu et al., 2024).
Alcohol/substance abuse is another risk factor. Drinking too much alcohol can raise blood pressure and contribute to high triglycerides. Alcohol and recreational drug use contribute to cardiovascular disease development, including CAD, ranging from subclinical atherosclerosis to fatal acute coronary syndromes. However, the risk of heart disease in people who drink moderate amounts of alcohol (i.e., one drink per day for women, two drinks per day for men) is lower than in nondrinkers.
Elevated total homocysteine (tHCY) levels pose an increased risk of cardiovascular disease by causing abnormal endothelial cell function and thrombosis. HCY can be lowered by combining folate ingestion with vitamin B supplementation. Higher than normal tHCY levels are also prognostic of an increased risk of death, particularly in the case of NSTEMI.
Plasma homocysteine is a nonprotein amino acid that contains sulfur. HCY is directly associated with cardiovascular diseases such as CAD, hypertension, acute MI, and aortic atherosclerosis. Elevated HCY levels are also related to cardiac dysrhythmias such as recurrence of atrial fibrillation after cardioversion, prolonged QT intervals, and p-wave dispersion as a precursor for newly occurring atrial fibrillation (Medline Plus, 2022a).
Nutrition is also an important factor. Eating habits can affect other controllable risk factors such as cholesterol, blood pressure, diabetes, and weight. Evidence has shown that including a diet rich in vegetables, fruits, whole-grain and high-fiber foods, fish, lean protein, and fat-free or low-fat dairy products may lower a person’s risk for developing CAD. AHA guidelines place emphasis on foods and an overall eating pattern rather than on percentages of food components such as fat (see box below).
The recommended average for U.S. adult calorie consumption is 2,500 calories for men and 1,800 calories for women. Dietary habits affect multiple cardiovascular risk factors, including both established risk factors (e.g., systolic blood pressure, diastolic blood pressure, LDL-C levels, HDL-C levels, glucose levels, and obesity/weight gain) and novel risk factors (e.g., inflammation, cardiac arrhythmias, endothelial cell function, triglyceride levels, lipoprotein(a) levels, and heart rate).
AHA DIET AND LIFESTYLE RECOMMENDATIONS
- Eat a variety of fresh, frozen, and canned vegetables and fruits without high-calorie sauces or added salt and sugars. Replace high-calorie foods with fruits and vegetables.
- Choose fiber-rich whole grains for most grain servings.
- Choose meat, poultry, and fish without skin and prepare them in healthy ways without added saturated and trans fats. If you choose to eat meat, look for the leanest cuts available and prepare them in healthy and delicious ways.
- Eat a variety of fish at least twice a week, especially fish containing omega-3 fatty acids (e.g., salmon, trout, and herring).
- Select fat-free (skim) and low-fat (1%) dairy products.
- Avoid foods containing partially hydrogenated vegetable oils to reduce trans fats in the diet.
- Limit saturated fat and trans fat and replace them with “better” fats (monounsaturated and polyunsaturated). To lower blood cholesterol, reduce saturated fat to no more than 5%–6% of total calories. For someone consuming 2,000 calories a day, that is about 13 grams of saturated fat.
- Cut back on beverages and foods with added sugars.
- Choose foods with less sodium, and prepare foods with little or no salt. To lower blood pressure, aim to eat no more than 2,400 milligrams of sodium per day. Reducing daily intake to 1,500 mg is desirable because it can lower blood pressure even farther. If one cannot meet these goals right now, even reducing sodium intake by 1,000 mg per day can benefit blood pressure.
- For those who drink alcohol, drink in moderation. That means no more than one drink per day for a woman and no more than two drinks per day for a man.
- Follow the American Heart Association recommendations when eating out, and keep an eye on portion sizes.
(AHA, 2024f)
MEDITERRANEAN DIET
The Mediterranean diet has been studied and shown to have a positive effect on heart health. The diet is characterized by:
- High intake of monounsaturated fatty acids, primarily from olives and olive oil
- Daily fruits, vegetables, whole-grain cereals, and low-fat dairy products
- Weekly intake of fish, poultry, tree nuts, and legumes
- Lower intake of red meat, approximately twice a month
- Moderate daily consumption of alcohol, normally with meals
Adherence to the diet is associated with improved HDL cholesterol and triglyceride levels. Adherence to the diet has been shown to result in prevention of CAD and a significant reduction in mortality from ischemic heart disease. The Mediterranean diet can be adopted by most population groups and cultures and is cost-effective (Mayo Clinic, 2024b).
PLANT-BASED DIET
Plant-based diets (vegetarian and vegan) are believed to prevent CAD and other cardio-metabolic disorders such as stroke, type 2 diabetes, and obesity. Vegetarian and vegan diets are healthful, effective for weight and glycemic control, and provide cardiovascular benefits including reversing atherosclerosis and decreasing blood lipids and blood pressure. The American Heart Association (AHA) rated plant-based and vegetarian diets as 86 out of 100 on a heart healthy scale for containing a wide variety of fruits and vegetables, mostly whole grains rather than refined grains, legumes, nuts, liquid plant oils instead of tropical oils, and plant sources of protein. The vegan diet scored somewhat lower at 78 out of 100 because, while it matches the above characteristics of the vegetarian diet, it is so restrictive as to be difficult for many people to follow (AHA, 2023a).
DAILY ASPIRIN THERAPY
Aspirin can be taken to prevent heart disease and stroke in some individuals. The U.S. Preventive Services Task Force recommends that adults between the ages of 50–69 with a ≥10% chance of developing cardiovascular disease within the next 10 years take a low-dose (81 mg) aspirin every day. These recommendations apply only when the benefit of aspirin use outweighs the potential harm of gastrointestinal hemorrhage or other serious bleeding. Patients should always discuss aspirin use and dosage with their healthcare provider (USPSTF, 2022).
LIFE’S SIMPLE 7
Based on extensive research, the AHA developed the “Life’s Simple 7” program. Its seven steps are:
- Manage blood pressure.
- Control cholesterol.
- Reduce blood sugar.
- Get active.
- Eat better.
- Lose weight.
- Stop smoking.
High blood pressure is a risk factor for heart disease, stroke, and renal disease. Elevation of LDL cholesterol contributes to plaque formation and CAD. Consistently high serum glucose levels can cause cardiac, renal, neurological, and eye damage. Daily physical activity may increase longevity and quality of life. A heart-healthy diet helps to prevent cardiovascular disease. Weight loss improves the cardiac, pulmonary, vascular, and musculoskeletal burden and reduces blood pressure. Smoking increases the risk of cardiovascular and pulmonary diseases and increases blood pressure (AHA, 2022; CDC, 2024f).
ASSESSMENT, SCREENING, AND DIAGNOSIS
Chest pain is the most common symptom of CAD. When this occurs, it is vital for the healthcare worker to assess this very carefully with a detailed description of the pain. History is an equally vital part of understanding every aspect of CAD, including history of episodes of chest pain, medical history, social history, and family history. Laboratory work, ECGs, cardiac arteriography, and imaging or radiography are the most common means used to diagnose CAD and disease severity.
Chief Complaint: Chest Pain
Chest discomfort is a key identifying symptom of coronary artery disease, particularly in men. When a man with coronary artery disease comes to the office, clinic, or hospital with heart symptoms, the typical chief complaint is chest discomfort. Most often, the patient does not describe this discomfort as pain but instead as heaviness, pressure, squeezing, smothering, or a burning sensation.
By contrast, a woman with coronary artery disease is more likely to complain of symptoms such as nausea or abdominal discomfort; neck, throat, or jaw pain; shortness of breath; or weakness or fatigue rather than the more classic symptom of chest pain. Coronary ischemia should therefore be considered in women who appear to be acutely ill even if they do not complain of chest pain (Morata & Flynn, 2024).
ANGINAL PAIN
Chest discomfort or chest pain can originate from many places other than the heart, but the characteristic pain of angina almost always points to ischemia of heart muscles. The pain may be retrosternal, left pectoral, or epigastric.
Classic symptoms associated with angina include:
- Chest pain or discomfort
- Pain in arms, neck, jaw, shoulder, or back, accompanying chest pain
- Nausea
- Fatigue
- Shortness of breath
- Sweating
- Dizziness
(Johns Hopkins, 2024)
Onset and Provocation
Anginal pain is caused when the myocardium receives insufficient oxygen. Most activities have predictable oxygen requirements, and with stable angina the patient gets chest discomfort at predictable levels of activity that subside with rest. With unstable angina, people experience chest discomfort at rest and at unpredictable times that is unrelieved by rest or medications.
Any situation that increases heart rate can trigger angina in people with coronary artery disease. Exercise is a classic cause of anginal pain: hurrying, walking up an incline, walking against a strong cold wind, working with the arms extended above the shoulders, and sexual activity are all exercises that can produce ischemic heart pain. Strong emotions or nightmares stimulate the heart through the sympathetic nervous system, and these too can cause angina.
In the case of stable angina, although the amount of exertion needed to produce chest pain is predictable, the threshold for angina will vary during the day and with the weather and temperature. After a heavy meal, for example, blood flow is diverted to the gastrointestinal organs from the heart and brain, and less exertion than usual can cause angina. Lying down changes the dynamics of blood flow, and some people get angina when they get in bed at night. Women with chronic stable angina are more likely than men to get chest discomfort when they are resting or sleeping or when they are in stressful situations.
Other medical conditions can precipitate angina in a person with coronary artery disease. Anemia, systemic infections, pneumonia, or atrial fibrillation change the balance between the heart’s need for oxygen and the available supply.
Time Course
During assessment it is essential to determine the duration of anginal pain to establish the nature of the cause. As previously stated, the chest discomfort of stable angina typically lasts 1–5 minutes and rarely persists for as long as 10 minutes. The angina begins dully and then fades away as the patient stops and rests. Nitroglycerin tablets or sprays will usually end or lessen stable angina in a few minutes or less.
Unstable angina lasts for more than 10 minutes, and with myocardial infarctions, the pain can last for hours if untreated. When rest does not relieve classic anginal pain, then it is more likely that the patient has an acute coronary syndrome such as unstable angina or an MI.
Quality
The quality or sensation of angina has a special character. Rather than saying “pain,” patients most often use words such as squeezing, tightening, constricting, pressing, or strangling, or they clench their fists to describe the feeling of heart ischemia. They may say that they feel like there is “a band across my chest,” “a heavy weight in the center of my chest,” or “a vise that is tightening my chest.”
Location
When asked, “Where do you get this uncomfortable feeling?” patients with angina usually put a hand or fist over their sternum in the middle of their chests and say “Inside here!” meaning retrosternally. When asked, “Does this discomfort extend anywhere else?” angina patients will often say that the feeling extends to the left shoulder, to the inside (ulnar) half of either or both arms, to the neck and jaw, or sometimes to the middle of the upper back. Additionally, women with angina may complain of pain or discomfort in the abdominal area (Harding et al., 2022).
The pain or discomfort of angina is broad, and patients do not point to it with a finger, saying “It’s right here.” Also, patients rarely feel angina above the jaw, below the umbilicus, in the lower right chest, or localized below the left nipple. Moreover, the examiner usually cannot reproduce the pain by pushing gently on the skin or the chest wall.
| One commonly used method for quickly assessing the patient with chest pain is referred to as PQRST, mimicking electrocardiography waves. | ||
| (Harding et al., 2022; Morata & Flynn, 2024) | ||
| P | Provocation/precipitating events | Provocation/precipitating events What events or activities precipitated the pain or discomfort (e.g., argument, exercise)? |
|---|---|---|
| Q | Quality of pain | What does the pain or discomfort feel like (e.g., pressure, dull, aching, tight, squeezing, heaviness)? |
| R | Region/radiation of pain | Can you point to where the pain or discomfort is? Does the pain or discomfort radiate to other areas (e.g., back, neck, arms, jaw, shoulder, elbow)? |
| S | Severity of pain | On a scale of 0–10, with 0 indicating no pain and 10 being the most severe pain you can imagine, what number would you give the pain or discomfort? |
| T | Timing/treatment | When did the pain or discomfort begin? Has it changed since that time? Have you had pain or discomfort like this before? |
History
In addition to a description of individual occurrences of angina, the overall history of these episodes is important. Chronic stable angina gives predictable episodes of chest discomfort over many months, although the exact pattern of the episodes differs from patient to patient. In some patients, episodes of chest pain may occur several times a day. In others, there may be symptom-free intervals of weeks, months, or years. Occasionally, anginal attacks gradually decrease or disappear if adequate collateral coronary circulation (i.e., growth of new blood vessels) develops; this does not mean that the disease has gone away.
In contrast, acute coronary syndromes give unpredictable or steadily worsening episodes of ischemic symptoms. As acute coronary syndromes are developing, the symptoms may change from being occasional to happening constantly. An MI may give prolonged severe chest discomfort and continuous fatigue.
The chest discomfort of chronic stable angina is predictable for a given patient. Therefore, any changes in the pattern or the intensity of angina should be considered serious (Harding et al., 2022).
NON-CAD CAUSES OF CHEST PAIN
Chest discomfort is a classic symptom of myocardial ischemia. It is also a key symptom of other medical problems, the most common of which are gastroesophageal diseases.
| Origin | Causes |
|---|---|
| (Harding et al., 2022) | |
| Cardiovascular |
|
| Pulmonary/chest trauma |
|
Musculoskeletal |
|
| Gastrointestinal |
|
| Infectious |
|
| Neurologic |
|
Other Symptoms of CAD
In addition to chest pain, other symptoms are frequently caused by myocardial ischemia. These symptoms include:
- Shortness of breath, especially when it feels localized to the middle of the chest
- Weakness and tiredness
- Faintness or dizziness
These three symptoms are especially common in older (age >75 years) patients and in patients with diabetes when they have episodes of heart ischemia.
Other general symptoms that may accompany or replace angina are:
- No chest pain, but discomfort in the shoulders, inside (ulnar side) of the left arm, neck, or lower jaw
- Indigestion or nausea
When accompanying angina, certain additional symptoms signal potential emergencies. For example, chest pain with fatigue, sweating, and nausea or vomiting suggests myocardial infarction.
WOMEN AND MYOCARDIAL ISCHEMIA SYMPTOMS
Healthcare professionals should be alert to the fact that women are more likely than men to present with the following as the primary symptoms of an MI:
- Dyspnea
- Gastrointestinal complaints (nausea and vomiting)
- Back pain or pressure
- Jaw pain
- Shortness of breath
- Fatigue
Women also are more likely to attribute cardiac symptoms to other causes (such as influenza, stress, and normal aging) and may delay reporting symptoms (Harding et al., 2022).
SILENT MIs
Not all patients with myocardial ischemia have symptoms. Angina is a very common indicator of myocardial ischemia, and the characteristics described above are frequent and typical. Patients with all forms of CAD can have atypical feelings of chest discomfort or anginal equivalents. Moreover, ischemia severe enough to cause myocardial infarctions can occur without any chest pain, giving what are called silent MIs (asymptomatic myocardial ischemia). Often, the MI is discovered by ECG when the patient is seen for other problems (Mankad, 2024).
CASE
Celia Brown is a 62-year-old female with a family history of CAD and a previous history of smoking one pack per day for 20 years (i.e., 20 pack-years). Mrs. Brown has arrived at the emergency department with complaints of anxiety, dizziness, weakness, and ongoing fatigue.
The nurse on duty is Robert, who questions Mrs. Brown about her current medications, which include Zocor, Atenolol, and Xanax. As Robert continues to triage Mrs. Brown, he is initially concerned that she may be having an anxiety attack since she also seems short of breath and is perspiring. He asks probing questions about any pain that she is noticing. Mrs. Brown reports that she has been having some pain in her jaw and thought it was a tooth problem and that she has had more heartburn lately and felt nauseous with cold sweats.
She tells Robert that she called her doctor with her concerns a week ago and was instructed to continue to take her Xanax and start taking an over-the-counter antacid to help with the heartburn symptoms. Mrs. Brown states that “she originally thought she might have the flu, but now she knows that there is something wrong, as she has had the symptoms for a couple of weeks.”
Although this patient has a few classic symptoms of an anxiety attack, Robert also recognizes that Mrs. Brown may be having cardiac symptoms, as women with angina do not always present with obvious complaints of chest pain. Robert discusses the case with the ED physician. Together, they continue to question Mrs. Brown about her symptoms. When asked if she had tried anything to relieve her symptoms, the patient stated that she has been taking Tylenol for the jaw pain and antacids for the heartburn symptoms.
The ED team proceeds with a cardiac evaluation along with other testing in order to rule out myocardial ischemia. Mrs. Brown’s evaluation shows that she is experiencing anginal pain. She is admitted for continuing monitoring and medical management.
Patient History
PAST MEDICAL HISTORY
The past medical history of patients with CAD may suggest that they have or are at high risk for atherosclerosis. The primary elements in a person’s medical history that should alert the clinician to the possibility of an increased risk for atherosclerotic CAD include:
- Hyperlipidemia
- Hypertension
- Diabetes
- Metabolic syndrome
- Family history of CAD
- Tobacco use
- Fatty diet
- Male gender
- Advanced age
- Lack of physical activity
- Obesity
Past medical history should also include any previous hospitalizations, drug or food allergies, and a psychosocial history.
Ischemia
When taking a medical history, the healthcare provider may find that atherosclerosis of the coronary arteries has already revealed itself. A patient with CAD may already have had heart ischemia, such as myocardial infarctions. (See also above regarding analysis of angina.)
Peripheral Artery Disease
Atherosclerosis is a whole-body disease. Patients with coronary artery disease will often have indications of atherosclerosis in arteries outside the heart. For example, they may have a history of intermittent claudication (a result of atherosclerosis in the leg arteries), strokes or transient ischemic attacks (results of atherosclerosis in the carotid arteries), or abdominal aortic aneurysms.
Lipid Abnormalities
High levels of blood lipids predispose a patient to atherosclerosis. High levels of LDL cholesterol or low levels of HDL cholesterol can cause atherosclerosis. A patient with CAD will likely already have a diagnosis of high cholesterol.
Hypertension
High blood pressure is another major risk factor for developing atherosclerosis. The stress of an elevated blood pressure (BP) causes endothelial injury to the arteries that increases the rate of atherosclerosis. Atherosclerosis causes narrowed, thickened arterial walls and decreases elasticity of vessels. More force is needed to pump blood through diseased coronary arteries. This increased force causes a higher BP. This added workload results in left ventricular (LV) hypertrophy and decreased stroke volume with each contraction. A patient with CAD may already be taking antihypertensive medicines (Harding et al., 2022).
Diabetes
Diabetes puts a patient at high risk of developing CAD. People with diabetes experience CAD at an earlier age than the general population. Diabetes, especially type 2, tends to increase the level of blood cholesterol and to worsen atherosclerosis. People with diabetes have an increased tendency for endothelial dysfunction that may also increase the production of fatty streaks in the arterial walls. They also have abnormal lipid metabolism, causing high cholesterol and triglyceride levels.
The progressive increase in diabetes in the U.S. population contributes to the concurrent increase in CAD. Both diseases are also related to the aging U.S. population. Patients who have undiagnosed or poorly controlled diabetes are at highest risk. The diabetes is often diagnosed for the first time when the person has an MI (Harding et al., 2022).
FAMILY HISTORY
Patients are much more likely to develop coronary artery disease if they inherit a genetic propensity for the disease. When assessing a patient for CAD, a good indicator of this propensity is the existence of first-degree (parents, siblings, and offspring) relatives who have had an acute coronary syndrome, such as an MI, at an early age. For men, this would be when they were younger than 45 years, and for women, it would be when they were younger than 55 years.
There may also be a familial history of environmental or behavioral risk factors (e.g., tobacco use or alcohol abuse) or genetic risk factors (e.g., obesity, diabetes, or hypertension) that may contribute to the increased possibility of the occurrence of CAD within a family.
Similarly, racial and ethnic groups share a large percentage of their genetic variables within the group. This may explain why some racial/ethnic groups have a higher incidence of CAD than others. The higher incidence may also partly be attributable to other characteristics within the group related to dietary or other health practices (Harding et al., 2022).
SOCIAL HISTORY
Two features of a patient’s lifestyle may put them at high risk for developing CAD: smoking or other tobacco use and a high-fat diet. Assessment should include taking a careful history of current or previous smoking as well as asking about dietary habits.
Smoking one or more packs of cigarettes a day for several years doubles a person’s chance of dying from CAD. A person who stops smoking can reduce this extra risk. The lungs will clear themselves of damage caused by smoking over the course of several years. Likewise, a diet high in cholesterol, saturated fats, and trans fats increases a patient’s chances of developing artery problems from atherosclerosis, while low-fat diets or diets containing only polyunsaturated fats may reduce the risk. Unlike stopping smoking, a change in diet will not necessarily clear the arteries of the deposits of plaque.
PACK-YEARS
Pack-years is a specific measurement of smoking taken in a medical history. The history taker asks for the number of packs of cigarettes smoked per day, on the average, multiplied by the number of years the patient has been smoking. This is based on a commercial pack of cigarettes containing 20 cigarettes, regardless of the brand or tar/nicotine content. For example, a person who smokes two packs per day for 30 years would be documented as having a 60 pack-year history.
Physical Exam Components
A patient with CAD who presents to the emergency department with serious cardiac symptoms can show many abnormalities on physical examination, whereas a patient with CAD who comes to the clinic or office for a regular checkup may have only a few signs of the underlying disease. During a routine physical examination, the following findings would fit with a diagnosis of CAD.
WEIGHT
Body mass index (BMI) takes weight and height into consideration by including overall body size rather than a single indicator. BMI is calculated as the weight in pounds, divided by the height in inches squared, with this figure multiplied by 703. Below are the accepted weight parameters measured in BMI:
| BMI | Status |
|---|---|
| <18.5 | Underweight |
| 18.5–24.9 | Normal weight |
| 25.0–29.9 | Overweight |
| ≥30 | Obese |
Patients with excess intra-abdominal or visceral fat (an “apple-shaped” build) are more likely to have atherosclerotic cardiovascular disease. Waist circumference is a good measure of intra-abdominal fat content: a waist circumference of >102 cm (>40 inches) in men or >89 cm (>35 inches) in women is considered in the high-risk range for cardiovascular disease (Mayo Clinic, 2023c).
VITAL SIGNS
During a routine office visit, the pulse may have a normal rate and rhythm in a patient with CAD. Tachycardia (heart rate >100 beats per minute) is common, however, when a patient is suffering from an episode of myocardial ischemia as a result of the stress hormones released. Bradycardia (heart rate <60 beats per minute) during an acute coronary syndrome can be an ominous sign because of the drop in cardiac output.
During a physical examination during a routine office visit or in a medical facility, the clinician can recommend aerobic exercises to improve physical and cardiac fitness and increase collateral circulation. The goal of aerobic exercise is to increase the resting heart rate to 50%–75% of the maximum heart rate appropriate for the patient’s age. The following chart indicates a target heart rate zone based on the age of adult patients:
| Age | Target (beats per minute) |
Maximum (beats per minute) |
|---|---|---|
| (Mayo Clinic, 2024c) | ||
| 25 | 100–170 | 200 |
| 35 | 93–157 | 185 |
| 45 | 88–149 | 175 |
| 55 | 83–140 | 165 |
| 65 | 78–132 | 155 |
Patients with CAD often have hypertension (BP ≥135/85), and the higher the blood pressure, the greater the risk of heart disease. Hypotension during a myocardial infarction is also an ominous sign because of the possible increased damage to the myocardial tissue secondary to the higher afterload.
The respiration rate is usually normal (12–20 breaths per minute) in a routine office visit, but patients will breathe more rapidly under the stress of heart ischemia secondary to stress hormones.
SKIN
No unusual sweating is expected on a routine office visit, but acute coronary syndromes, especially myocardial infarctions, are often accompanied by profuse sweating (diaphoresis). The skin will also show signs of hypoxia with cyanosis, pallor, mottling, and an increase in the occurrence of decubitus ulcers and other skin lesions that do not heal readily.
HEAD AND NECK
The blood vessels of the retina may show the effects of hypertension or atherosclerosis (i.e., widened light reflections from the arteries, copper- or silver-colored arteries, white sheaths along the arteries, venous tapering or “nicking” at arterial-venous crossings, hemorrhages, or papilledema). Diabetes, which worsens CAD, produces a characteristic retinopathy.
Atherosclerotic plaques can produce local blood turbulence, which will sometimes cause a bruit that can be heard when listening to the carotid arteries. CAD that evolves into heart failure may cause jugular vein distension due to congested blood vessels.
Hypoxia may affect the individual’s ability to think or reason, orientation, and level of consciousness. Carotid arteries blocked by atherosclerotic plaques may cause confusion, hallucinations, irritability, memory loss, restlessness, pupil response, and reduced muscle strength.
THORAX
The pain of heart ischemia is usually diffuse and “somewhere inside.” If a patient’s chest pain can be reproduced by the examiner pressing on some point along the chest wall, the pain is unlikely to be angina. (In some patients with myocardial infarctions, however, broad regions of the chest become tender.)
On a routine exam, the lungs of a patient with CAD can be clear and unremarkable. With myocardial infarction, on the other hand, the patient may be breathing rapidly and may complain of shortness of breath. When ischemia has brought on some degree of heart failure, valve dysfunction, or dysrhythmia, patients can have fluid in their lungs, and crackles or coarse breath sounds may be auscultated. Chest expansion may be asymmetrical due to guarding while breathing, secondary to pain.
A routine physical exam of a patient with CAD may find no overt heart problems. If the patient has a history of ischemic episodes, there may be several adverse findings. Previous heart surgeries will have midline chest scars. Hypertension or heart failure may have caused cardiomegaly. Murmurs suggest valve or papillary muscle damage, and gallops suggest heart wall damage (Morata & Flynn, 2024).
An ischemic heart is more susceptible to dysrhythmias. Infarction causes necrotic tissue that is electrically and chemically unstable.
ABDOMEN
CAD is a risk factor for aortic aneurysms. A patient with an aortic aneurysm is at higher risk for rupture in the case of a patient with CAD with epithelial inflammation and ruptured atherosclerotic plaques in the arterial wall. An already weakened vessel wall, because of the aneurysm, is more vulnerable to internal rupture in the case of CAD (Cleveland Clinic, 2022a). Rupture of an aortic aneurysm is a medical emergency and requires immediate attention. Bruits from other major abdominal arteries, such as the renal arteries, can be due to atherosclerosis.
EXTREMETIES
Peripheral edema may be secondary to heart failure due to chronic ischemic heart disease. Atherosclerosis can cause weakened peripheral pulses. Diabetes can produce neuropathies, which show up as a decrease in the patient’s ability to sense stimuli in the feet and/or hands with paradoxical, concurrent extremity pain.
GENITOURINARY
When CAD has progressed to a concurrent heart failure, fluid balance is an essential aspect of the physical health examination. In addition to the peripheral edema noted above, sluggish circulation may cause compromised renal function secondary to decreased renal perfusion. Retained fluid as evidenced by peripheral edema and decreased urinary output may cause an increase in blood pressure that will place more stress on arterial wall atherosclerotic plaques, promoting rupture. Compromised circulation may contribute to reduced libido in women and erectile dysfunction in men in CAD, as in diabetes (Morata & Flynn, 2024).
Laboratory Studies
A patient being evaluated for CAD is given several laboratory tests. Certain tests are especially helpful in assessing a patient’s risk of serious heart damage from atherosclerosis. These include blood tests of lipid levels, complete blood count, fasting glucose levels, A1C, creatinine and other metabolic levels, and the possible presence of cardiac markers (e.g., troponin), which are indicators of recent heart cell damage.
BLOOD LIPIDS
High serum cholesterol levels markedly increase a patient’s risk for developing atherosclerosis-induced heart injury. The LDL (“bad”) fraction of cholesterol is the specific culprit. Patients with CAD often have one or more lipid levels in the unhealthy range.
Certain desired lipid levels have been increased somewhat in recent years with new research. The box below shows both healthy and unhealthy fasting blood lipid levels for patients with no evidence of CAD and little or no risk factors. These are different from the lipid levels cited earlier in the course, as those are not specifically fasting nor clear evidence of CAD.
| Lipid |
Optimal (mg/dL) |
Unhealthy (mg/dL) |
|---|---|---|
| Total cholesterol | <200 | >240 |
| HDL cholesterol | ≥60 | <40 (men) <50 (women) |
| LDL cholesterol | <160 | >160 |
| Triglycerides | <190 | >200 |
In the presence of CAD or significant risk factors, some target lipid levels are recommended to be even lower:
- LDL <100 mg/dL
- Triglycerides <130 mg/dL
(Morata & Flynn, 2024)
FASTING PLASMA GLUCOSE
Patients with diabetes are twice as likely to develop CAD and other types of heart disease and two to four times as likely to die of heart disease. People with diabetes develop heart disease, including CAD, at an earlier age because of scarring to the interior of blood vessels caused by high blood sugars. Diabetes will manifest as a fasting plasma glucose level of ≥100 mg/dL when measured on at least two different days. Tight control of the serum glucose will help to prevent the development of CAD when added to a program of a low-fat, low-carbohydrate diet and physical activity (Healthline, 2023).
BLOOD UREA NITROGEN (BUN) / SERUM CREATININE
Renal disease worsens atherosclerosis. The levels of BUN and creatinine in a patient’s blood can be used to screen for several kidney problems. When the CAD is complicated by heart failure, the kidneys are more likely to fail or will fail more quickly.
CARDIAC MARKERS
When myocardium is damaged, intracellular molecules leak into the bloodstream. After a myocardial infarction, specific heart proteins (cardiac markers) can be detected in a patient’s blood within hours and then for many days afterward. The standard cardiac markers are the cardiac troponin molecules. Other commonly measured proteins are the creatinine kinase molecules. Cardiac markers are used for diagnosing and following emergency cardiac events and are not measured at routine checkups for CAD.
| Marker | Normal Level | Duration of Elevation after MI |
|---|---|---|
| (Morata & Flynn, 2024; Pagana et al., 2022) | ||
| Creatinine kinase (CK-2-MB) |
0.3–4.9 ng/mL |
|
| Myoglobin | <72 ng/mL (men) <58 ng/mL (women) |
|
| Cardiac troponin I (cTnI) | <0.03 ng/mL |
|
| Cardiac troponin T (cTnT) | <0.1 ng/mL |
|
Creatinine kinase-2 (CK-2) was formerly the definitive diagnostic test for MI. This has been replaced by serum troponin levels. The troponin levels remain elevated for so much longer than the CK-2, it is possible to discover an MI has occurred when the patient presents for diagnosis after a delay of even several days.
Troponin I and troponin T are released into the blood subsequent to a myocardial infarction (MI). A normal serum troponin level is 0–0.4 ng/mL (nanogram/ milliliters) while a level >0.40 ng/mL is indicative of a probable MI. Physicians will usually order a series of serum troponin levels, usually every six hours apart, to document the progression of myocardial damage over time. Other diseases and disorders that may contribute to an elevated serum troponin are sepsis, renal failure, chronic kidney disease, heart failure, undergoing chemotherapy, cardiac infection, myocarditis, cardiac damage secondary to recreational drugs such as cocaine, and trauma such as that caused by a hard blow to the chest (Villines, 2023).
Serum myoglobin elevation is indicative of inflammation. It is not a specific determinant of an MI, but the early release may allow the practitioner an early diagnosis.
SERUM ELECTROLYTES
Electrolytes are closely associated with cardiac contractility and conduction. Small changes from the normal serum levels in either direction may cause dysrhythmias, particularly with potassium and magnesium. Normal serum levels and panic levels of the most significant electrolytes are shown in the table below.
| Electrolyte | Normal Range | Panic Value |
|---|---|---|
| (Morata & Flynn, 2024) | ||
| Potassium | 3.5–5.0 mEq/L | <2.5 or >6.6 mEq/L |
| Calcium L | 8.5–10.2 mg/d | <7.0 or >12.0 mg/dL |
| Magnesium | 1.7–2.2 mg/dL | <0.5 or >3.0 mg/dL |
Electrocardiogram (ECG)
Twelve-lead electrocardiography is the standard method for identifying dysrhythmias and conduction problems. In terms of CAD, the ECG is a quick, accurate, and noninvasive way to detect myocardial injury, ischemia, pericarditis, pulmonary diseases, left ventricular hypertrophy, and the presence of prior myocardial infarction. In the presence of any chest pain, this diagnostic test will be performed before any others.
An MI changes the electrical properties of the region of the heart muscle that has been affected, and these changes can be seen in the ECG. The location of the ischemic heart region can often be identified by the segments of the wave pattern that have changed. The segments of the electrical wave pattern produced during a heartbeat have been named, and changes in the ST segment and the T wave are the clearest indicators of a myocardial infarction (Morata & Flynn, 2024).
About one quarter of patients with stable angina will have a normal ECG wave pattern when they are resting. To determine the degree of heart ischemia that a patient with chronic stable angina suffers when the heart is stressed, an ECG can be taken while the patient exercises, typically walking on a treadmill or pedaling a bicycle. Not all patients with CAD show ECG changes during such “stress testing” (see below).
Stress Testing
Stress testing is a noninvasive procedure that directly assesses the ability of a patient’s heart to cope with exercise. A stress test is a controlled way to increase the myocardial oxygen demand to find the threshold beyond which coronary arteries supply insufficient blood, causing myocardial ischemia. The lower the threshold (i.e., the smaller the stress) at which symptoms appear, the worse the patient’s coronary artery disease (Harding et al., 2022).
Stress tests can confirm that a patient’s complaint of chest discomfort is actually anginal pain. The tests can also establish the level of activity that causes chest discomfort. Subsequent stress tests can objectively monitor both the progression of the CAD and the efficacy of treatments.
EXERCISE STRESS TESTING
The preferred heart stressor is graded exercise, which consists of having the patient walk on a treadmill or ride on a stationary bike, progressively increasing the speed and inclination of the device to cause exercise stress while the patient is connected to a cardiac monitor, an automatic blood pressure cuff, and a pulse oximeter. This allows the examiner to monitor and record continuous data about heart rate, dysrhythmias, blood pressure, and capillary oxygen levels while the heart undergoes physical stress.
As part of exercise stress testing, patients are instructed as follows:
- About procedures that will take place before, during, and after the test
- To withhold certain medications prior to the test (e.g., beta blockers may limit the patient’s ability to increase heart rate during the test)
- To avoid caffeine for 24 hours prior to the procedure, otherwise the test may be cancelled
- To wear comfortable clothing and shoes for the test
- To report any level of discomfort or other symptoms that occur during testing
- To immediately report any chest pain, leg pain, shortness of breath, or fatigue during testing
- That they will return to their hospital room or home as soon as their heart rate and rhythm return to their pre–stress test levels
(Morata & Flynn, 2024)
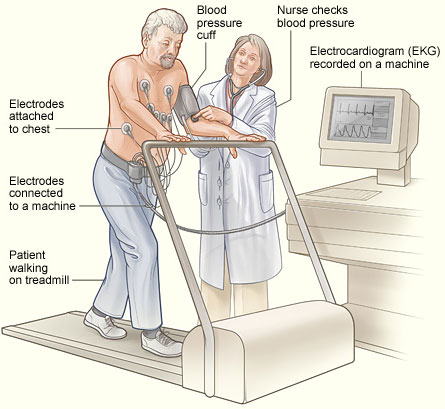
Stress testing uses graded exercise in a supervised session to assess the heart’s response to increases in its workload. (Source: NHLBI.)
During a stress test, symptoms of heart problems—such as angina, shortness of breath, severe fatigue, lightheadedness, or fainting—can appear when patients exceed their tolerated exercise threshold. At the same threshold, signs of heart problems—such as gallops, dysrhythmias, hypotension, inappropriate increases or decreases in heart rate, dyspnea, pulmonary rales, or cyanosis—can also appear.
In addition to watching for these symptoms and signs of cardiac problems, the stress test examiner uses more objective monitoring. The typical objective monitor is electrocardiography (ECG), which shows the rate and rhythm of the heart’s electrical wave pattern, and echocardiography, which follows changes in the heart’s anatomy and functionality during exercise.
ECG stress testing is most useful in the following two clinical scenarios:
- When trying to make a diagnosis of CAD in an unclear case
- When measuring the exercise limitations imposed by a patient’s CAD
Some ECG stress tests give false positives, so the test is not recommended for routine examinations of people who are not likely to have CAD. This most commonly occurs in patients who experience an increase in blood pressure while performing the stress test. The blood pressure subsequently returns to normal when the patient is in the resting phase after stress testing and is not related to the appearance of chest pain. Some ECG stress tests give false negatives, and an ECG stress test that appears normal cannot be used to discard an otherwise convincing diagnosis of CAD based on symptoms, history, and risk factors (Harding et al., 2022).
ANSWERING PATIENT QUESTIONS
Q: What is a stress test?
A: In a stress test, a person exercises in a safe place to see how well their heart handles increased activity. Usually, you walk on a treadmill or pedal a bicycle while a technician monitors your pulse rate, blood pressure, and electrocardiogram (ECG).
You will probably be asked to come to the hospital in comfortable clothes and soft shoes. When you arrive in the exercise room, electrode pads will be stuck to the skin of your chest, and the wires will be attached to an ECG machine, which records the electrical activity of your heart.
Then you will exercise—slowly at first and gradually harder. Your heart rate will get faster, your blood pressure will go up, and you will breathe more heavily. Meanwhile, the technician will keep an eye on the electrical activity of the heart. If you feel any heart symptoms, the test will be stopped. The goal is to measure exactly how much work (stress) your heart can cope with and, if your heart has difficulty, what specific heart problem may be occurring.
PHARMACOLOGIC STRESS TESTING
When patients cannot tolerate exercise, their heart can be stressed with a specific drug in a monitored and controlled setting. This is done in conjunction with radionuclide scintigraphy and/or echocardiography. A physician is present at all of this type of stress tests because of the possibility of induced cardiac pain or life-threatening dysrhythmias, and the tests are tailored to the individual patient’s health. Patients who are unable to undergo strenuous exercise or are incapacitated (e.g., leg fracture) and unable to run on a treadmill or ride a stationary bike may be good candidates for this type of stress testing.
Dipyridamole (Persantine) or dobutamine (increasing heart rate and contractility) is administered intravenously. Regadenoson or adenosine may be given to cause vasodilation of normal coronary arteries. Depending on the patient’s history, thallium or sestamibi (a radioactive tracer) may also be administered with the stress test. The drugs will stimulate the heart to react as if the patient were exercising.
A partially or completely blocked coronary artery will be unable to dilate under these conditions, and this will be visible as hypoperfusion on radionuclide scanning or as hypokinesis (poor movement) on echocardiography (Morata & Flynn, 2024).
The tracer drugs travel through the bloodstream to the heart, where they are picked up by the muscle cells. The areas of the heart that lack adequate blood supply pick up the tracer very slowly or not at all. Baseline images are compared with images taken three to four hours later. A cardiologist will determine if areas of the heart have suffered permanent damage from previous ischemia.
Imaging Tests
Images of the heart and the coronary arteries can be obtained in a variety of ways. The least invasive techniques are chest X-rays, CT scans, and echocardiograms. Another technique, coronary arteriography, produces excellent views of the coronary arteries, but it is an invasive procedure, using arterial catheters, with potentially hazardous side effects.
CHEST X-RAYS
A chest X-ray is usually performed in the anteroposterior (AP) and lateral views and shows the size and shape of the heart and the condition of the lungs. Patients with CAD can have normal chest X-rays, and usually chest X-rays do not help to diagnose CAD. Sometimes, chest films will show consequences of the disease, such as cardiomegaly, cardiac positioning, cardiac abnormalities, aortic aneurysms, aortic dissections, or fluid infiltrating the pulmonary or pericardial spaces.
CORONARY ARTERY CALCIUM (CAC)
CAC is a newer type of diagnostic X-ray that scans the heart to measure the amount of calcified plaque in the coronary arteries. This noninvasive procedure can provide an early diagnosis of CAD, possibly in asymptomatic patients. This gives care providers the opportunity to initiate early treatment for prophylaxis, such as one aspirin tablet per day or starting a regime of statins or other anticholesterol/antilipid medications.
Results of the CAC can also be used for risk stratification (putting patients into groups of similar complexity and care needs), therefore predicting the risk of a future MI.
CAC can be used as an easy way to diagnose those with subclinical coronary artery plaques. Most recently, CAC is used to predict risk in borderline or intermediate levels of heart disease, particularly among people at higher risk such as those with a history of preeclampsia and early menopause (AHA, 2023b).
ECHOCARDIOGRAPHY
An echocardiogram uses noninvasive ultrasound technology to show the size and thickness of the atria and ventricles of the heart. It also shows the heart valves in action. Used during stress testing for CAD, echocardiography can indicate which heart walls or valves are most affected by ischemic episodes. Echocardiographic stress tests are not recommended as screening tools, but many physicians use these tests to confirm a clinical diagnosis of CAD in unclear cases. Echocardiography can be used to measure ejection fraction, which is the amount of blood ejected from the left ventricle during systole. (Normal ejection fraction is 55%–70%) (Harding et al., 2022).
Transesophageal echocardiography (TEE) takes an ultrasonic image from a view below the heart. It is performed by inserting a flexible gastroscope into the esophagus. TEE is specifically used to view prosthetic heart valves, mitral valve function, aortic dissection, vegetative endocarditis, tumors, and emboli. The patient is NPO for six to eight hours before the procedure and until the gag reflex returns post procedure. Side effects include sore throat, dysphagia, neck and shoulder pain, and a rare occurrence of esophageal perforation (Harding et al., 2022).
CORONARY ARTERIOGRAPHY
Coronary arteriography (also called coronary angiography or cardiac catheterization) is an invasive procedure that uses X-rays to follow dye injected into the heart or the coronary arteries via arterial or venous sheaths. This is used to measure pressures in the ventricles, measure cardiac output, and quantify coronary arterial patency by displaying the velocity of blood flow as the dye passes through the arteries. Coronary arteriography gives as definitive a diagnosis of arterial narrowing and blockage as is possible without major surgery.
The high cost, mortality rate, and morbidity rate limit its use as a routine diagnostic tool. The procedure holds a high possibility of hemorrhage when the catheter through which the dye is injected is removed. Coronary arteriography is most often used in CAD patients when preparing them for possible bypass grafts or other heart operations. Coronary arteriography is also used when other tests cannot determine the cause of debilitating cardiac symptoms of ischemia (Golla et al., 2023).
CORONARY COMPUTERIZED TOMOGRAPHY ANGIOGRAPHY (CCTA)
CCTA is a noninvasive form of imaging that provides an accurate diagnosis of obstructive and nonobstructive (<50% occlusion in a coronary artery) disease, risk stratification, and early initiation of appropriate treatment. This diagnostic method has proved particularly effective for use in women in spite of their smaller epicardial blood vessels.
In the PROMISE trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain), results showed that when CCTA is used on women, there are more effective prognostic results than with stress testing (exercise electrocardiography, exercise imaging, or pharmacologic imaging). There is also less radiation produced.
Radiation exposure is particularly a concern with the proximity of diagnostic imaging to breast tissue. Initially, there were concerns with the use of CCTA for women and the amount of exposure. Advances in technology have significantly reduced the amount of radiation from CCTA, particularly compared to other myocardial perfusion imaging techniques such as positron emission tomography (PET) and single-photon emission computerized tomography (SPECT) (Harding et al., 2022; Rassmussen et al., 2022).
OTHER STUDIES
Computed tomography (CT) scanning is another common imaging tool in coronary artery disease. Coronary calcium scoring involves administration of a cardiac CT scan to collect information about the presence, location, and extent of calcified plaque in the coronary arteries. Because calcium is a marker for CAD, the coronary calcium score (a number reflecting the degree and extent of calcium deposits in the walls of the coronary arteries) can be a useful prognostic tool in coronary artery disease (Harding et al., 2022).
Cardiac magnetic resonance imaging (MRI) is a noninvasive diagnostic test used to evaluate tissues, structures, and blood flow. It is used to diagnose CAD, aortic aneurysms, congenital heart disease, left ventricular function, tumors, blood clots, and pericardial disorders. The advantages are that the patient is not exposed to ionizing radiation and that dye can be injected to enhance results. Disadvantages are that implanted metal (e.g., pacemakers, defibrillators, or cochlear implants) may prohibit this test being performed, as the powerful magnet involved may pull too strongly on the metal, dislodging it. Another disadvantage may be that an enclosed MRI chamber can trigger claustrophobia in the patient (Harding et al., 2022).
Magnetic resonance angiography (MRA) uses MRI technology combined with injected contrast dye to assess for areas of narrowing or blockages in the coronary arteries. This technology is not as precise as coronary arteriography.
MANAGEMENT OF ACUTE CAD
Patients with CAD may have mild symptoms (stable angina) that can be monitored and treated over time. Other patients may present with chest pain, dyspnea, profuse sweating, extreme fatigue, or other acute symptoms that may need to be seen in an emergency department and evaluated immediately.
Emergency Treatment
Emergency treatment for patients with cardiac arrest caused by CAD can be guided by the American Heart Association’s “chain of survival,” a series of actions that, when put into motion, can reduce the patient’s chance of dying from cardiac arrest. The six links in the adult out-of-hospital chain of survival are:
- Recognition of cardiac arrest and activation of the emergency response system
- Early cardiopulmonary resuscitation (CPR) with an emphasis on chest compressions
- Rapid defibrillation
- Advanced resuscitation by emergency medical services and other healthcare providers
- Post–cardiac arrest care
- Recovery (including additional treatment, observation, rehabilitation, and psychological support)
(AHA, 2024d)
Unless patients have already been diagnosed with stable angina and recognize that they are having a typical short-lived anginal attack, they should call 911 and be transported quickly by emergency responders to an emergency department whenever experiencing an episode of chest pain.
BEFORE THE HOSPITAL
Because quick treatment of cardiac arrest caused by MI is so beneficial, bystanders should start cardiopulmonary resuscitation (CPR) as soon as they see someone collapse, call 911, and use an automated external defibrillator (AED) if one is available.
Emergency response professionals (EMTs or nurses) who encounter patients experiencing chest pain or a sudden onset of dyspnea should treat the symptoms as myocardial ischemia and begin active interventions.
According to the ACLS Training Center (2023), the immediate actions to treat acute coronary syndrome include:
- Monitor and support CABs (circulation, airway, breathing).
- Monitor vital signs.
- Monitor the cardiac rhythm.
- Administer CPR, if necessary.
- Use a defibrillator, if necessary.
- Administer oxygen as needed to maintain the saturation at 94%–100%. If the patient has a history of COPD, administer oxygen if pulse oximetry falls below 90% on room air.
- Obtain a 12-lead ECG.
- Interpret or request an interpretation of the ECG. If ST elevation is present, transmit the results to the receiving hospital. Hospital personnel gather resources to respond to STEMI. If unable to transmit, the trained prehospital provider should interpret the ECG, and the cardiac catheterization laboratory team should be notified for STEMI activation based on that interpretation.
In addition to the above emergency procedures:
- Start an intravenous (IV) access for the purpose of administering emergency medications, if necessary.
- Have a conscious patient chew and swallow 325 mg of aspirin.
- Administer sublingual NTG by tablet or spray every five minutes until chest pain is relieved a maximum of three times.
If the pain is unrelieved by NTG, give a narcotic pain reliever such as fentanyl, hydromorphone (Dilaudid), or morphine sulfate. Morphine is the drug of choice for a myocardial infarction, given its properties of preload and afterload reduction.
ANSWERING PATIENT QUESTIONS
Q: I think I’m having a heart attack, but I’m not sure. Should I call my doctor? Should I drive to the hospital?
A: Don’t waste time calling your doctor, and don’t take any chances. Don’t drive yourself to the hospital. Instead, call 911 immediately. Emergency medical technicians can start to treat you on the way to the hospital. While you wait for the ambulance, if you can, chew one regular aspirin (325 mg) or four baby aspirins (81 mg each), then sit down and try to relax.
Q: But I would be embarrassed having an ambulance zooming up to my house with lights flashing and sirens blaring. I’d feel even worse if I wasn’t really having a heart attack.
A: Of course, those are normal feelings. The paramedics in the ambulance and the healthcare professionals in the emergency department know that it isn’t easy for a person to figure out if they are really having a heart attack. They also know that when people wait too long to get help, they are more likely to die. No one will give you a hard time if you are not actually having a medical crisis.
If there is even a small chance that you could have a heart attack, your primary care provider may have already warned you. Your life is worth more than a little embarrassment, so call 911 if there is any possibility that you might be having a heart problem.
IN THE EMERGENCY DEPARTMENT (ED)
Medical Screening Exam (MSE)
When a patient experiences ischemic heart symptoms, it is potentially a life-threatening emergency. Screening of patients with acute chest pain by the medical team in the ED includes the following assessment steps, coordinated by the ED physician in conjunction with the nurse conducting the initial medical screening examination:
- Upon presentation to the ED with symptoms suggestive of an acute coronary syndrome, the patient is immediately taken to a room.
- The ED physician assesses for and, if necessary, reverses circulatory system failure and respiratory insufficiency.
- When clinically stabilized, the patient is assessed for immediate life-threatening conditions (medical crises associated with chest pain):
- Cardiovascular
- Acute, massive myocardial infarction (MI)
- Pulmonary embolism
- Aortic dissection
- Cardiac tamponade
- Pulmonary
- Pulmonary embolus
- Tension pneumothorax
- Cardiovascular
- Working together with the ED physician, nurses’ responsibilities may include assessing the patient’s complaints, symptoms, and vital signs. Patients presenting with a complaint of chest pain are evaluated with a focused history and physical examination conducted by a physician. Chest pain typically requires additional diagnostic testing, including ECG, chest X-ray, and blood tests for cardiac markers of ischemic heart injury.
ED nurses monitor the patient’s basic vital signs and cardiac monitor at regular intervals for the development of any change in hemodynamics or dysrhythmias.
Older patients (>75 years), patients with diabetes, and female patients are more likely to present with the sudden onset of dyspnea and fatigue as the primary symptoms of an acute coronary syndrome, and new dyspnea can be the equivalent of chest pain in these individuals. Nausea and vomiting may also accompany these symptoms.
Evaluation of Stabilized Patients
After stabilizing patients, an MSE protocol for chest pain/sudden dyspnea is implemented.
It is important for the team of providers in the ED to remember that one third of people with acute myocardial infarction do not mention chest pain as their chief complaint. Many patients are more likely to describe other symptoms as their primary complaint, even when they are suffering an MI.
Atypical presentations tend to come from patients with diabetes, older adults, women, patients of non-White ethnicities, and patients with dementia. Besides dyspnea, atypical symptoms include nausea; profuse sweating; fainting; and pain in the neck, shoulder, arms, or upper abdomen.
To begin the medical screening of a patient with stable chest pain, the ED physician working with a stabilized adult patient may order an immediate 12-lead ECG to rule out (r/o) a STEMI. It is thought that patients with STEMIs usually have a completely blocked artery, whereas patients whose infarctions do not produce significant ST elevations have an incompletely blocked artery (NSTEMI) (Harding et al., 2022; Singh et al., 2023).
Treatment of STEMI
Fast treatment gives the best outcome for all myocardial infarctions. Certain types of myocardial infarction will benefit dramatically from quick reperfusion therapies, drugs, and other techniques that open the blocked arteries and restore blood flow.
Heart damage does not happen all at once after the blockage of a coronary artery; a myocardial infarct continues to enlarge over five to six hours if the blockage is not reduced or removed. After the initial infarction, an area of ischemia known as a corona (crown) surrounds the infarcted tissue. It is this ischemic tissue that may become infarcted if reperfusion of the area is not established.
For these reasons, the AHA criteria recommend that EDs aim for reperfusion within 90 minutes of admission to the hospital, with an emphasis of treating STEMI patients within 90 minutes. The mortality rate from a STEMI can be decreased by about half if the blocked arteries are reopened in the first 90 minutes after the symptoms begin. Quick reperfusion therapy will also reduce the amount of permanent muscle damage resulting from a STEMI.
Since reperfusion may be accomplished by thrombolytic drugs or dilation of the blocked artery, this 90-minute duration is referred to as “door-to-balloon” time. If the patient is initially admitted to a non-PCI hospital (i.e., lacks the capacity to perform a percutaneous coronary intervention), the “door-to-balloon” or “door-to-device” time must include the transfer to a PCI hospital (Singh et al., 2023).
When a STEMI is identified, a reperfusion plan is formulated for the patient by the healthcare team. The two major choices for reopening a blocked artery are pharmacologic and mechanical. The pharmacologic option is administration of a fibrinolytic or thrombolytic drug therapy, unless contraindications exist, to weaken and disrupt the damaging clot. The mechanical option consists of a percutaneous coronary intervention (PCI), also known as PTCA (percutaneous transluminal coronary angioplasty), meaning balloon angioplasty with or without the placement of a stent, to break up or remove the clot. The ideal time from when the ACS patient enters the ED until they reach the heart catheterization lab for a PCI is 90–120 minutes. Most recently, PCI is performed more often than administration of thrombolytics (Singh et al., 2023).
Thrombolytic or fibrinolytic drugs may be given after a STEMI to dissolve a blood clot that is completely occluding a coronary artery. These drugs are also familiarly called “clot busters.” They are administered as soon as possible after a diagnosis is made and this form of reperfusion is chosen. They are given slowly by intravenous infusion over a period of 30–90 minutes, depending on the drug. AHA guidelines recommend a 30-minute “door-to-needle” time from the prescriber’s order to the administration of the drug to be (Singh et al., 2023).
The outcomes of PCIs are considered to be more effective than thrombolytic or fibrinolytic drugs. The possible side effects of thrombolytics/fibrinolytics are considered to be potentially more serious than PCIs because of the potential for hemorrhage. These are not the only factors that determine which method will be used to treat ACS, as only 39% of hospitals in the United States have cardiac catheterization laboratories, and patients aren’t always able to reach these hospitals within the recommended 90- to 120-minute window (Vallabhajosyula et al., 2021).
Two possible side effects of thrombolytics are so potentially dangerous that they are only administered in an ED or an ICU where critical care nurses can closely monitor the patients. The more common side effect is reperfusion dysrhythmias. This is an accelerated idioventricular rhythm, or a heart rhythm initiated in the ventricles at a rate faster than the inherent rate of the ventricles (>70 beats per minute). The cause is a rapid return of blood perfusing the ischemic myocardial tissue. For this reason, the patient’s heart is monitored continuously, although the rhythm usually returns quickly to normal.
Thrombolytics dissolve the blood clot that is blocking one or more coronary arteries. They may also dissolve other blood clots, causing hemorrhage. This is a rare but much more dangerous potential side effect. For this reason, patients who are given thrombolytics or fibrinolytic drugs are monitored in the ICU setting.
Commonly used thrombolytic agents include:
- Tissue plasminogen activator (TPA)
- Alteplase
- Reteplase
- Tenecteplase
- Lanoteplase
(Harding et al., 2022)
CONTRAINDICATIONS FOR THROMBOLYTICS
Absolute (will never be given):
- Previous intracranial bleeding
- Vascular malformation
- Intracranial tumor
- Ischemic stroke within the past three months
- Closed head or facial injury within the past three months
- Intracranial or intraspinal surgery within the past two months
- Severe uncontrolled hypertension
- Active internal bleeding
- Suspected aortic dissection
Relative (may be given after evaluation):
- Active peptic ulcer disease
- Use of oral anticoagulants
- Pregnancy
- Ischemic stroke <3 months ago
- Dementia
- Intracranial pathology
- Noncompressable vascular punctures
- Recent (2–4 weeks) internal bleeding
- Major surgery <3 weeks
- History of chronic, severe, poorly controlled hypertension
- Current BP >180/110
- Traumatic or prolonged (>10 minutes) cardiopulmonary resuscitation
(Harding et al., 2022)
Percutaneous coronary interventions (PCIs) are an invasive form of reperfusion therapy. They have more potential risks and are more expensive than drug therapy. They must be performed in a facility with a cardiac catheterization laboratory (cath lab) or interventional radiography department (IR), often limiting the ability to perform reperfusion within the desired 90-minute goal. A cardiologist usually performs these. (See also “Percutaneous Coronary Intervention” later in this course.)
Distinguishing a STEMI from an NSTEMI infarction is important. Either reperfusion technique will benefit STEMI patients when done quickly. Patients without the characteristic ECG changes of STEMI may have either NSTEMI infarction or unstable angina, which also must be treated but not necessarily in the same emergent manner.
Additional diagnostic tests coordinated by the triage physician for all chest pain/sudden dyspnea patients include a chest X-ray. As the patient is evaluated, the nurse continuously makes assessments for changes on the telemetry monitor and in symptoms, vital signs, and blood oxygen levels, since these are important indicators of a worsening medical condition. ECGs may also be repeated. Serum cardiac markers will be measured to determine whether reperfusion has caused a release of protein into the blood.
Stabilized patients who are unlikely to have an acute coronary syndrome still need to be evaluated for the cause of their chest pain. The ED physician conducts a thorough patient history to uncover underlying causes for chest pain. Among the causes that are considered include pneumonia, pulmonary embolism, pneumothorax, pericarditis, myocarditis, rib fracture, costochondral separation, esophageal spasm, aortic dissection, renal calculus, splenic infarction, abdominal disorders, or chest injuries (Morate & Flynn, 2024).
CASE
Nelson Martinez is a slightly overweight, 56-year-old Hispanic male with a history of hypertension, CAD, and chronic stable angina. At a family gathering in a local park, he joined in on a soccer game but started to feel nauseated and short of breath after running around for a few minutes. Because he has a history of heart disease and angina, his wife called 911, and an ambulance brought him to the closest emergency department.
Mr. Martinez was admitted to the ED 30 minutes after his angina symptoms emerged. He described his initial symptoms as shortness of breath, nausea, and arm pain to the nurse in the ED. The nurse recognized these as signs of possible myocardial ischemia and immediately initiated the chest pain protocol along with the medical team. This included a chest X-ray, a 12-lead ECG, starting an IV, administering 325 mg of oral aspirin to be chewed, drawing blood, and initiating oxygen therapy. The nurse communicated this to Mrs. Martinez and explained the process of assessment of the chest pain in order to stabilize her husband’s angina. Mrs. Martinez was reassured by the nurse that she did the right thing by calling 911, since quick treatment can improve outcomes.
The ECG revealed a ST elevation myocardial infarction (STEMI). The physician ordered the administration of a fibrinolytic drug to achieve reperfusion. The nurse working with Mr. Martinez explained the indication for the therapy to the patient and his wife by educating them about ST elevations and the need to treat symptoms quickly in order to provide blood supply to the heart muscle. The nurse helped Mr. Martinez to sign a consent for the procedure after the possible risks and benefits of the procedure were explained. She reassured Mr. Martinez and his wife that he would be monitored on a cardiac monitor continuously during and after the procedure and that he would be observed and have frequent vital signs taken the entire time the medication was infusing.
Because of the quick action by Mrs. Martinez and the identification of the STEMI, Mr. Martinez was treated within 60 minutes after his first symptoms appeared. His condition was stabilized, and he was admitted to the cardiology service for further monitoring of his symptoms.
Goals of ED Care for Patients with Acute Coronary Syndromes
For all patients with acute coronary syndromes, the primary goals of care include:
- Revascularize the coronary artery.
- Stabilize heart rhythm.
- Preserve myocardial tissue and function.
- Reduce cardiac workload.
- Provide pain relief.
While the type of acute coronary syndrome is being identified, the following medical treatments may be ordered for the patient:
- Supplemental oxygen, to ensure that the existing blood supply is oxygenated to at least 94% unless more is needed to treat chest pain.
- Antiplatelet drugs. Aspirin taken daily reduces the mortality from an acute myocardial infarction, unless contraindications exist (such as coagulopathies), and all conscious patients with a possible acute coronary syndrome should have chewed and swallowed 325 mg of nonenteric-coated aspirin. Chewing causes the aspirin to absorb and start taking effect more quickly. Aspirin can also be given as a suppository if the patient is not able to take medications orally.
- Fibrinolytic drugs (TPA, alteplase, tenecteplase, lanoteplase, or reteplase), unless contraindicated, to weaken and disrupt the damaging clot in the coronary artery. Thrombolytic therapy can be used within 12 hours of the onset of symptoms (MedlinePlus, 2022b).
- Vasodilators, to increase blood flow to heart muscle and reduce the force needed to pump blood through the arterial system. The standard vasodilator for heart arteries is nitroglycerin, which can ease ischemic pain and can also reduce mortality rates. In the ED, nitroglycerin is administered either sublingually, by spray, or via IV drip. (Certain patients, such as those with hypotension, require graded doses of nitroglycerin and careful monitoring.)
- Beta blockers, such as atenolol, esmolol, metoprolol, or the newer nebivolol, to lessen the oxygen requirements of the heart by slowing the heart rate and lowering the arterial tension against which the heart is working (diastolic blood pressure). By reducing the cardiac workload, beta blockers also serve to reduce oxygen consumption, reserving a greater amount of oxygen to be available to the myocardium. Beta blockers also reduce the risk of developing heart dysrhythmias, which can accompany heart ischemia. The use of beta blockers has been shown to minimize the size of infarcts and to reduce mortality rates by as much as 40%.
- Angiotensin-converting enzyme (ACE) inhibitors to patients with evolving MI with ST segment elevation or left bundle-branch block, to reduce blood pressure, also reducing cardiac workload.
- Antidysrhythmic drugs, such as amiodarone, lidocaine, vasopressin, and epinephrine, to stabilize the heart rhythm if the patient has dysrhythmias.
- Transcutaneous pacing or defibrillation, if serious dysrhythmias continue and drugs are ineffective.
- Glycoprotein IIb/IIIa inhibitors (such as abciximab) in conjunction with daily aspirin, to reduce platelet aggregation if a patient continues to have unstable angina or acute chest pain.
- Anticoagulants, to keep new blood clots from forming. Heparin and the low-molecular-weight heparins (e.g., enoxaparin) are often used to lower the risk that unstable angina will progress to myocardial infarction. Heparin administration requires careful monitoring for bleeding, and when the drug is stopped, the patient must be watched for “rebound” ischemic episodes that sometimes occur during the subsequent 24 hours. Low molecular-weight heparins, when given in only therapeutic doses, are much less likely to cause bleeding as a side effect.
- Analgesics (pain relievers), such as morphine sulfate, to reduce chest pain and to reduce the sympathetic nervous system’s demands on the heart muscles.
- Laser angioplasty, atherectomy, or stent placement (see also “Percutaneous Coronary Intervention” below).
- Emergency cardiac surgery, for patients who are unable to undergo percutaneous interventions (see also “Coronary Artery Bypass Graft” below).
(Harding et al., 2022)
ANSWERING PATIENT QUESTIONS
Q: My doctor says my medicine is a beta blocker. What’s that, and what is it blocking?
A: A beta blocker is a drug that slows your heart rate and lowers your blood pressure. This kind of drug blocks the stress caused by the nerves that make you tense when you are frightened. It decreases the workload of the heart and prevents arrythmias and another heart attack.
Treating Stable CAD in the ED
Some patients who arrive to the ED with chest discomfort will have stable angina instead of an acute coronary syndrome. As with all patients with possible heart ischemia, these patients follow a similar treatment protocol, including aspirin, nitroglycerin, a beta blocker, supplemental oxygen, and a blood draw to search for cardiac marker molecules.
In patients with stable angina, the symptoms that brought them to the ED should resolve and not return over the two to three hours that they are being monitored. Their ECG and vital signs will remain normal for that patient for the next few hours, and repeated blood tests will find no cardiac marker molecules.
If the evaluation of noncardiac causes of their chest discomfort identifies no serious problems, these patients do not need further medical treatment in the ED. Instead, they can be monitored and followed as an outpatient by a CAD treatment team.
Care of the patient in the ED includes collaborative care with a multidisciplinary team approach. Team members may include emergency medical personnel, nurses, a cardiologist, respiratory therapists, radiology technologists, venipuncture technicians, and a rehabilitation specialist.
Nursing assessment and care of the cardiac patient in the ED is as follows:
- Assess and monitor vital signs (blood pressure, heart rate, temperature, oxygen saturation, respiratory rate, and heart and breath sounds), communicating any changes to the care team.
- Perform continuous cardiac monitoring of the patient via telemetry until discharge.
- Assess and monitor pain symptoms. Include severity, location, possible cause, what relieves the pain, and duration of pain; medications administered; a reevaluation of severity; and any related symptoms.
- Obtain a 12-lead ECG to assess heart rate and dysrhythmias (monitor at admission for a baseline and during acute episodes of angina).
- Assess and monitor urine output hourly or with each voiding.
- Monitor oxygen saturation status continuously and make changes as needed.
- Provide patient education regarding medications administered and any procedures anticipated; include family present in any education and provide brochures.
- Communicate changes and status updates to family as needed.
- Assess the patient and family for any ongoing psychosocial needs and refer to appropriate supportive services as needed (e.g., medical social worker, community resources, psychologist, counselor, or clergy).
Interventional Cardiac Procedures and Surgery
Treatment for patients with stable coronary artery disease includes both medical therapy and lifestyle modification. In some cases, however, surgery or an invasive intervention to increase blood flow to ischemic areas can be added to the treatment program to improve a patient’s heart function. The general term for these procedures is coronary revascularization, which is commonly performed throughout the United States.
Coronary revascularization should be considered for patients who still have debilitating angina after optimal medical therapy. The two types of coronary revascularization procedures are percutaneous coronary interventions (PCI) and coronary artery bypass grafts (CABG).
- PCI is usually indicated for patients with significant narrowing of one or two major coronary arteries when the left ventricle is functioning normally.
- CABG is indicated for patients with more than two arterial constrictions, left main coronary artery disease, failed medical management, or poor candidacy for a PCI, such as a long obstruction or one that is hard to access. A CABG is also considered when the patient has had a failed PCI and is still having chest pain.
There are other therapies for patients whose medical treatment does not improve the symptoms of their coronary artery disease but who are not good candidates for either PCI or CABG. The alternatives include laser transmyocardial revascularization that uses a high-energy laser to create channels in the myocardium to allow alternative blood flow to ischemic tissue (Harding et al., 2022).
In external enhanced counterpulsation, cuffs are applied to the legs and inflated sequentially distally to proximally during early diastole, with deflation at the onset of systole. This creates a retrograde aortic flow, causing diastolic augmentation and resulting in increased coronary perfusion, increased venous return, and improved cardiac output (Harding et al., 2022).
PERCUTANEOUS CORONARY INTERVENTION (PCI)
PCI, also commonly known as coronary angioplasty or simply angioplasty, is used to unclog blocked coronary arteries. If PCI is recommended, the patient may be transferred to an interventional radiology suite or cardiac catheterization laboratory, where the procedure takes place. The procedure involves threading a catheter into the constricted region of a coronary artery and expanding a tiny balloon to flatten the plaque back against the walls of the artery, creating a larger opening to improve blood flow.
A metal stent is left in the region of the flattened plaque to hold the artery open. The stent is coated with medications that are slowly and continuously released into the artery. These are called drug-eluting stents. The drugs help prevent the artery from becoming blocked with scar tissue that can form in the artery and to prevent blood clots or further plaque build-up from forming around the stent (Harding et al., 2022).
Typically, the PCI catheter is inserted under local anesthesia using X-ray fluoroscopy. The PCI catheter is threaded through the femoral or radial artery into the heart to the area where the coronary artery is narrowed. The procedure can take between 30 minutes and two hours.
PCI gives a sufficient increase in blood flow to initially reduce angina in >95% of cases. Approximately one fifth of treated arteries narrow again within six months, and angina returns within six months in about 1 in 10 patients. There are approximately 950,000 PCIs performed in the United States annually (Ghandakly et al., 2024).
In-stent restenosis (narrowing) is a continued concern with coronary angioplasty. Recent studies have shown that using drug-eluting stents (DES) is currently the most effective method for preventing the need for another reperfusion procedure. In one study, a DES using thrombolytic medication reduced the target lesion revascularization by 74% a year after implantation compared to bare metal stents (BMS) (Koźlik et al., 2023).
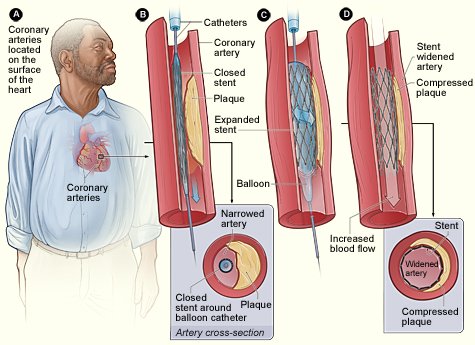
In PCI, a catheter is threaded into the region of the artery that is narrowed by plaque. A balloon near the tip of the catheter is inflated, flattening the plaque against the arterial wall and widening the space inside the artery. A wire support (stent) is left in place to hold the artery open. (Source: NHLBI.)
Care of a patient during and after PCI includes primary nursing assessments and measures as follows:
- Perform assessment and compare to baseline: vital signs; pulse oximetry; heart and breath sounds; neurovascular assessment of extremity used for procedure; assessment of catheter insertion site for hematoma, bleeding, signs of infection, and bruit.
- Assess neurovascular status of involved extremity every 15 minutes for the first hour, then according to institutional policy.
- Check for bleeding at catheter insertion site every 15 minutes for the first hour, then according to institutional policy.
- Report changes in neurovascular status of involved extremity or any bleeding.
- Monitor ECG for dysrhythmias or other changes (e.g., ST segment elevation or depression).
- Monitor patient for chest pain and other sources of pain or discomfort (e.g., back, vascular access site).
- Monitor IV infusions of antianginals (e.g., nitroglycerin) and antiplatelet medications (e.g., eptifibatide).
- Teach patient and caregiver about discharge drugs (e.g., aspirin, clopidogrel, lipid-lowering drugs).
- Teach patient and caregivers about discharge activity and care, including signs and symptoms to report to HCP (e.g., access site complications, return of chest pain).
(Harding et al., 2022)
CORONARY ARTERY BYPASS GRAFT (CABG)
Coronary artery bypass surgery is the most common open-heart operation performed in the United States, with approximately 400,000 procedures performed each year. CABG may be contraindicated in elderly patients and patients with end-stage kidney disease, lung disease, and peripheral vascular disease, as they are at higher risk for complications.
The procedure involves attaching an unclogged blood vessel to a blocked coronary artery beyond the obstruction. One or both internal thoracic (also called internal mammary) arteries can be rerouted, or a piece of the saphenous vein or the radial artery can be made into a conduit.
The surgery is done under general anesthesia and takes between three to six hours. Usually, the procedure is done by temporarily stopping the heart and oxygenating the blood with a cardiopulmonary bypass machine. When patients have no other serious disease, there is <1% mortality from a first-time CABG surgery.
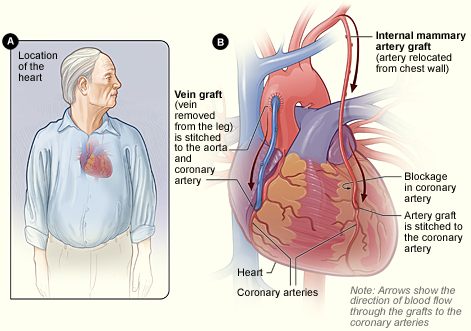
In CABG revascularization surgery, blood is routed past blockages in coronary arteries. Figure B shows how veins and artery bypass grafts are attached to the heart. (Source: NHLBI.)
There are several types of bypass surgery:
- Conventional (on-pump, arrested heart) CABG
- “Beating heart” (off-pump) OPCAB
- Minimally invasive direct coronary artery bypass (MIDCAB)
- Robotic or totally endoscopic coronary artery bypass (TECAB)
(Ghandakly et al., 2024)
Conventional Coronary Artery Bypass Graft
Conventional (or “on-pump”) CABG is performed on an arrested (stopped) heart through a midsternal, longitudinal incision down the patient’s chest. The patient’s heart is stopped with medications, and blood is routed to a heart-lung bypass machine, which removes CO2 and supplies oxygen, thus bypassing the processes carried out by the heart and lungs. The reoxygenated blood is returned to the body.
The procedure involves the revascularization of ischemic myocardial tissue by implanting one or both saphenous veins, a radial artery, or a mammary artery on both sides of a seriously blocked coronary artery, bypassing the blockage to provide a new source of blood flow to the previously deprived cardiac tissue. Use of the internal mammary artery has a better history of long-term patency than saphenous veins and is usually used for left anterior descending artery bypass. The radial artery is more patent than the saphenous veins but is prone to spasms unless calcium channel blockers or long-acting nitrates are given (Harding et al., 2022).
When saphenous veins are used, it is necessary to reverse them before implantation so that the valves do not impede blood flow.
After the bypass is performed, the patient is gradually taken off the bypass pump. Pacemaker wires and chest and mediastinal tubes are inserted. The patient’s incision is then closed with a spiral suturing technique.
A conventional CABG is done for patients who have the following conditions:
- Left main coronary occlusion >50%
- At least three-vessel CAD
- Advanced CAD
- Diabetes mellitus
- Refractory angina pectoris
- Heart failure associated with ischemic coronary disease
- Lesions not amenable to PCI
- Failed PCI
The patient may need blood transfusions (donor blood, blood harvested during the procedure and returned to the patient, or self-donations made in advance of surgery) to replenish blood volume, red blood cells, or platelets. Blood drained from the chest tubes postoperatively may be harvested, filtered, and transfused back into the patient. To reduce oxygen demand, the patient is placed in therapeutic hypothermia.
“Beating Heart” / Off-Pump Coronary Artery Bypass
In “beating heart” or off-pump coronary artery bypass (OPCAB) surgery, the heart is not stopped, the heart-lung bypass machine is not used, and the patient remains at normal or only slightly lowered temperature. The surgery is performed while the heart is still beating by placing mechanical stabilizers on the heart. The surgical procedure of using an internal mammary artery or saphenous veins to divert blood flow to the myocardium is identical to the traditional CABG with a bypass pump. The operating time is shorter, and the procedure is associated with fewer adverse postoperative outcomes, such as:
- Less blood loss
- Less renal dysfunction
- Less postoperative atrial fibrillation
- Fewer neurological complications
- Less incidence of stroke
- Less evidence of infection
Indications for this type of surgery include patients who have diabetes, lung disease, kidney disease, a previous history of stroke, or any other comorbidities that would put the cardiac patient at higher risk for surgery on a heart-lung or cardiopulmonary bypass pump. Fewer than 20% of patients undergoing CABG receive OPCAB surgery. Early studies showed somewhat higher mortality and the need for the proposed patients to have coronary artery lesions with no greater than 50% obstruction.
Beating heart surgery often allows patients to be discharged from the hospital more quickly than with conventional CABG, and the avoidance of the heart-lung machine has been shown to reduce the need for transfusions (Ghandakly et al., 2024; Harding et al., 2022).
Minimally Invasive Direct Coronary Artery Bypass
A MIDCAB procedure is used to bypass either the left anterior descending artery or the right coronary artery. Unlike traditional CABG, MIDCAB does not require a sternotomy or the use of a CPB pump. Instead of a long, midsternal incision, a MIDCAB includes several small incisions between the ribs or a mini thoracotomy. The surgeon then inserts a small camera via a thoracoscope or small robotic arms through the incisions. During the procedure, the surgeon sits at a console and controls the robotic instruments to perform the CABG.
The robotic assistance is used to dissect the left internal mammary artery and separate it from the chest. Using a mechanical stabilizer at the operative site, the left internal mammary artery is sutured to the blocked artery and bypasses the blockage, providing improved blood flow to the myocardium.
Robotic arms have been in use for this type of surgery for approximately 18 years. Da Vinci was the first company that built robotic arms and made them available for surgery; they remain the brand most commonly used for robotic surgery.
The mini-thoracotomy and totally endoscopic port-only approaches to perform this type of CABG reduce surgical trauma while providing cosmetic benefits and preserving chest wall, muscle, and function. The robotic approach has been shown to improve postoperative outcomes for patients, including less pain, a positive reduction of the recovery period, and a more rapid return to full activity for the patient (Ghandakly et al., 2024; Harding et al., 2022).
This procedure is not indicated for everyone and requires specialized training for the surgeon.
Totally Endoscopic Coronary Artery Bypass
A TECAB is another type of CABG surgery done without the use of a CPB pump or with the pump using a femoral vein approach. It is used for limited bypass grafting, usually only one coronary artery. With no incision or sternotomy, the TECAB has a much lower incidence of adverse postoperative outcomes such as infection, blood loss, pain, and recovery time. The patient typically requires a much shorter hospital stay (Ghandakly et al., 2024).
Transmyocardial Laser Revascularization
A transmyocardial laser revascularization is an indirect method of revascularization. It is used for patients with advanced CAD who are not good candidates for a more traditional CABG surgery and who have persistent, significant angina despite all other medical therapy having been attempted. The procedure uses a high-energy laser to create channels in the heart muscle to allow blood flow to ischemic areas. The procedure is generally done using left thoracotomy surgery. It can be used as an adjunctive therapy when bypass grafts cannot be placed to help reduce angina symptoms (Boston University, 2024; Harding et al., 2022).
Postoperative Care and Management
Care of the patient in the postoperative setting includes collaboration and a team approach. Team members may include respiratory therapists, nurses, a cardiologist, a cardiothoracic surgeon, an anesthesiologist, and rehabilitation specialists. Postoperative care after a CABG is as follows:
- Transfer patient immediately to the ICU; the nurse-patient ratio is 1:1 in at least the first 24–36 hours post op.
- Attach the patient to a cardiac monitor, mechanical ventilator connected to an endotracheal tube, temporary pacemaker with epicardial wires, pulmonary artery or Swan-Ganz catheter, arterial line in the radial artery, mediastinal and pleural chest tubes connected to drainage containers connected to wall suction (one container for each chest tube), indwelling (Foley) catheter, nasogastric tube, and one or two intravenous (IV) lines all connected to automatic IV pumps.
- Monitor vital signs, watching for signs of hemodynamic changes such as severe hypotension, decreased cardiac output, and shock.
- Administer IV fluids rapidly and vasopressive agents intravenously in the case of severe hypotension.
- Initiate warming procedures according to hospital protocol.
- Assess and record vital signs every five minutes (if unstable) to 15 minutes until the patient’s condition is completely stable.
- Administer medications as ordered and titrate according to patient response.
- Monitor ECG for any heart rate changes or dysrhythmias.
- Evaluate and assess the patient’s peripheral pulses, capillary refill time, and skin temperature.
- Auscultate heart sounds, noting and reporting any changes.
- Monitor chest tube drainage including color, odor, consistency, and negative pressure. Observe chest tubes and drainage systems for patency, change collection chambers when appropriate, and assist surgeon with chest tube removal, two to three days post op after drainage has sufficiently diminished.
- Assess breathing and breath sounds, monitor ventilator settings, and check arterial blood gas (ABG) results every two hours. Suction the endotracheal tube for secretions when necessary. Assist the anesthesiologist or respiratory therapist with extubation when the patient is ready.
- Monitor the mean arterial pressure (MAP), pulmonary artery pressure (PAP), central venous pressure (CVP), continuous arterial pressure, and cardiac output as ordered. Once hemodynamically stable, remove PAP, CVP, and arterial line and transfer the patient to a step-down or telemetry unit.
- Measure strict intake and output (I & O) and assess for any electrolyte imbalances.
- Assess the patient’s pain and provide pain medications as needed.
- Monitor the patient for signs and symptoms of stroke, pulmonary embolism, pneumonia, and impaired renal function.
- Encourage incentive spirometry, coughing, and deep breathing (while splinting the incision) after the patient is weaned from the ventilator. The patient is usually extubated six hours post op and then transferred to a step-down unit if hemodynamically stable.
- Reinforce or change surgical dressing when indicated, noting color, odor, consistency, and amount of drainage.
- Assist with range-of-motion exercises to enhance peripheral circulation and prevent formation of thrombus.
- Assist with ambulation per postoperative protocol. This will be initiated after the patient is extubated but before the chest tubes or any other tubes are removed.
- Provide information and emotional support to the patient and family or friends.
(Harding et al., 2022)
COMPLICATIONS IN THE ACUTE POSTOPERATIVE PERIOD
Adverse events can occur in the postoperative period. It is important for nurses to assess the patient for postoperative complications, which may include hypothermia, atrial fibrillation or other dysrhythmias, stroke, cognitive decline (including delirium), surgical site infections, depression, and acute renal failure that may persist long after the patient has been stabilized after surgery. Traditional CABG is associated with the most complications largely due to the prolonged time spent on the cardiopulmonary bypass pump and the cooling of the blood while on the pump.
Surgical Site Infection
Surgical site infections are the most common postoperative complication in CABG patients. The risk of deep sternal wound infections is increased if a patient has a history of diabetes, smoking, obesity, and COPD. Infection rates and a risk for sepsis also increase with the use of blood transfusions, prolonged intubation, and surgical re-exploration. Careful nursing assessment for any signs or symptoms of infection includes monitoring patient temperature, pain, swelling, and incision site redness/discharge (Harding et al., 2022).
Atrial Fibrillation
Postoperative atrial fibrillation (AF) is the most common post-CABG dysrhythmia, occurring in 20%–50% of all CABG patients. Factors that may increase a patient’s risk include peripheral artery disease, COPD, valvular heart disease, previous cardiac surgery, male gender, and advanced age. First-line treatment includes beta blockers and amiodarone. It is highly recommended that the beta blockers be started or restarted as soon as possible after surgery to prevent atrial fibrillation. A prolonged episode of atrial fibrillation will lengthen the hospital stay, especially if the patient is on warfarin for atrial fibrillation. If the atrial fibrillation is determined to be chronic, it will be necessary to start the patient on a carefully monitored regimen of anticoagulation possibly for the rest of their life (Harding et el., 2022).
Stroke
Postoperative stroke usually occurs in elderly patients. Risk factors include age, previous stroke, diabetes, hypertension, and female gender. Along with vital signs, nursing assessment includes postoperative neuro status checks in addition to any functional or cognitive changes that may be due to sudden stroke (Harding et al., 2022).
Preventative measures include early and progressive ambulation, sequential compression devices applied to the lower legs or feet, and scrupulous care of needle or IV or arterial line insertion sites in order to reduce the risk of a deep vein thrombosis that could cause a stroke if it became dislodged and circulated.
Cognitive Decline
Postoperative delirium and cognitive decline may occur in a small number of patients who have undergone a CABG. The patient may experience memory impairment, difficulty concentrating, poor language comprehension, and decreased social integration. Nursing assessment includes monitoring for any cognitive changes, especially in patients at high risk. Risk factors for cognitive decline include preexisting cerebral vascular disease, central nervous system disorders, and cognitive impairment (Harding et al., 2022).
Depression
Symptoms of anxiety and depression peak before any heart surgery and again two weeks after and may persist up to four months after discharge. Pain, fatigue, and sleep disorders are common after a CABG and may be partly due to the lack of postoperative physical activity. Cardiac rehabilitation is highly recommended.
Preoperative depression may increase the occurrence of rehospitalization, heart failure, stroke, myocardial infarction, and death. Postoperative depression following CABG affects 30%–40% of all patients, is a risk factor for increased morbidity and mortality, and can occur weeks after discharge. Postoperative depression also increases rehospitalizations for up to six months and cardiac events for eight years after surgery. In a study of over 1 million patients, 5.6% were readmitted within 14–90 days after a discharge after a CABG (NIMH, 2024; Stenman et al., 2022).
Depression is strongly linked to patients with low physical activity and limited mobility. Thus, it is important to initiate physical activity and rehabilitation as soon as possible following a cardiac event. Nurses should place an emphasis on educating patients and their families about the development of depressive symptoms and provide resources and strategies to address depression.
SYMPTOMS OF DEPRESSION
- Persistent sad, anxious, or “empty” mood
- Feelings of hopelessness or pessimism
- Irritability
- Feelings of guilt, worthlessness, or helplessness
- Loss of interest or pleasure in hobbies and activities
- Decreased energy or fatigue
- Moving or talking more slowly
- Feeling restless or having trouble sitting still
- Difficulty concentrating, remembering, or making decisions
- Difficulty sleeping, early-morning awakening, or oversleeping
- Appetite or weight changes
- Thoughts of death or suicide, or suicide attempts
- Aches or pains, headaches, cramps, or digestive problems without a clear physical cause or that do not ease with treatment
(NIMH, 2024)
CASE
Mrs. Crawford is a patient in the cardiac unit who is recovering from coronary artery bypass surgery. She is 72 years old, with a daughter and son who live in the local area. Her family has been visiting her throughout her hospitalization. Mrs. Crawford’s husband died two years ago from lung cancer.
Fatima is her nurse today and is preparing Mrs. Crawford for discharge in one to two days. Fatima is reviewing the discharge plans and all of Mrs. Crawford’s instructions and medications. Fatima also reviews Mrs. Crawford’s exercise program and verifies her appointments to see a physical therapist to continue her cardiac rehabilitation program.
As Fatima discusses the transition to the home environment, Mrs. Crawford states, “It’s pretty lonesome around the house with my husband gone. My daughter stops by when she can, but she is busy with her work, so she does not have much time.” Mrs. Crawford has not progressed as well as expected with her physical activity level in the hospital. Fatima is concerned that Mrs. Crawford may not do well with recovery if she does not progress with her exercise program. She is also concerned that Mrs. Crawford may be depressed.
In order to understand more about how Mrs. Crawford is coping, Fatima asks a couple of questions to assess depressive symptoms. Fatima asks Mrs. Crawford about her sleep habits, social contacts, and hobbies. When asked directly, “Do you feel like you are ‘blue’ or depressed after this heart surgery?” Mrs. Crawford starts to cry. She says, “I just am not sure if I want to go home alone. I am afraid, and I have no one to be with me. I miss my husband.”
With this feedback, Fatima discusses her assessment with the cardiologist in charge of Mrs. Crawford’s care in the hospital. Mrs. Crawford is started on Zoloft therapy (an SSRI, which is safe to take with her cardiac meds). Fatima reviews the plan with Mrs. Crawford and her family members and what depressive symptoms they should be aware of. Fatima also reiterates how important it will be to continue her rehabilitation program because exercise and physical activity may potentially lift her spirits. Fatima also encourages Mrs. Crawford to be in close contact with friends, which will give her the opportunity to get out of the house and be with others.
Mrs. Crawford seems to understand the importance of following her rehabilitation plan. Fatima confirms her follow-up appointment in one week with the team. Mrs. Crawford and her family verbalize their understanding of the discharge plans.
Acute Renal Failure
Incidence of acute renal failure after CABG occurs in a significant number of patients. A small percentage of patients will go on to need dialysis, possibly temporarily.
Some risk factors for renal failure post-CABG are not modifiable, such as advanced age, hypertension, hyperlipidemia, and peripheral vascular disease. Other factors are specific to anesthetic, high-volume blood transfusion, aortic cross-clamping, and ICU management.
Careful nursing monitoring includes kidney function (urinary output, creatinine clearance, and other kidney function tests), especially for those patients at high risk, including those with preexisting renal dysfunction, decreased cardiac output, insulin-dependent diabetes, peripheral artery disease, advanced age, African race, and female gender.
Complications in Older Adults
Older adults, including those over age 80, tolerate elective CABG fairly well. The incidence of postoperative complications is higher than in younger patients, possibly because of a larger number of comorbidities. These complications may include dysrhythmias, stroke, postoperative cognitive dysfunction, and infection. Despite the increased incidence of complications, the benefits of the surgery typically outweigh the risks (Harding et al., 2022).
POSTOPERATIVE PHYSICAL AND OCCUPATIONAL THERAPY
Frequently, a patient with physical limitations following a cardiac event will be referred to a rehabilitation specialist. Based on the assessment and evaluation, the physical or occupational therapist will create an individualized treatment plan that includes the patient’s goals for treatment and addresses their specific physical limitations. Research studies suggest that nonpharmacologic interventions such as exercise training and psychoeducation have a positive physiologic and psychological effect in early outpatient rehabilitation.
Physical Therapy
The primary outcome goal in post-CABG cardiac rehabilitation is physical function. This can be measured by the 6-Minute Walk Test, which assesses functional, aerobic capacity as indicated by heart rate, blood pressure, and a self-evaluated Borg rate of perceived exertion (RPE). The secondary outcomes are mental health and increased physical activity that will be encouraged and continued when the patient is discharged from the hospital.
Early and frequent physical therapy, starting as soon as one day after surgery, can help restore a normal pattern of daily functioning in a patient post CABG. A customized physical therapy program may include exercises for range of motion, muscle strengthening, and coordination. Exercises will vary depending on the patient’s baseline condition (Sears, 2022).
INITIAL PHYSICAL THERAPY EVALUATION
Patients who are stable after a cardiac event will have an initial evaluation while recovering in the hospital. Elements of a physical therapy evaluation include assessments of:
- Medical history
- Heart rate
- Blood pressure
- Oxygen saturation
- Upper body strength and range of motion
- Lower body strength and range of motion
- Level of functional mobility and ability to perform self-care
- ECG measurements at rest and during activity
- Other measurements of baseline functional status (e.g., Timed Up-and-Go [TUG] Test or 6-Minute Walk Test)
Exercise is a key component of physical rehabilitation and focuses on maintaining and improving strength, endurance, balance, and overall functional mobility. When the postoperative patient can tolerate it and has had most surgery-related tubes removed, exercise may include but is not limited to walking with the help of parallel bars, using a treadmill that can be adjusted to include an inclined surface, or riding a stationary bicycle. When patients tolerate these exercises well, they may progress to stair-climbing on a wooden platform containing two or three steps.
These exercises will be done while the patient is connected to a cardiac monitor to observe stress-induced dysrhythmias. Should they occur, the exercises will immediately be stopped and the patient returned to their room. Additionally, if dyspnea, dizziness, or chest pain occurs during exercise, the exercise is stopped immediately and the patient’s cardiac status reassessed. Before hospital discharge, patients are reassessed so that an individual home exercise program can be taught to the patient and any caregivers (Isaac, 2023a).
(See also “Cardiac Rehabilitation Phases” below).
Occupational Therapy
Occupational therapists (OTs) have specialized knowledge and skills that address the limitations that patients may experience with the performance of basic activities of daily living (ADLs) and instrumental activities of daily living (IADLs). Driving, for example, is particularly complex, requiring an integration of visual, physical, and cognitive tasks (Isaac, 2023b). After a CABG, the physician usually informs the patient when it is safe to begin driving again. This period may extend six weeks or more postoperatively depending on the patient’s surgical wound healing and pain level.
COMMON ACTIVITIES EVALUATED DURING POSTOPERATIVE RECOVERY
ADLs:
- Eating
- Dressing
- Bathing
- Grooming
- Toileting
- Transfers (such as moving between the bed, chair, and bathtub or shower)
IADLs (require more complex cognitive functioning):
- Preparing meals
- Telephone calling
- Writing
- Working on a computer
- Managing finances
- Accurately following a daily drug regimen
- Cleaning, doing laundry
- Grocery shopping and other errands
- Traveling as a pedestrian or by public transportation
- Driving
The occupational therapy process begins with a thorough evaluation that identifies the baseline status of the patient and any secondary disabilities, the need for any assistive devices, home safety, and any areas of the patient’s occupation or skills that may be difficult for the patient to perform. Patients are evaluated for any limitations that require intervention and for any strengths that can be used to compensate for weaknesses. Limitations may involve motor function, sensation, cognition, or psychosocial function.
Evaluators determine the activities (e.g., ADLs, work responsibilities, leisure activities, social integration, or learning and comprehension) for which patients want or need help. The OT may use an evaluation instrument such as the Barthel Index, Katz Index, Functional Independence Measure (FIM), or Patient Reported Outcomes Measurement Information System (PROMIS) to measure a client’s ability before establishing a plan of care.
Before developing a cardiac rehabilitation program, an occupational therapist observes patients doing each activity of the daily routine to learn what is needed to ensure safe, successful completion of the activities, with methodologies that are intended to become progressively more complex and challenging. Therapists can then recommend ways to eliminate or reduce maladaptive patterns and to establish routines that promote function and health. Specific performance-oriented exercises are also recommended. Therapists emphasize that exercises must be practiced and motivate patients to do so by focusing on exercise as a means of becoming more active at home and in the community.
Patients who are planning to return to a work environment may need to adjust their work schedules and place limits on physical activities (such as lifting) depending on the type of work in which they are engaged. Patients are taught creative ways to facilitate social integration activities (e.g., how to get to a museum, movie theater, or church without driving). They are also given instructions about how to use hearing aids or other assistive communication devices in different settings. Important strategies may include how to travel safely with or without a cane or walker. Therapists may suggest new activities such as volunteering in foster grandparent or mentoring programs, in schools, in libraries, or at hospitals. Patients are taught strategies to compensate for their limitations, such as to sit when gardening or showering. The therapist may identify various assistive devices that can help patients do many activities of daily living (Isaac, 2023b).
STERNAL PRECAUTIONS
Sternal precautions may help the patient recover from a sternal incision and prevent separation of the breastbone as it heals. The patient should follow these precautions for four to six weeks until the sternal incision is well healed.
A newer form of sternal precautions is referred to as “Keep the Move in the Tube (KMIT).” This terminology helps the patient to visualize a tube encircling the body around the shoulders and torso. Any load-bearing movements require that the arms must be kept within the imagined tube. Nonload-bearing movements (such as bathing, dressing, and toileting) can be made freely. While in the “tube,” both arms should be used if movement causes any pain.
Physical and occupational therapy strategies to assist the patient in protecting the sternum include instructions on the following:
- Scooting to the edge of the sitting surface prior to rising from a chair
- Actively engaging the leg muscles instead of relying upon momentum when moving from sitting to a standing position
- Avoiding pulling on the handrail for support when navigating stairs
- Rolling over in bed and gradually sitting up without using the arms for assistance
- Using assistive devices if recommended (e.g., walker or quad cane)
- Holding a heart pillow during transfers to avoid putting pressure on the arms
- Holding the heart pillow tightly to the chest during laughing or sneezing to “splint” the incision like a broken bone
- Utilizing strategies to assist with performing ADLs such as bathing, dressing, and brushing hair
- Performing arm exercises with weights: one arm at a time to prevent pressure on the sternum, no more than 1- to 2-pound weights, no higher than shoulder level
(Cardiac Sciences, 2023; Sears, 2023)
POSTOPERATIVE CARDIAC REHABILITATION GOALS
Studies show it is in the best interests of CABG patients to undergo active exercises and strengthening in order to help prevent postoperative complications and enable the patient to return home more quickly. Cardiac rehabilitation (CR) is universally recognized as a means to promote decreased mortality, morbidity, and disability, and increased quality of life in all cardiac patients, including after surgery. Despite the proven beneficial effects of cardiac rehabilitation, participation is often less than optimal due to cost, location, and difficulties with traveling to reach therapy services.
There are four phases of CR. In the immediate postoperative period, phase I of cardiac rehabilitation is initiated. The interventions in phase I rehabilitation after CABG are the results of investigation in the physical or the psychological perspective. Cardiopulmonary bypass has a temporarily negative effect on the physical level of function. The effect of respiratory exercise preoperatively and in the early postoperative period after CABG surgery has been examined using different techniques that are found to be very effective. CR programs must be supervised and monitored by trained rehabilitation professionals. The goals of cardiac rehabilitation are to maximize strength, prevent regression of CAD, and reduce the likelihood of future cardiac problems.
Comprehensive rehabilitation programs that include exercise, education, counseling, and help with lifestyle changes can:
- Increase exercise tolerance
- Decrease cardiac-related symptoms (such as angina and shortness of breath)
- Improve blood lipid levels
- Reduce stress
- Make it easier to stop smoking
- Improve mood
Many patients with CAD who are over age 60 and have had an MI or heart surgery may be fearful of exercise. They are likely to have deficits in muscle strength and balance.
The first step in reassuring patients is to educate them about the disease in general and their current condition. General advice should include a review of the symptoms of ischemia, rules on managing an episode of angina or dyspnea, and an explanation of which specific symptoms require a quick trip to an ED. When possible, the patient’s family should be included when educating the patient.
Patients with signs and symptoms of depression are statistically less likely to complete their cardiac rehabilitation programs, and it is important to identify these patients and to get the appropriate help for them early in the program.
Rehabilitation specialists advise patients on how to safely resume their normal activities after discharge from the hospital. For example:
- Daily walking can be encouraged immediately.
- Sternal precautions may be recommended (see above).
- Patients can often resume their previous level of sexual activity in two weeks, depending on their individual tolerance for exercise. (If patients have no symptoms of angina, dyspnea, or palpitations with moderate-exertion physical activity, this is a good indication that they will not have symptoms during sexual intercourse.)
- Routine driving can usually be resumed in approximately six weeks in those states that allow it. Some states consider a postoperative CABG to be a disqualifying condition and require that a cardiologist approve fitness for driving.
- Patients can return to work with recommended modifications to their schedule or duties as needed.
(AHA, 2023c; Harding et al., 2022; Isaac, 2023b)
(See also “Cardiac Rehabilitation” below.)
CASE
Mr. Townsend, age 65, is recovering in the hospital from CABG surgery to reopen a blocked coronary artery. The day after his surgery he is visited by a nurse and a physical therapist, each of whom brief him on the cardiac rehabilitation regimen he is about to undergo. The patient expresses anxiety about having to undergo cardiac rehabilitation so soon, but the nurse and physical therapist reassure him that the regimen will be helpful in several different ways, such as maintaining muscle mass, promotion of respiratory hygiene and promotion of bowel motility. They explain that it will be manageable and that it will start slowly.
Mr. Townsend starts his early-stage (phase I) cardiac rehabilitation the following day. Upon performing an initial evaluation of Mr. Townsend’s functional mobility status and activity tolerance, the physical therapist teaches him how to transfer from the bed to the chair without causing physical stress to his incision or using his arms. Later, the physical therapist helps Mr. Townsend to get out of bed and walk a short distance to the door of his room and back to his bed. This exercise is repeated twice more throughout the day. The therapist talks to Mr. Townsend about his plan for home-based mobility following discharge and offers individualized home safety recommendations.
The nurse introduces Mr. Townsend to the occupational therapist, who helps him begin taking care of his ADLs, such as personal care, bathing, eating, and understanding the timing and administration of new medications. The occupational therapist addresses planning for his transition to the home environment by asking Mr. Townsend about safety issues, anticipated barriers in the home, and assistive devices needed or used in the past. Together, they plan strategies to address showering at home.
On the following day, after his physical therapist has determined the level of assistance needed for Mr. Townsend to ambulate safely, his nurse helps him venture out to the corridor, and he is able to walk slowly to the nurses’ station, which is 60 feet from his room. Mr. Townsend takes two more corridor walks that day and four similar walks on each of the following two days.
By the time he is discharged on the fifth post-surgical day, Mr. Townsend is able to walk in the hallway for 10 minutes at a time. The nurse reinforces the safety recommendation instructions from the physical therapist and reviews the detailed instructions with Mr. Townsend with regard to continuing with his exercise plan while at home. The nurse has Mr. Townsend repeat back to him how he will adapt his ADLs to accommodate his temporary postoperative mobility restrictions and his plans to work toward regaining his preoperative level of function.
Mr. Townsend has consistent support at home from his wife, and the nurse schedules a home health visit twice weekly for one month in order to monitor his progress. He is also scheduled to see his doctor and his physical therapist for an outpatient follow-up visit in one week to initiate his long-term outpatient cardiac rehabilitation program.
DISCHARGE PLANNING AND EDUCATION
Discharge planning following a cardiac event or procedure may include the following patient education and instructions:
- Monitor for signs of infection (redness, swelling, discharge, drainage, fever, or sore throat).
- Recognize the symptoms to be reported.
- Know how to take care of any dressings or incisions, including what to do to protect the operative site during bathing/showering.
- Understand the warning signs for arterial reocclusion (angina, dizziness, dyspnea, rapid or irregular pulse, and shortness of breath).
- Monitor body weight and notify the primary care provider of any weight gain of more than 3 lbs (1.4 kg) in one week.
- Follow any special dietary instructions (especially any sodium and cholesterol restrictions).
- Restrict any lifting to <10 lbs for four to six weeks.
- Maintain a good sleep routine, with at least eight hours of sleep each night and short rest periods throughout the day.
- Participate in an exercise program and cardiac rehabilitation recommendations, including specific restrictions and when activities can be resumed.
- Follow any lifestyle modifications recommended (smoking cessation, nutrition, and exercise programs).
- Understand the dose, indication, frequency, and side effects of all prescribed medications.
- Understand the follow-up plan of care, including visits with cardiology, the surgeon, and the primary care provider.
(Harding et al., 2022)
COMPREHENSIVE MANAGEMENT OF CHRONIC CAD
A patient who has chronic CAD should be enrolled in a long-term treatment plan. These patients include people with chronic stable angina and people with stable coronary artery disease after having been treated for acute coronary syndromes.
Outpatient Monitoring and Guidance
Each patient is an individual and will need an individualized treatment program. Such programs include educating the patient and family on medications, therapeutic lifestyle changes, possible revascularization (reperfusion) surgery, and treatment of associated disorders.
The primary goals of care for patients with CAD include strategies that focus on stabilizing any progression of disease and improving symptoms while improving physical function, quality of life, and psychosocial well-being.
LONG-TERM GOALS FOR TREATMENT
- Support the patient in living a comfortable life without pain and with the fewest possible restrictions.
- Prevent the development of an acute coronary syndrome.
- Slow or reverse the degree of atherosclerosis.
- Reduce the cardiovascular risk factors in the patient’s life, where possible.
(Harding et al., 2022)
Medications
Drug therapy is a key part of the treatment of coronary artery disease. To reduce the likelihood of developing obstructive clots, patients who have CAD or are at high risk of developing CAD should take antiplatelet drugs daily. To lessen the work of the heart, most patients with CAD also take beta blockers. For relief of angina, nitrates are prescribed.
The standard medication therapies for CAD include:
- Antiplatelet therapy
- Beta-adrenergic blocking agents (beta blockers)
- Angiotensin-converting enzyme (ACE) inhibitors / angiotensin II receptor blockers (ARBs)
- Calcium channel blocking agent
- LDL-lowering drug, when needed
- Nitrates (short- and long-acting)
Medications are essential to the care of cardiac patients. Older adult patients with CAD who do not take their prescribed medications regularly are twice as likely to develop acute coronary syndromes. By asking patients at each visit whether they are taking their medicines all the time and having them describe their dosing regime, it is possible to intervene and to lower the risk of serious complications.
ASPIRIN AND OTHER ANTIPLATELETS
Long-term antiplatelet therapy makes acute ischemic episodes less likely in all forms of coronary artery disease. Aspirin is the first-line antiplatelet drug unless the patient has an aspirin allergy or a history of or risk for gastrointestinal or other forms of bleeding. It inhibits cyclooxygenase, which produces thromboxane A2, a potent platelet activator.
The initial dose is typically between 65 mg and 325 mg, and then 81 mg to 325 mg per day. It should be continued indefinitely unless contraindicated. Clopidogrel (Plavix), plasugrel (Effient), or cangrelor (Kengreal) can be added for up to 12 months to increase the inhibition of clot formation, and these drugs can also be given to patients when aspirin is contraindicated. Any patient taking anticoagulants should avoid foods high in vitamin K, as these may interfere with the therapeutic effect of the medications (Harding et al., 2022).
Newer research, however, supports that aspirin may be efficacious only in preventing secondary atherosclerotic cardiovascular disease (when there has already been an episode of a myocardial infarction or a stroke), in which case the benefits outweigh the risks. It is believed that aspirin is less likely to prevent primary atherosclerotic cardiovascular disease (ASCVD). Therefore, the use of aspirin prophylactically for primary ASCVD is becoming controversial because the benefits in preventing an MI or stroke may not outweigh the risks unless the patient’s risk score is very high (ACC, 2022).
Ticagelor (Brilinta) is a newer antiplatelet medication that has proved to be effective in preventing blood clots in patients with CAD and, therefore, the occurrence of first MIs and CVAs. Ticagelor has shown better efficacy than clopidogrel (Plavix) but less than plasugrel (Effient), which is almost as new. In some studies, clopidogrel has fewer side effects of bleeding and dyspnea (Drugs.com, 2024).
Patients may need to discontinue antiplatelet or anticoagulant therapy for up to 10 days before undergoing elective surgery, as not doing so can lead to cancellation or postponement of the operation or, worse, cardiac events or other potentially catastrophic developments, such as bleeding during or following surgery. Patients considering elective surgery should therefore coordinate their antiplatelet/anticoagulant regimens with their primary care provider, cardiologist, and surgeon (Harding et al., 2022).
NITROGLYCERIN
Nitrates, such as nitroglycerin, dilate blood vessels throughout the body. By lowering the arterial resistance to blood flow, nitrates ease the work of the heart by lowering the blood pressure, and by dilating the coronary arteries, they increase the blood flow to the myocardium. Nitrates may also prevent or control vasospasm.
Nitroglycerin relieves the pain of angina, and if taken approximately five minutes before exercise or stress, it can prevent angina. The nitroglycerin in sublingual tablets is absorbed quickly and completely, and it generally works within two to three minutes and lasts for half an hour. All patients with angina should be given sublingual nitroglycerin with specific instructions about its use, including the potentially fatal risk of taking it with certain drugs (e.g., Cialis, Viagra, Levitra, and other nitrates). Nitroglycerin is also available as an oral spray and as long-lasting tablets, ointment, and patches.
The long-lasting forms of the drug are used to prevent angina and will also help to control hypertension as a therapeutic side effect. The alternate forms of the drug and their dosages are as follows:
- Tablets: 0.3, 0.4, 0.6 mg
- Capsules: 2.5, 6.5, 9 mg
- Spray: 0.4 mg/spray
- Transdermal patch: 0.1, 0.2, 0.3, 0.4, 0.6, 0.8 mg/hour
- Ointment: 2%
- Infusion solution: 25, 50, 100 mg/250 mL
- Injectable solution: 5 mg/mL
(Harding et al., 2022)
| (Adapted from Ogbru, 2023.) | |
| Purpose |
|
| When to use |
|
| How to use |
|
| What to expect if it works | Chest discomfort should decrease in 1–5 minutes. |
| What to do if it does not work | If discomfort does not decrease after taking one tablet, call 911 immediately and report chest pain. Alternately, for those used to taking nitro, take up to three tablets before calling 911. |
| Typical side effects |
|
| Side effects to report immediately to primary care provider |
|
| Drugs that can be taken before or after nitroglycerin (to prevent or treat headache) |
|
| Drugs not to be taken with nitroglycerin | Erectile dysfunction medicines (Viagra, Cialis, Levitra) |
| Storage |
|
BETA BLOCKERS
Beta-adrenergic blocking agents (beta blockers) are antihypertensive drugs that also reduce heart rate contractility and reduce afterload. This takes effect by inhibiting sympathetic nervous stimulation of the heart. By this action, beta blockers reduce the heart’s demand for oxygen. Beta blockers lower the incidence of episodes of angina and also reduce the likelihood of myocardial infarctions and death in CAD patients.
Special care must be taken when prescribing beta blockers to patients with asthma, other obstructive airway conditions (e.g., COPD), intermittent claudication, insulin-requiring diabetes, certain heart conduction problems, and clinical depression. When the side effects of beta blockers become a problem, calcium channel blockers, such as diltiazem or verapamil, or Ranolazine can be substituted, with similar effects.
Until recently, beta blockers were not given to patients with reactive airway disease such as COPD. Since 10% of the beta cells in the body reside in the lungs, adrenergic beta blocking agents can cause difficulty breathing. Several beta blockers are referred to as cardioselective in that they only work to block the beta 1 cells in the heart and spare the lungs. These cardioselective beta blockers include:
- Atenolol
- Esmolol
- Metoprolol
- Bisoprolol
ACE INHIBITORS
Angiotensin-converting enzyme (ACE) inhibitors, such as ramipril (Altace), are antihypertensive drugs that can reduce the likelihood of acute ischemic episodes, strokes, and death in patients with CAD. These drugs prevent angiotensin II from converting to angiotensin I, a powerful vasoconstrictor. The resulting vasodilation causes lowering of the blood pressure. They also cause endothelial dysfunction, reducing atherosclerosis formation.
STATINS
Lipid-lowering drugs are frequently prescribed for people with CAD. The statins atorvastatin (Lipitor) and simvastatin (Zocor) are the preferred lipid-lowering drugs for coronary artery disease, but some lipid abnormalities are treated with nicotinic acid or fibric acid. Patients with liver disease should not take statins.
High levels of LDL cholesterol initiate and worsen atherosclerosis. In patients with high blood levels of cholesterol, the first medical intervention is lifestyle changes, especially a low-fat diet and increased exercise (see below). When this does not lower a patient’s cholesterol to safe levels, lipid-lowering drugs are prescribed. These drugs reduce morbidity and mortality from CAD. Two infrequently occurring (<1%) side effects of statins are liver failure and rhabdomyolysis.
OTHER MEDICATIONS
Patients with moderate to severe depression may be prescribed antidepressant medications as part of their management program. Selective serotonin reuptake inhibitors (SSRIs), including sertraline and citalopram, are a form of antidepressant therapy safe to use with patients who have CAD (Harding et al., 2022).
ANSWERING PATIENT QUESTIONS
Q: I’m afraid of taking too many medicines. What natural remedies are safe to use for my coronary artery disease?
A: Your fears are understandable. All medicines have side effects, and all medicines can be dangerous in higher-than-recommended doses. If you are having side effects that make your life difficult or if you are worried about something, then talk directly to your primary care provider. Don’t be shy about telling them what is bothering you.
Natural remedies, such as herbs and plant or animal extracts, are chemicals just like the medicines that you are taking. “Natural” often means that the chemical is not as pure or as precisely measured as a prescription drug. When the natural remedy is not purified, you are taking all the impurities as well as the chemical—in fact, you don’t know exactly what you are taking, which can be dangerous. When the natural remedy is not as well measured as a prescription drug, you don’t know exactly how much you are taking, which can also be dangerous.
There are some herbs and other natural products that are especially dangerous for patients with coronary artery disease:
- Don’t take anything containing ephedra because it puts excessive strain on the heart. Ephedra is sometimes found in weight-loss products.
- Don’t take concentrated licorice or licorice root. Licorice is sometimes used as an herbal remedy for breathing or stomach problems, but it can cause high blood pressure and salt imbalances in your body.
- Don’t take danshen, evening primrose oil, garlic, ginkgo, ginseng, or St. John’s wort, as these may interfere with medications that you are taking for your CAD.
(Mayo Clinic, 2022)
Often, there are safe alternatives to the standard therapy for a disease. Talk with your primary care provider and ask for an alternative that is safe.
Cardiac Rehabilitation
Cardiac rehabilitation (CR) is a proven method of reducing morbidity and mortality in patients with CAD, particularly in those who have undergone cardiac surgery. (See also “Postoperative Cardiac Rehabilitation Goals” earlier in this course.)
A broad CR program may include exercise training, dietary counseling, medication management, tobacco cessation counseling, or psychosocial assessment interventions. CR has proven highly effective in helping those with heart disease and recovering from heart surgery to increase physical strength, reduce their weight, reduce LDL cholesterol, manage stress, reduce blood pressure, resume activities of daily living, and reduce cardiac symptoms and additional cardiac tissue damage (Kolominsky et al., 2021; Sears, 2022).
Hospitalization for a cardiac event or surgery is often the time when phase I of cardiac rehabilitation begins. Once the patient is discharged, referral to an outpatient rehabilitation program is initiated. Patients may begin formal outpatient cardiac rehabilitation programs as early as 10 days postoperatively depending upon their condition. Cardiac rehabilitation may last up to three to six months or longer.
Cardiac rehabilitation may begin in an acute care hospital. Rehabilitation hospitals or units may provide the most extensive and comprehensive care and should be a consideration for patients who have good potential for recovery and can participate in and tolerate aggressive therapy.
Rehabilitation can also be offered in nursing homes or in the home environment with a less intensive approach that lasts longer and may be better suited to patients less able to tolerate therapy (e.g., frail or older adult patients). However, one of the disadvantages of home-based CR is the lack of access to specialized exercise equipment such as weights, treadmills, balance balls, and resistance machines. One approach that has proven highly effective is to use the patient’s own body weight in the form of push-ups, squats, sit-to-stands, and balancing/stretching exercises, often largely negating the need for more specialized exercise equipment (Kolominsky et al., 2021).
Ideally, the patient’s care is coordinated by a multidisciplinary team, all of whom see the patient regularly. For patients recovering from myocardial infarctions or surgical cardiac procedures, the team should include cardiac rehabilitation specialists. Cardiac rehabilitation specialists may include a cardiologist, nurse educator, nurse practitioner, dietitian, exercise physiologist, occupational therapist, physical therapist, psychologist, and psychiatrist who are all specifically trained in cardiac rehabilitation programs. Also, family members may need help learning how to adjust to the patient’s disability and how to safely and effectively help the patient (NHLBI, 2022).
Cardiac rehabilitation models are continuing to evolve to meet a variety of age groups and needs. Advanced age is associated with a higher prevalence of CAD as well as increased morbidity and mortality. Cardiac rehabilitation programs designed to meet the needs of older patients (>65 years of age) should include strength, balance, coordination, and flexibility. Evidence-based programs show that older patients can realize positive benefits from an exercise-based cardiac rehabilitation program to increase functional capacity, glucose control, quality of life, enhanced ability to perform ADLs, and reduced incidence of hospitalization.
CARDIAC REHAB DURING COVID-19
The global pandemic caused by the COVID-19 virus in 2020 necessitated widespread suspension of face-to-face contact for healthcare practices that were considered nonessential. Prior to this, home-based CR was proving to be very effective and less costly, as it provided instruction for those for whom transportation to a CR center proved prohibitive. CR patients in an exercise-based home program were more likely to start sooner after discharge and continue longer.
With lockdown initiated during the pandemic, CR patients were no longer able to consistently receive physical therapy personnel in their homes. Teleconferencing communication was put into use to connect patients and healthcare workers. Assessments were also conducted via telephones. Where there was a lack of access to computers or internet, the technology was made available by loaning computers to the patients who needed them. The structured exercises were considered low or moderate level to prevent unsupervised cardiac injury. Early exercises included walking with pedometers that measured progress. The practitioner’s inability to observe patients resulted in a more cautious approach for safety (Kolominsky et al., 2021).
In recent studies, exercise-based CR was found to be highly cost-effective, with physiology and symptomology results similar to traditional CR. The beneficial effects of exercise for cardiac patients are weight reduction and decreased cardiovascular mortality and recurrent cardiac events. CR/PR has also been found to increase longevity, reduce hospital admissions, and improve the quality of life of CAD patients who participate (Dibben et al., 2023).
Tai chi has been found to be an effective exercise for inclusion in CR programs. Although not widely accepted by the medical community as a successful adjunct to the rest of CR therapy, tai chi has been found to out-perform other exercises for stress management, lowering blood pressure, and improving balance (Corliss, 2023).
The 2018 Bipartisan Budget Act provided for physician assistants, nurse practitioners, and clinical nurse specialists to supervise patients in CR/PR beginning in 2022 and updated in 2024. This will make cardiac rehabilitation more accessible and less expensive, and the associated increase in the numbers of practitioners is hoped to greatly decrease wait times (MLN, 2024).
CARDIAC REHABILITATION PHASES
Cardiac rehabilitation may be divided into four phases:
Phase I: Inpatient
The first phase of cardiac rehab takes place before the patient is discharged from the hospital. This phase generally consists of evaluation and assessment of the patient’s condition, motivation, and risk factors, accompanied by education and discharge planning. Much of the evaluation is done by physical therapists and occupational therapists (discussed earlier in this course).
The patient is gradually introduced to exercise on day two of cardiac rehab, with an intensity of exercise up to four metabolic equivalents (METS) (i.e., four times the resting metabolic rate, or four times the amount of oxygen consumed at rest; 1 MET = 3.5 liters of oxygen). Ideally, by day four, the patient will be walking in the corridor for five to ten minutes, three to four times per day.
Phase II: After Discharge
Phase II is a supervised phase that occurs in an outpatient setting such as a physical therapy clinic or a physician’s office. The patient is given clear instructions on their individualized exercise plan. The rehabilitation team may include the following professionals who work closely with the patient: exercise physiologists, occupational therapists, and physical therapists.
If a patient is considered home bound, a home physical therapy evaluation is completed and a program of home exercises outlined for the patient. The initial mode of exercise is usually walking on level ground, with an intensity goal of 2–4 METS or a score of 11–12 on the RPE scale (i.e., moderate intensity). Patients are generally advised to stay indoors for the first day or two because they may expect to feel fatigued or anxious, though patients with uncomplicated coronary artery disease may be advised to increase their walking distance progressively to 3–5 kilometers per day after four to six weeks.
During phase II rehabilitation, exercises may include:
- Treadmill walking
- Stationary bike
- Using an upper body ergometer (UBE)
- Rowing
- Upper- and lower-body strengthening using free weights
- Stretching
As the patient gains strength, these same exercises may progress in intensity and duration as the patient transitions from phase II to phase III (Sears, 2022).
Phase III: Outpatient Exercise Program
The goal of this phase is to enable the patient to exercise safely in a structured environment and to understand the benefits of exercise. Before starting an exercise program, it is common for a patient to undergo an exercise stress test until symptoms become apparent. The exercise test can be used as either a diagnostic or prognostic tool or as a test of functional capacity.
Cardiac patients should exercise in the low to moderate range of exercise intensity, corresponding to 60%–75% of maximum heart rate or 60%–70% of maximum heart rate reserve, which is equivalent to a score of 12–14 on the RPE scale. (The maximum heart rate is usually calculated by subtracting the patient’s age from 220. The heart rate reserve is calculated by subtracting the resting heart rate from the maximum heart rate.) The outpatient exercise program may last from 8 to 12 weeks, and patients generally attend two to three times per week (Mayo Clinic, 2023b).
Exercises during this phase promote total physical conditioning and include:
- Treadmills
- Cycle and arm ergometers
- Stair climbers
- Rowing machines
The exercise session should be preceded by a warm-up period lasting approximately 15 minutes, and the session itself lasts for 30–35 minutes, followed by a 10-minute cool-down period.
While the above exercises are largely aerobic in nature, resistance training can also be used in patients at low to moderate risk. However, patients are advised to spend some time on aerobic-type exercises before they initiate resistance exercise.
Phase IV: Independent Ongoing Conditioning
In this phase, the patient exercises independently and maintains the clinically recommended lifestyle modifications on an ongoing basis. Increased physical activity and enhanced physical fitness can promote cardiovascular health, provided the patient remains consistent with their prescribed exercise program. Indeed, the change in exercise behavior that the patient achieves must be lifelong in order to have any lasting benefit.
The physical therapist’s evaluation of a patient undergoing cardiac rehabilitation may include:
- Sternal precautions and scar mobility
- Exercise endurance level
- Assessment of range of motion and strength
- Assessment of gait, balance, and mobility
- Functional mobility tests (6-Minute Walk Test, TUG Test)
(Cleveland Clinic, 2023a; Sears, 2022)
The occupational therapist’s role in evaluating and treating patients during cardiac rehabilitation may include:
- Evaluating physical and psychological functioning, independence, self-care skills and other activities of daily living such as cooking, showering, and laundering
- Home safety evaluation
- Self-care skills training, including vital signs
- Recommendations for home management tasks and instrumental activities of daily living
- Teaching, strategies, and tools for health management (e.g., medication reminders, stress management, and appointment schedules)
(Stephenson, 2021)
EXERCISE PROGRAMS
Formal cardiac exercise programs are supervised and tailored to the abilities of the individual patient, and these programs increase exercise levels appropriately but gradually. Physical conditioning from a regular exercise program generally:
- Improves the body’s metabolism as well as conditioning the cardiac musculature
- Increases the amount of activity a patient can do before developing chest discomfort
- Assists with losing weight and maintaining weight loss
- Makes smoking cessation easier
- Improves lipid levels
- Lowers blood pressure
- Increases feelings of well-being
- Increases the chances of surviving a subsequent myocardial infarction
LIFESTYLE MODIFICATIONS
For patients with existing CAD, lifestyle changes will improve their quality of life and their sense of well-being as well as slow or even reverse their illness. Patients may have modifiable risk factors that will put them at increased risk for continued medical problems related to CAD (see also “Preventable Risk Factors” earlier in this course).
Smoking cessation, reducing dietary calories and fats (especially saturated fats), and increasing exercise can significantly reduce a patient’s risk of further developing atherosclerotic cardiovascular disease. Therapeutic lifestyle changes are also the cornerstones of the treatment of diabetes, obesity, hypertension, insulin resistance, and most dyslipidemias (Harding et al., 2022).
Smoking Cessation
Smoking injures cells throughout the body. Smoking contributes to the development of atherosclerotic cardiovascular disease, insulin resistance, type 2 diabetes, dyslipidemia, a variety of cancers, many lung diseases, gastrointestinal diseases, reproductive problems, osteoporosis, cataracts, age-related macular degeneration, hypertension, dental plaque formation, and hypothyroidism.
Patients should be educated on the medical consequences of smoking and strongly advised to stop smoking. It may be difficult for smokers to quit on their own. Counselors working with patients should encourage them to set a goal for a specific date they will begin to wean themselves from cigarettes. Patients may be referred to programs that include support, counseling, and the availability of antismoking medications.
Part of patient education about smoking is the information that nicotine is a very addictive drug. E-cigarettes, vaping, low-tar and -nicotine cigarettes, and cigars all contain addicting dosages of nicotine.
Secondhand smoke is the name for the effects on nonsmokers who are exposed regularly to exhaled smoke. Inhaling smoke in this form is known to cause many of the same health problems that smokers incur. Recent studies show that e-cigarettes and vaping cause the same problems as secondhand smoke.
Weight Management
The ideal goal for a patient’s body mass index (BMI) should be between 18.5–24.9 kg/m2, and the waist circumference should be <40 inches for men and <35 inches for women. Excess weight strains the heart, and excess fat leads to continuous high levels of blood lipids. Weight loss improves blood lipid profiles and helps lower blood pressure in overweight and obese people. For coronary artery disease patients who are overweight, weight loss can reduce the severity of their angina (CDC, 2024j).
Nutrition
Eating nutritiously will slow the development of atherosclerosis. Simply reducing the calories in a patient’s diet will improve the lipid profile, and reducing the amount of dietary fat will improve lipid levels even further.
For a heart-healthy diet, it is especially important to remove or limit foods that are high in saturated fats and trans fats, red meats, processed meats, refined carbohydrates, and sweetened beverages. It can be difficult to eliminate these foods completely; portion control is more manageable. Instead, diets should focus on fresh fruits, vegetables, and whole grains. In addition, daily plant sterols and 25+ g/day of soluble and insoluble fiber (oat bran, beans, soy products, psyllium) are recommended. Moderate alcohol intake (≤20 g/day in men or ≤10 g/day in women) is associated with a reduced incidence of coronary artery disease events, although the mechanism behind this benefit is not well understood (Cleveland Clinic, 2022b).
When choosing foods, it is important to read nutrition labels. The following are practical suggestions and information about managing the fats in one’s diet and maintaining a healthy diet:
- Monounsaturated and polyunsaturated fats are safe in moderate amounts (examples of monounsaturated fats are olive oil and canola oil; examples of polyunsaturated fats are soybean oil, corn oil, sunflower oil, and the oils in nuts).
- Fish usually have healthy oils, particularly omega-3, which is found in cold-water fish such as tuna, salmon, trout, sardines, and herring.
- Poultry without the skin has less fat than most cuts of beef.
- Beef, pork, chicken with skin, whole milk cheeses, and dairy products contain high amounts of cholesterol and saturated fat.
- Whole-grain foods, fruits, beans, and vegetables are healthy foods and high in fiber.
- Select seven to nine fruits and vegetables per day in various colors.
- Limit sweets, desserts, and sugary soda to a few times a month.
- Drink alcohol in moderation.
- Practice portion control.
(Cleveland Clinic, 2022b)
ANSWERING PATIENT QUESTIONS
Q: I’ve heard that trans fats are bad for your heart. What are trans fats?
A: Trans fats are the worst type of fats for your heart and arteries. Trans fats are also called hydrogenated fats. Most trans fats are manmade and added to processed foods to make the food last longer. On ingredient labels, trans fats are usually called partially hydrogenated oils or fats, and on nutrition labels, they are listed as trans fats (usually a subclass of saturated fats). Trans fats are often found in vegetable shortenings, margarines, cakes, crackers, cookies, snack foods (potato chips, corn chips, popcorn), and foods like fried potatoes that have been cooked in partially hydrogenated oils. In 2015 the U.S. Food & Drug Administration released its final determination that trans fats (partially hydrogenated oils) are not “generally recognized as safe” (GRAS) (Cleveland Clinic , 2023b).
Dental care and tooth replacement are an often-forgotten part of improving a patient’s diet, and oral health problems can indirectly increase the risk of developing cardiovascular disease. For instance, the bacteria from periodontal disease can cause an increase in inflammation. Inflammation from gum disease may then contribute to atherosclerotic plaque formation and coronary artery disease (AHA, 2024e).
Nutritional evaluation, counseling, and monitoring are essential to helping patients improve their diet. However, it is unrealistic to expect that a single nutritional educational session or program will result in long-term adherence to a sensible diet. Moreover, patients may find it difficult to absorb a large amount of information in a short period of time. Some patients, particularly those with comorbidities such as diabetes, obesity, or heart failure, as well as those from culturally and linguistically diverse backgrounds, may require more nutritional information and counseling than they can obtain in the context of a group program.
These factors make it especially important for patients and their family members to consult with a dietitian on a regular basis. Many hospitals offer preventive and therapeutic nutrition classes with an emphasis on cardiovascular health.
Emotional Support
For many patients, adjusting to the lifestyle changes needed to manage CAD can take time. Some patients may feel anxious or depressed and lose touch with their support system. Patients may also need to be away from their work for several weeks or months during treatment and recovery.
Counseling may be helpful for patients with depressive symptoms. Antidepressants may also be helpful for patients who have more severe or chronic symptoms. Occupational therapists can help in teaching new skills if a patient needs to modify activity levels because of their work or vocation.
Patients should be encouraged to learn stress reduction strategies that work for them. These may include mind–body techniques such as tai chi, yoga, journaling, guided imagery, or other creative outlets.
PATIENT EDUCATION GOALS
Patients should be taught the basics of the disease. They should learn that their sensitivity to ischemia will vary during the day. Angina is more likely in the early morning, with activities, just after meals, and according to the weather (cold weather is more stressful).
Patients can control their angina by the way they live their daily lives. Heart ischemia is brought on when the heart muscle is asked to work hard. Many tasks that cause chest pain can be done without discomfort simply by doing them more slowly or in smaller increments.
Instruction and education from both physical and occupational therapists can assist patients if modifications are needed in activities of daily living in order to prevent ischemic symptoms. Walking, climbing stairs, vacuuming, raking, and lifting can all be done in a more leisurely way. Washing, carrying, and lifting should be done with fewer items. In their jobs, cardiac patients may have to learn to allot more time to each task.
For some people, anger, frustration, and other strong emotions can cause ischemic episodes. These patients need help in calming their emotions, and they should be referred to therapy programs that emphasize behavioral modification and that provide practical coping techniques for stressful situations. In addition, relaxation techniques, mental focusing strategies, guided imagery, and yoga have all proven useful in reducing stress for patients with coronary artery disease.
Patient and family education regarding the management of CAD may include the following:
- Understanding the warning signs of angina:
- Chest pain (may be described as heaviness, tightness, pressure, aching, burning, numbness, fullness, or squeezing)
- Pain or discomfort in other areas of the upper body, including the arms, left shoulder, back, neck, jaw, or stomach
- Difficulty breathing or shortness of breath
- Sweating or “cold sweat”
- Fullness, indigestion, or a choking feeling (may feel like heartburn)
- Nausea or vomiting
- Lightheadedness, dizziness, extreme weakness, or anxiety
- Rapid or irregular heart beats
- Calling 911 for severe chest pain that does not go away after five minutes
- Decreasing risk factors, including:
- Smoking or the use of tobacco products
- High blood cholesterol
- High blood pressure
- Uncontrolled diabetes
- Sedentary lifestyle
- Being obese or overweight
- Stress
- High-fat diet
- Taking medications as directed
- Understanding that cardiac procedures may be needed (now or in the future) to treat unstable disease
- Knowing the importance of regular visits to the cardiologist
(Cleveland Clinic, 2023c)
CASE
Linda Ortiz, a 60-year-old Hispanic woman with a history of type 2 diabetes and hypertension, was recently diagnosed with coronary artery disease after complaints of chest pain. She has come to the doctor’s office for a follow-up appointment two weeks after an episode of angina that brought her to the emergency department.
Ms. Ortiz tells her nurse practitioner that she has been compliant with her newly prescribed drug regimen of daily aspirin, an ACE inhibitor, and a statin. However, when Leilani, the NP, asks her about her lifestyle, Ms. Ortiz admits that she has been having a hard time adhering to the recommended lifestyle changes.
Leilani counsels Ms. Ortiz about the importance of smoking cessation, regular exercise, and a healthy diet. As Leilani talks with Ms. Ortiz, she also provides her with an educational brochure about the benefits of implementing lifestyle changes, including an example of heart-healthy food choices and a diary for Ms. Ortiz to record and track her daily activities, food intake, and medication doses.
Leilani and Ms. Ortiz agree on setting a goal to stop smoking within two months, and Leilani prescribes a mild anti-anxiety medication to help her quit. She also helps Ms. Ortiz establish some exercise goals, starting with moderate walking 15 to 20 minutes every day and gradually progressing to more prolonged and challenging exercise.
Ms. Ortiz has some hesitation with this exercise goal, stating, “I just don’t feel like I have the strength to start exercising.” After discussing this, Leilani refers Ms. Ortiz to a physical therapist for evaluation of her mobility status and exercise tolerance and to assist her in establishing and implementing a realistic long-term physical fitness and functional mobility regimen.
Finally, Leilani has Ms. Ortiz schedule another follow-up appointment in four weeks so they can track and assess her progress on these lifestyle changes.
CONCLUSION
Coronary artery disease continues to be one of the most common health problems in the United States and around the globe. Great strides are being made in understanding how to prevent and treat CAD. Treatment outcomes are also improving, which means that more patients are now living with CAD. Therefore, it is vital for all healthcare professionals to understand the key components of managing CAD. Patient, family, and community education is essential to promote further improvement in managing CAD and treatment outcomes.
Prevention of CAD begins with understanding individual risk factors and implementing therapeutic lifestyle changes. Weight loss, improved diet, medications, smoking cessation, prevention of inflammation, and regular physical exercise are the elements of the initial treatment program. Drugs and interventional procedures are used to treat those components of CAD that do not improve sufficiently with therapeutic lifestyle changes alone.
A multidisciplinary team manages patients with CAD to assure that they have success in treating, managing, and living with the best quality of life and outcomes possible. Cardiac rehabilitation (CR) is an evidence-based program that optimizes the most recent research to inform patients, families, and healthcare workers how to treat cardiac patients with CAD, after an MI, and after a CABG to provide them with the most active and healthy life possible.
If conservative medical management does not suffice to treat CAD adequately, more invasive methods are available. A percutaneous coronary intervention (PCI), drug-emitting stent (DES), or CABG may be warranted.
RESOURCES
About coronary artery disease (CDC)
American College of Cardiology
Cardiovascular diseases (World Health Organization)
Coronary artery disease (Cleveland Clinic)
Coronary artery disease/coronary heart disease (American Heart Association)
REFERENCES
NOTE: Complete URLs for references retrieved from online sources are provided in the PDF of this course.
ACLS Training Center. (2023). Acute coronary syndromes algorithms. https://www.acls.net
ACLS.com. (2024). Myocardial infarction: Prognosis and predictors of mortality. https://resources.acls.com
American College of Cardiology (ACC). (2022). New USPSTF recommendation on aspirin in CVD: No for primary prevention, yes for secondary prevention. https://www.acc.org
American Heart Association (AHA). (2024a). Heart disease and stroke statistics update fact sheet at-a-glance. https://www.heart.org
American Heart Association (AHA), Go Red for Women. (2024b). Warning signs and symptoms of heart attack and stroke. https://www.goredforwomen.org
American Heart Association (AHA). (2024c). Healthy and unhealthy blood pressure ranges. https://www.heart.org
American Heart Association (AHA). (2024d). Out-of-hospital chain of survival. https://cpr.heart.org
American Heart Association (AHA). (2024e). American Heart Association’s healthy smiles, healthy hearts. https://www.heart.org
American Heart Association (AHA). (2024f). American Heart Association diet and lifestyle recommendations. https://www.heart.org
American Heart Association (AHA). (2023a). Here’s how 10 popular diets scored for heart health. https://www.heart.org
American Heart Association (AHA). (2023b). Coronary artery calcium (CAC) test. https://www.heart.org
American Heart Association (AHA). (2023c). What can I expect when I go home after heart surgery? https://www.heart.org
American Heart Association (AHA). (2022). American Heart Association’s Life’s Simple 7: Lifestyle recommendations, polygenic risk, and lifetime risk of coronary heart disease. https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.121.053730
American Heart Association (AHA). (2021). What is metabolic syndrome? https://www.heart.org
Boston University. (2024). Transmyocardial laser revascularization (TMR). https://www.bumc.bu.edu
Cardiac Sciences. (2023). Keep your move in the tube. Alberta Health Science. https://myhealth.alberta.ca
Centers for Disease Control and Prevention (CDC). (2024a). About women and heart disease. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2024b). Heart disease facts. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2024c). Health effects of cigarettes: Cardiovascular disease. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2024d). LDL and HDL cholesterol and triglycerides. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2024e). Risk factors for high cholesterol. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2024f). High blood pressure facts. https://www.doi.cdc.gov
Centers for Disease Control and Prevention (CDC). (2024h). Benefits of physical activity. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2024i). National diabetes statistics report. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2024j). About body metabolism index (BMI). https://www.cdc.gov
Cleveland Clinic. (2023a). Cardiac rehab. https://my.clevelandclinic.org
Cleveland Clinic. (2023b). Trans fat has been banned but that doesn’t mean that you’re free from it. https://health.clevelandclinic.org
Cleveland Clinic. (2023c). Coronary artery disease. https://my.clevelandclinic.org
Cleveland Clinic. (2022a). Aortic aneurysm. https://my.clevelandclinic.org
Cleveland Clinic. (2022b). How to follow a heart healthy diet. https://my.clevelandclinic.org
Corliss J. (2023). For mellow movement that helps your heart, try tai chi. Harvard Health Publishing. https://www.health.harvard.edu
Dibben GO, Faulkner J, Oldridge N, Rees K, Thompson DR, et al. (2023). Exercise-based cardiac rehabilitation for coronary heart disease: A meta-analysis. European Heart Journal, 44(6), 452–69. https://doi.org/10.1093/eurheartj/ehac747
Dorwart L. (2022). Angina facts and statistics: What you need to know. Verywell health. https://www.doi.verywellhealth.com
Drugs.com. (2024). Brillinta vs. Plavix: What’s the difference? https://www.drugs.com
Ecgwaves. (2024). Myocardial ischemia: Reactions, ECG, and development of infarction. https://ecgwaves.com
Elendu C, Amaechi DC, Elendu TC, Jingwa KA, Okoye OK, Fiemotonghan BE, Chirinos GA, et al. (2024). Relationship between stress and coronary artery disease: A comprehensive review. Medicine, 103(5), Article e37066. https://doi.org/10.1097/MD.0000000000037066
Ghandakly EC, Iacona GM, & Bakaeen FG. (2024). Coronary artery surgery: Past, present, and future. Rambam Maimonides Medical Journal, 15(1), Article e0001. https://doi.org/10.5041/RMMJ.10515
Golla MSG, Brown KN, & Nagendra G. (2023). Percutaneous transluminal coronary angiography. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
Harding MM, Kwong J, Hagler D, & Reinisch C. (2022). Lewis medical-surgical nursing assessment and clinical management of clinical problems (12th ed.). Elsevier.
Harvard Health Publishing. (2023). Takotsubo cardiomyopathy (broken-heart syndrome). https://www.doi.health.harvard.edu
Healio.com. (2024). CHA2DS2-VASc Score. https://www.healio.com
Healthline. (2023). Diabetes and coronary artery disease: How are they related? https://www.healthline.com
Isaac J. (2023a). Cardiac rehabilitation. Merck Manual. https://www.merckmanuals.com
Isaac J. (2023b). Occupational therapy (OT). Merck Manual. https://www.merckmanuals.com
Jellis C. (2023). What a family history of heart disease means to your health. https://health.clevelandclinic.org
Johns Hopkins Medicine. (2024). What is angina pectoris? https://www.doi.hopkinsm edicine.org/health/conditions-and-diseases/angina-pectoris
Kolominsky J, Rifai MA, Patel J, & Shapiro MD. (2021). Cardiac rehabilitation and implications during the COVID-19 era. American College of Cardiology. https://www.acc.org
Koźlik M, Harpula J, Chuchra PJ, Nowak M, Wojakowski W, & Gąsior P. (2023). Drug-eluting stents: Technical and clinical progress. Biomimetics (Basel), 8(1), 72. https://doi.org/10.3390/biomimetics8010072
Macias-Konstantopoulos WL, Collins KA, Diaz R, Duber HC, Edwards CD, et al. (2023). Race, healthcare, and health disparities: A critical review and recommendations for advancing health equity. Western Journal of Emergency Medicine, 25(5), 906–18. https://pmc.ncbi.nlm.nih.gov
Mankad R. (2024). Silent heart attack: What are the risks? Mayo Clinic. https://www.mayoclinic.org
Mayo Clinic. (2024a). Cholesterol test. https://www.mayoclinic.org
Mayo Clinic. (2024b). Nutrition and healthy eating. https://www.mayoclinic.org
Mayo Clinic. (2024c). How fit are you? See how you measure up. https://www.mayoclinic.org
Mayo Clinic. (2023a). Acute coronary syndrome. https://www.mayoclinic.org
Mayo Clinic. (2023b). Nutrition and healthy eating. https://www.mayoclinic.org
Mayo Clinic. (2023c). Obesity. https://www.mayoclinic.org
Mayo Clinic. (2023d). Exercise intensity: How to measure it. https://www.mayoclinic.org
Mayo Clinic. (2022). Herbal supplements and heart medicines may not mix. https://www.mayoclinic.org
Medline Plus. (2022a). Homocysteine test. https://medlineplus.gov
MedlinePlus. (2022b). Thrombolytic therapy. https://medlineplus.gov
Medicare Learning Network (MLN). (2024). Pulmonary rehabilitation, cardiac rehabilitation, & intensive cardiac rehabilitation expansion of supervising practitioners. MLN Matters, 13513. https://www.cms.gov
Morata TL & Flynn MMB. (2024). Sole’s introduction to critical care nursing (9th ed.). Elsevier-Evolve.
National Heart, Lung, and Blood Institute (NHLBI). (2023). What is angina? https://www.doi.nhlbi.nih.gov
National Heart, Lung, and Blood Institute (NHLBI). (2022). Heart treatments. https://www.nhlbi.nih.gov
National Institute of Mental Health (NIMH). (2024). Depression. https://www.nimh.nih.gov
Ogbru O. (2023). What is nitroglycerin and what is it used for? MedicineNet. https://www.medicinenet.com
Pagana K, Pagana T, & Pagana T. (2022). Mosby’s diagnostic and laboratory tests (7th ed.). Elsevier.
Rasmussen LD, Fordyce CB, Nissen L, Hill CL Jr, Alhanti B, et al. (2022). The PROMISE minimal risk score improves risk classification of symptomatic patients with suspected CAD. JACC Cardiovascular Imaging, 15(8), 1442–54. https://doi.org/10.1016/j.jcmg.2022.03.009
Sears B. (2023). Sternal precaution after open heart surgery. VeryWellHealth. https://www.verywellhealth.com
Sears B. (2022). The four phases of cardiac rehab. VeryWellHealth. https://www.verywellhealth.com
Sekkarie A, Fang J, Hayes D, & Loustalot F. (2024). Prevalence of self-reported hypertension and antihypertensive medication use among adults—United States, 2017–2021. MMWR, 73, 191–8. http://dx.doi.org/10.15585/mmwr.mm7309a1
Singh A, Museedi AS, & Grossman SA. (2023). Acute coronary syndrome. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
Stenman M, Jeppsson A, Pivodic A, Sartipy U, & Nielsen SJ. (2022). Risk of depression after coronary artery bypass grafting: A SWEDEHEART population-based cohort study. European Heart Journal Open, 2. https://doi.org/10.1093/ehjopen/oeac015
Stephenson B. (2021). Benefits of occupational therapy for cardiac issues. https://blog.rehabselect.net
U.S. Preventive Services Task Force (USPSTF). (2022). Aspirin use to prevent cardiovascular disease: Preventative medicine. https://www.uspreventiveservicestaskforce.org
Vallabhajosyula S, Verghese D, Bell MR, Murphree DH, Cheungpasitporn W, et al. (2021). Fibrinolysis vs. primary percutaneous coronary intervention for ST-segment elevation myocardial infarction cardiogenic shock. ESC Heart Failure, 3, 2025–35. https://doi.org/10.1002/ehf2.13281
Villines Z. (2023). What is the normal range for troponin levels? MedicalNewsToday. https://www.medicalnewstoday.com
Wang Y, Leifheit EC, & Krumholz HM. (2022). Trends in 10-year outcomes among Medicare beneficiaries who survived an acute myocardial infarction. JAMA Cardiology, 7(6), 613–22. https://jamanetwork.com
World Health Organization (WHO). (2024a). Prevalence of overweight among adults, BMI >= 25 (age-standardized estimate) (%). https://www.who.int
World Health Organization (WHO). (2024b). Prevalence of obesity among adults, BMI >= 30 (age-standardized estimate) (%). https://www.who.int
World Health Organization (WHO). (2024c). Obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
Williamson L. (2023). The connection between menopause and cardiovascular disease risks. American Heart Association News. https://www.heart.org
Yow AG, Rajasurya V, & Sharma S. (2024). Sudden cardiac death. StatPearls Publishing. https://www.doi.ncbi.nlm.nih.gov






Customer Rating
4.9 / 1003 ratings