Substance Abuse Education for Delaware Nurses
Best-Practice Prescribing, Drug Diversion, and Impaired Practice
Online Continuing Education Course

Course Description
MANDATORY DELAWARE NURSING CEU. Fulfills the requirement for 3 contact hours of continuing nursing education on substance abuse for DE nurses, focusing on prescription drug abuse and diversion, challenges in managing chronic pain, and best practices for prescribing controlled substances. ANCC-accredited provider; accepted by the Delaware Board of Nursing.
Course Price: $30.00
Contact Hours: 3
Pharmacotherapeutic Hours: 0.75
Course updated on
April 23, 2025
"Extremely informative CEU. It had great state-specific information." - Gail, RN in Delaware
"Presentation was excellent and very informative as to Delaware's requirements and on the national drug abuse crisis." - Louise, RN in Delaware
"Very thorough, informative, and easy to follow. Just the right amount of statistics to back information." - Lisa, RN in Delaware
"Material was presented well and writing style held my interest." - Marsha, RN in Delaware
Substance Abuse Education for Delaware Nurses
Best-Practice Prescribing, Drug Diversion, and Impaired Practice
Copyright © 2025 Wild Iris Medical Education, Inc. All Rights Reserved.
LEARNING OUTCOME AND OBJECTIVES: Upon completion of this continuing education course, you will better understand best-practice prescribing of controlled substances, how to prevent drug diversion, and how to administer an opioid antagonist. Specific learning objectives to address potential knowledge gaps include:
- Identify components of best-practice prescribing for opioid medications.
- Summarize the CDC and Delaware Guideline for Prescribing Opioids for Chronic Pain.
- Explain various strategies designed to prevent prescription drug misuse and diversion.
- Describe considerations for the use of the opioid antagonist naloxone.
- Discuss chemical dependency and impairment in the workplace.
TABLE OF CONTENTS
SCOPE OF THE PROBLEM
Overdoses are the leading injury-related cause of death in the United States. According to provisional data from the National Center for Health Statistics, an estimated 107,543 people died from drug overdoses in 2023, with 81,083 (75%) of those deaths involving an opioid. This represents a decrease of 3% from estimated deaths in 2022 (CDC, 2024a). In 2022, the drug overdose death rate in Delaware was 55.3 per 100,000, which was fourth highest in the nation following West Virginia, the District of Columbia, and Tennessee (CDC, 2025).
The crisis is far from resolved. Data indicate that a large number of individuals continue to misuse prescription drugs. The National Survey on Drug Use and Health estimates that in 2023:
- 8.6 million people ages 12 and older misused prescription pain relievers.
- 828,00 people ages 12 and older misused prescription or illegally made fentanyl.
- 48.5 million people ages 12 and older had a substance use disorder in the past year. (SAMHSA, 2023a)
One of the biggest challenges in healthcare practice is providing safe and appropriate pain care without contributing to the crisis of prescription drug misuse, drug diversion, and drug overdose deaths. Nurses in particular are in a unique position to address this problem since they care for more patients than any other health profession. Nurses who understand the risks associated with prescription drug abuse will be better prepared to identify and intervene with patients and colleagues who may be at risk.
COMMONLY USED TERMS
- Illicit drug use
- Use and misuse of illegal and controlled drugs, including any use of marijuana or hashish, cocaine, crack, heroin, hallucinogens, inhalants, methamphetamine, as well as misuse of prescription pain relievers, tranquilizers, stimulants, and sedatives; excludes use of over-the-counter drugs
- Opioids
- Drugs that act on opioid receptors in the spinal cord and brain to reduce the intensity of pain-signal perception intensity; include the illegal drug heroin, illegally made fentanyl, and pain medications available legally by prescription, such as oxycodone, hydrocodone, codeine, morphine, prescribed fentanyl, and others (CDC, 2024b)
- Opioid use disorder (OUD)
- A problematic pattern of opioid use that causes significant impairment or distress; diagnosed based on specific criteria such as unsuccessful efforts to cut down or control use or use resulting in social problems and a failure to fulfill obligations at work, school, or home, among other criteria; preferred term over opioid abuse or dependence or opioid addiction
- Overdose
- Injury to the body (poisoning) that happens when a drug is taken in excessive amounts
- Prescription drug misuse (or nonmedical use of prescription drugs)
- The use of prescribed medications in any way not directed by a doctor, including use without a prescription; use in greater amounts, more often, or longer than instructed; use to get high; diversion; concurrent use of alcohol, illicit substances, or nonprescribed controlled medications; or use in any other way not directed by a doctor
- Prescription drug diversion
- Obtaining or using prescription drugs illegally
- Substance use disorder (SUD)
- A cluster of cognitive, behavioral, and physiological symptoms indicating that the individual continues using the substance despite harmful consequences or impaired ability to function in day-to-day life; can cause changes in brain function and distorted thinking and behaviors; preferred term over addiction or substance abuse
(Becker & Starrels, 2024; CDC, 2024b, 2024c; APA, 2024; SAMHSA, 2023a)
BEST-PRACTICE OPIOID PRESCRIBING
The serious and deadly consequences of the opioid crisis prompted the medical community to reevaluate chronic pain treatment and prescribing practices. Responsible prescribing involves individual prescribers following best practices and taking action to balance the risks and benefits of opioid pain management for each patient. Three important components to responsible prescribing include:
- Thorough patient evaluation, including risk assessment
- Treatment plan design that incorporates functional goals
- Periodic review and monitoring
Best practices are also described in evidence-based guidelines for prescribing opioids for chronic pain released in 2016 by the Centers for Disease Control and Prevention (CDC) and updated in 2022 (Dowell et al., 2022) as well as in the Delaware Prescription Opioid Guidelines for Health Care Providers (DDPH, 2017).
Patient Evaluation and Risk Assessment
A thorough patient assessment is critical prior to prescribing opioid medication for chronic pain. It is important to properly diagnose the condition to determine if opioid medication is an appropriate treatment. A comprehensive pain assessment includes a history of the pain, behavioral observations, past medical history, medications, family history, a physical examination, and if necessary, diagnostic testing.
PAIN HISTORY
A pain assessment begins with the history of the problem and can be obtained from written documents and from interviews with the person in pain as well as family members and other caregivers. Pain is a subjective symptom, and pain assessment is, therefore, based on the patient’s own perception of pain and its severity.
Because pain is subjective, a self-report is considered the gold standard, or the best, most accurate measure of a person’s pain. One method to obtain a complete pain history is the PQRST assessment (see box).
PQRST PAIN ASSESSMENT
Provocation/Palliation (P)
- What were you doing when the pain started?
- What caused the pain?
- What seems to trigger it (e.g., stress, position, certain activities)?
- What relieves it (e.g., medications, massage, heat/cold, changing position, being active, resting)?
- What aggravates it (e.g., movement, bending, lying down, walking, standing)?
Quality/Quantity (Q)
- What does the pain feel like (e.g., sharp, dull, stabbing, burning, crushing, throbbing, nauseating, shooting, twisting, stretching)?
Region/Radiation (R)
- Where is the pain located?
- Does the pain radiate, and if so, where?
- Does the pain feel like it travels/moves around?
- Did it start somewhere else and is now localized to another spot?
- Is it accompanied by other signs and symptoms?
Severity Scale (S)
- How severe is the pain on a scale of 0–10, with 0 as no pain and 10 as the worst pain ever?
- Does the pain interfere with activities?
- How bad is the pain at its worst?
- Does it force you to sit down, lie down, slow down?
- How long does an episode last?
Timing (T)
- When or at what time did the pain begin?
- How long did it last?
- How often does it occur (e.g., hourly, daily, weekly, monthly)?
- Is the pain sudden or gradual in onset?
- When do you usually experience it (e.g., daytime, night, early morning)?
- Are you ever awakened by it?
- Does it ever occur before, during, or after meals?
- Does it occur seasonally?
(Crozer Health, 2022)
BEHAVIORAL OBSERVATIONS
Most people who are experiencing pain usually show it either by verbal complaint or nonverbal behaviors or indicators. It is important, however, to remember that people in pain may or may not display behaviors that are considered an indication of “being in pain,” and making judgments about their honesty is inappropriate. Nonverbal indicators of pain may include:
- Restlessness or pacing
- Groaning or moaning
- Crying
- Gasping or grunting
- Nausea or vomiting
- Diaphoresis
- Clenching of the teeth and facial expressions
- Tachycardia or blood pressure changes
- Panting or increased respiratory rate
- Clutching or protecting a part of the body
- Unable to speak or open eyes
- Decreased interest in activities, social gatherings, or old routines
(Toney-Butler, 2023)
TYPES OF PAIN
- Acute: Pain that has lasted for less than a month; usually starts suddenly and has a known cause, like an injury, trauma, surgery, or infection; normally lessens as the body heals
- Subacute: Pain that lasts at least one month but not more than three months; includes conditions such as low back pain, neck pain, broken bones, muscle sprains or strains, dental pain from infection or tooth extraction, pain due to kidney stones, acute episodic migraines, and pain after surgery
- Chronic: Pain that lasts more than three months; can be caused by a disease or condition, injury, medical treatment, inflammation, or unknown reasons; can result from acute or subacute pain that is not effectively managed
(CDC, 2024b)
RISK ASSESSMENT
When clinicians assess patients with chronic pain, it is important to recognize two categories of risk due to opioid therapy: medical conditions that increase their risk for adverse events (e.g., respiratory depression) and risk of misuse, abuse, or addiction.
Risk of Adverse Events
Risk due to medical conditions are assessed and documented as part of the patient’s history and physical examination and the treatment plan adjusted accordingly to reduce risk of adverse events with opioid therapy. Older adults may be at higher risk because of cognitive decline and increased potential for falls. Patients with impaired renal or hepatic function, cardiopulmonary disease, mental health conditions, obesity, and sleep apnea are also at higher risk for adverse consequences when prescribed opioid medications.
Risk for Misuse, Abuse, and Addiction
Variables that have been associated with a higher risk for misuse, abuse, and addiction include history of addiction in biological parents, current drug addiction in the family, regular contact with high-risk groups or activities, and personal history of illicit drug use or alcohol addiction. (See also “Recognizing Aberrant Drug-Related Behaviors” later in this course.)
The use of screening tools is recommended, and multiple tools are available that can help healthcare providers to assess these risks. Examples of screening tools to assess opioid risk or misuse include:
- Opioid Risk Tool: Administered at initial visit prior to beginning opioid therapy; questions address age, family, and personal history of substance abuse, history of preadolescent sexual abuse, and psychological diseases (see below)
- Screening to Brief Intervention (S2BI): A series of questions regarding frequency-of-use in adolescent patients of substances most commonly used
- Tobacco, Alcohol, Prescription medication, and other Substance use (TAPS): A combined screening and brief assessment that addresses use-related behaviors and generates a risk level for each substance class
- Brief Intervention tool: A 26 item “yes–no” questionnaire to identify signs of opioid addiction or abuse
- CAGE, CAGE-AID, and CAGE-Opioid: CAGE (Cut down, Annoyed, Guilty, and Eye-opener) questionnaires consisting of four questions to assess for alcohol abuse and substance abuse (AID and Opioid versions)
- Current Opioid Misuse Measure (COMM): A 17-item patient self-report assessment designed to identify abuse in chronic pain patients in terms of aberrant behaviors associated with misuse, especially in patients who are already receiving long-term opioid therapy
- Diagnosis, Intractability, Risk and Efficacy (DIRE) tool: A clinician-rated questionnaire to predict patient compliance with long-term opioid therapy
- Screener and Opioid Assessment for Patients with Pain—Revised (SOAPP-R): A screening questionnaire addressing history of alcohol or substance abuse, cravings, mood disorders, and stress
- Urine Drug Test (UDT): Can be used to evaluate for the use of the medication prescribed and in detecting any unsanctioned drug use
- Rapid Oрioid Dependence Screen (RODS): An eight-item screening tool for οрiоid dependence; can be administered as a stand-alone instrument or as part of a comprehensive interview
- OWLS: A four-item self-administered screening tool to detect prescription ΟUD in persons prescribed long-term оpiοid therapy
(Dydyk, 2023; Strain, 2024)
(See also “Resources” at the end of this course.)
| Female | Male | |
|---|---|---|
| (Sources: Delaware Division of Professional Regulation, Partnership for a Drug Free America, and U.S. Surgeon General Turn the Tide Program.) | Family history of substance abuse | |
| Alcohol | 1 | 3 |
| Illegal drugs | 2 | 3 |
| Prescription drugs | 4 | 3 | Personal history of substance abuse |
| Alcohol | 3 | 3 |
| Illegal drugs | 4 | 4 |
| Prescription drugs | 5 | 5 | Age 16–45 years | 1 | 1 | History of preadolescent sexual abuse | 3 | 0 | Psychological disease |
| ADD, OCD, bipolar, schizophrenia | 2 | 2 |
| Depression | 1 | 1 |
|
Scoring totals
≤3 = low risk for future opioid abuse
4–7 = moderate risk
≥8 = high risk for opioid abuse
|
||
Treatment Plans
Responsible opioid prescribing requires clinicians to develop treatment plans that focus on patient-centered outcomes and functional goals that improve quality of life. A function-based treatment strategy that aims to maximize the patient’s quality of life and minimize the burden of their pain includes a mutual understanding between prescriber and patient covering the following principles:
- Complete elimination of all pain is often not possible.
- The goal of treatment is to successfully manage pain and not exclusively to reduce a pain scale score.
- Functional goals will be collaboratively set, with the aim of improving quality of life; these goals must be realistic, achievable, verifiable, and meaningful.
- Risks, benefits, side effects, and potential adverse consequences of opioid use will be fully disclosed.
- Education about safe use, storage, and disposal of opioid medication will be provided.
The treatment plan must be documented and include informed consent and patient education.
SAMPLE TREATMENT AGREEMENT FOR THE USE OF OPIOID MEDICATION
Opioids are controlled substances and there are numerous laws and regulations regarding the prescribing of them that your medical provider must adhere to. The following requests are considered standard best practice and help this health care practice and you comply with these laws and regulations.
The patient agrees:
- I will fill my prescriptions only at one pharmacy, located at __________________. I must contact my prescriber/provider if I wish to change pharmacies, and that change must be approved by my prescriber/provider.
- All prescriptions for pain medications will, except in an emergency, only come from my practitioner or the clinic.
- To reliably attend appointments with the practitioner.
- To not use any illegal substances, such as cocaine or marijuana, etc. while taking opioids.
- To not request earlier prescription refills if I decide to use more without the knowledge and consent of the prescriber/provider.
- To use other pain consultations/management strategies recommended by my provider, as necessary.
- To safely store the medication. (This is extremely important, as most of the prescription opioids now on the street were stolen from a prescribed user—use a locked box and do not keep them where others might see or have access to them.)
- That traveling with strong painkillers may pose problems. Before traveling, I will contact the appropriate travel authority (usually the consulate website of the country you are visiting) and obtain a note from my prescriber/provider.
- That lost, stolen, or spilled medications will not be replaced.
- To random, periodic urine drug tests, as required by law, no less than once every six months or more frequently at the prescriber/provider’s discretion.
The prescriber/provider agrees:
- To see you within a reasonable time frame for follow-up.
- To discuss the results of urine drug testing with you before making any decisions regarding your ongoing pain treatment.
- To offer you treatment for your pain through therapies other than opioids if these medications are found to cause you more harm than good.
Provider signature/date:__________________________
Patient signature/date:___________________________
(DDPH, n.d.-a)
SAMPLE NOTICE OF THE USE OF CONTROLLED SUBSTANCES FOR THE TREATMENT OF PAIN AND PATIENT’S INFORMED CONSENT
This will confirm that you have been diagnosed with:__________________________.
I have recommended treating your condition with the following controlled substance(s). There may be alternative methods of treatment available to you.
The material risks associated with taking these controlled substances include but are not limited to:
- Sedation that may interfere with your ability to drive and operate machinery safely
- Interference with breathing, which could become life-threatening
- Urinary and bowel function (constipation) serious enough to warrant urgent medical treatment
- Physical dependence
- The potential for addiction, abuse, and misuse
- Nausea, vomiting, itching, mood changes, muscle twitching, and allergic reactions
- Injury to the fetus or unborn child in a pregnant woman
- Overdose as a result of accidental exposure (especially in children)
- Neonatal opioid withdrawal (if I am a female and am or could be pregnant)
- Potentially fatal overdose resulting from interactions with alcohol and other drugs
Physical dependence is an inevitable consequence of chronic opioid use. This involves the body becoming used to having the medication present. If someone who is physically dependent on a medication discontinues the use of that medication suddenly, they may experience an uncomfortable withdrawal syndrome.
Addiction is not the same as physical dependence, although the two may overlap. Addiction involves the compulsive use of a substance, against a provider’s instructions, for unintended purposes. It may also involve unauthorized increases in medication or diversions of medication.
This will also confirm that I asked if you wanted a more detailed explanation of the proposed treatment, alternatives, and material risks and that you (check one):
- Were satisfied with the above description and did not want any more information.
- Requested and received, in substantial detail, further explanation of the treatment, alternative methods of treatment, and information about the material risks.
If this accurately represents our discussion and if you are satisfied with the explanation given, you must sign this document indicating your informed consent to use this controlled substance before commencement of treatment.
Patient signature/date:___________________________
Witness signature/date:__________________________
Provider signature/date:__________________________
(DDPH, n.d.-b)
Periodic Monitoring
It is critical to regularly reevaluate the appropriateness of continuing opioid therapy due to changes in pain etiology, health condition, progress toward functional goals, and addiction risk. To corroborate self-reports, review of data within the prescription drug monitoring program should be conducted at each visit (see “Prescription Drug Monitoring Programs” later in this course). Periodic monitoring should also include urine tests and pill counts when appropriate.
Clinicians must utilize screening and monitoring for all patients on chronic opioid therapy to document patient outcomes and progress toward functional goals. The Pain Assessment and Documentation Tool (PADT) is a practical tool that clinicians can use at each patient visit and incorporate into electronic records (see “Resources” at the end of this course). It offers a simple checklist to monitor the “Five As” of pain management (Dydyk, 2023).
| Analgesia | A reduction in pain |
| Activities of daily living | Improvement in level of function |
| Affect | Changes in mood |
| Adverse effects | Falls, decreased cognitive function, constipation, etc. |
| ADRBs | Aberrant drug-related behaviors |
Periodic monitoring timing will vary with each patient. CDC guidelines recommend monitoring every three months at the minimum and before refilling an opioid prescription at any time (Dowell et al., 2022).
CDC Clinical Practice Guideline for Prescribing Opioids for Pain
In 2022, the CDC updated its guidelines for prescribing opioids for the treatment of pain. The guidelines address four main issues, including:
- Making a determination about whether or not to initiate opioids for pain
- Selecting the appropriate opioid and determining the dosage
- Deciding the duration of the initial opioid prescription and conducting follow-up
- Assessing the risk and addressing the potential harms of opioid use with the patient
These recommendations aim to improve communication between clinicians and patients about the risks and effectiveness of pain treatment; improve pain, function, and quality of life for persons with pain; and reduce the risks associated with opioid pain treatment (including opioid use disorder, overdose, and death) as well as with other pain treatment.
The practice guidelines include 12 recommendations for clinicians who are prescribing opioids for outpatients ages 18 years and older with pain that is acute (duration of <1 month), subacute (duration of 1–3 months), or chronic (duration of >3 months), excluding pain management related to sickle cell disease, cancer-related pain treatment, palliative care, and end-of-life care.
- Nonopioid therapies are at least as effective as opioids for many common types of pain. Maximize the use of nonpharmacologic and nonopioid pharmacologic therapies appropriate for the condition and the patient, and only consider opioid therapy for acute pain if benefits are expected to outweigh risks to the patient. Discuss benefits and risks with the patient prior to prescribing opioid therapy.
- Nonopioid therapies are preferred for subacute and chronic pain. Maximize use of nonpharmacologic and nonopioid pharmacologic therapies as appropriate for the specific condition and patient. Consider opioid therapy if expected benefits are anticipated to outweigh risks, and work with the patient to establish treatment goals for pain and function. Consider how opioid therapy will be discontinued if benefits do not outweigh risks.
- When starting opioid therapy for acute, subacute, or chronic pain, prescribe immediate-release opioids instead of extended-release and long-acting (ER/LA) opioids.
- When opioids are initiated for opioid-naive patients with acute, subacute, or chronic pain, prescribe the lowest effective dosage. If opioids are continued for subacute or chronic pain, prescribe the lowest effective dosage. Avoid increasing dosage above levels likely to yield diminishing returns in benefits relative to risks.
- For those patients already receiving opioid therapy, carefully weigh benefits and risks and exercise care when changing opioid dosages. Work closely with patients to optimize nonopioid therapies while continuing opioid therapy. If benefits do not outweigh risk of continued opioid therapy, optimize other therapies and work closely with patients to gradually taper to lower dosages, or appropriately taper and discontinue opioids. Unless there are indications of a life-threatening issue such as warning signs of impending overdose (e.g., confusion, sedation, slurred speech), opioid therapy should not be discontinued abruptly, and clinicians should not rapidly reduce opioid dosages from higher dosages.
- When opioids are needed for acute pain, prescribe no greater quantity than needed for the expected duration of pain severe enough to require opioids.
- Evaluate benefits and risks with patients within 1–4 weeks of starting opioid therapy for subacute or chronic pain or of dosage escalation. Regularly re-evaluate benefits and risks of continued opioid therapy with patients.
- Before starting and periodically during continuation of opioid therapy, evaluate risks for opioid-related harms and discuss risks with patients. Work with patients to incorporate into the management plan strategies to mitigate risk, including offering naloxone.
- When prescribing initial opioid therapy for acute, subacute, or chronic pain and periodically during opioid therapy for chronic pain, review the patient’s history of controlled substance prescriptions using state prescription drug monitoring program data to determine whether the patient is receiving opioid dosages or combinations that put the patient at high risk for overdose.
- When prescribing opioids for subacute or chronic pain, consider the benefits and risks of toxicology testing to assess for prescribed medications as well as other prescribed and nonprescribed controlled substances.
- Use particular caution when prescribing opioid pain medication and benzodiazepines concurrently and consider whether benefits outweigh risks of concurrent prescribing of opioids and other central nervous system depressants.
- Offer or arrange treatment with evidence-based medications for patients with opioid use disorder. Detoxification on its own, without medications for opioid use disorder, is not recommended because of increased risks for resuming drug use, overdose, and overdose death.
(Dowell et al., 2022)
HOW TO CALCULATE MORPHINE MILLIGRAM EQUIVALENTS PER DAY (MME/DAY)
- Calculate the total daily amount of opioid the patient is prescribed.
- Convert each opioid to MMEs by multiplying the daily dosage for each opioid by its specific conversion factor (see table).
- Add all opioid MMEs together to obtain the patient’s MME.
| Opioid (doses in mg/day except where noted) |
Conversion Factor* |
|---|---|
| * Multiply the dose for each opioid by the conversion factor to determine the dose in MMEs. Dose conversions are estimated and cannot account for all individual differences in genetics and pharmacokinetics. (Dowell et al., 2022) |
|
| Codeine | 0.15 |
| Fentanyl transdermal (mcg/hr) | 2.4 |
| Hydrocodone | 1.0 |
| Hydromorphone | 5.0 |
| Methadone | 4.7 |
| Morphine | 1.0 |
| Oxycodone | 1.5 |
| Oxymorphone | 3.0 |
Example:
A patient with chronic back pain for more than three years is currently taking oxycodone 30 mg twice daily (BID). Calculate the MME.
- Calculate the total daily amount the patient is prescribed.
30 mg × 2 times daily (BID) = 60 mg/day - Multiply the total daily amount by the conversion factor for oxycodone.
60 mg/day × 1.5 = 90 MME per day
TAPERING OPIOID MEDICATIONS
An opioid-tapering flowchart is available from the U.S. DHHS that is useful in making determinations about ongoing opioid use or cessation. Tapering is recommended when:
- Pain improves
- The patient requests dosage reduction or discontinuation
- Pain and function are not meaningfully improved
- The patient is receiving higher opioid doses without evidence of benefit from the higher dose
- The patient has current evidence of opioid misuse
- The patient experiences side effects that diminish quality of life or impair function
- The patient experiences an overdose or other serious event (e.g., hospitalization, injury) or has warning signs for an impending event such as confusion, sedation, or slurred speech
- The patient is receiving medications (e.g., benzodiazepines) or has medical conditions (e.g., lung disease, sleep apnea, liver disease, kidney disease, fall risk, advanced age) that increase risk for adverse outcomes
- The patient has been treated with opioids for a prolonged period (e.g., years), and current benefit–harm balance is unclear
(U.S. DHHS, 2019)
Delaware Guidelines for Use of Controlled Substances for Treatment of Pain
In April 2017, Delaware issued regulations for safer opioid prescribing and better pain management practices, supporting the CDC Guideline for Prescribing Opioids for Chronic Pain, 2016 (DDPH, 2025). (See above for the 2022 update to the CDC guidelines.)
These regulations are aimed at stopping addiction before it starts by controlling the amount of opiates given to new patients and aggressively monitoring their treatment. These Delaware opioid prescribing rules stipulate that first-time opiate prescriptions are not to exceed a one-week supply. If further opiate prescriptions are deemed necessary, then further action must be taken by the prescriber, including a query of the statewide Prescription Monitoring Program database and a physical exam with discussion of relevant patient history and the risks of opiates (DDOS, 2017).
HelpIsHereDE.com
HelpIsHereDE.com provides Delaware healthcare providers with resources that can help curb the opioid epidemic. These include:
- What is expected of providers
- How to prescribe opioids safely
- How to use non-opioid treatments for pain management
- How to prevent substance use disorder
- How to identify and appropriately refer patients struggling with addiction
- Benzodiazepines prescribing guidelines
- Sample treatment agreement (see above)
- Sample informed consent form (see above)
(DDPH, 2025)
KEY ELEMENTS OF DELAWARE PRESCRIBING RULES
For an acute episode (injury or procedure):
- A first-time prescription to an adult patient for an acute episode cannot exceed a seven-day supply.
- No prescription to a minor can exceed a seven-day supply at any time.
- If professional judgment dictates more than a seven-day supply is necessary:
- Document the condition triggering the prescription.
- Query the Prescription Monitoring Program to obtain a prescription history.
- Indicate that a nonopiate alternative was not appropriate.
- Obtain informed consent, which must include information on:
- The drug’s potential for addiction, abuse, and misuse
- The risks of life-threatening respiratory depression associated with the drug
- The potential for fatal overdose as a result of accidental exposure, especially in children
- Neonatal opioid withdrawal symptoms
- The potential for fatal overdose when interacting with alcohol
- Other potentially fatal drug interactions, such as with benzodiazepines
- Administer a fluid drug screen, at the discretion of the provider.
- Conduct a physical examination, which must include a documented discussion to elicit relevant history, explain risks/benefits of opioid analgesics and possible alternatives, and establish other treatments tried or considered.
- Schedule periodic follow-up visits and evaluations to monitor progress, whether there is an available alternative to opiate use, and whether to refer the patient for a pain management or substance abuse consultation.
For chronic, long-term treatment with an opioid:
- Document the condition triggering the prescription, indicating that a nonopioid alternative was considered but not appropriate, obtain informed consent, and follow up with evaluations to monitor progress toward treatment goals.
- Query the Prescription Monitoring Program:
- At least every six months and more frequently if clinically indicated
- Whenever the patient is also being prescribed a benzodiazepine
- Whenever the patient is assessed to potentially be at risk for substance abuse or misuse
- Whenever the patient demonstrates loss of prescriptions, requests for early refills, or similar behavior
- Administer fluid drug screens at least every six months.
- Obtain a signed treatment agreement, which must include:
- The patient’s agreement to take medications at the dose and frequency prescribed, with a specific protocol for lost prescriptions and early refills
- Reasons for which medication therapy may be re-evaluated, tapered, or discontinued, including but not limited to violation of the treatment agreement or lack of effectiveness
- The requirement that all chronic pain management prescriptions are provided by a single practitioner or a limited, agreed-upon group of practitioners
- The patient’s agreement to not abuse alcohol or use other medically unauthorized substances or medications
- Acknowledgment that a violation of the agreement may result in action as deemed appropriate by the prescribing practitioner such as a change in the treatment plan, a referral to a pain specialist, or referral to an addiction treatment program
- The requirement that fluid drug screens be performed at random intervals at the practitioner’s discretion, but no less than every six months
(DDPH, 2017)
PREVENTING PRESCRIPTION DRUG MISUSE
Various actions by healthcare providers can help prevent prescription drug misuse and diversion. These include:
- Educating patients on safe use, storage, and disposal of medications
- Understanding which drugs are commonly misused or diverted
- Recognizing aberrant drug-related behaviors (ADRB) (behaviors that may be associated with misuse of prescription opioids)
- Detecting and responding to drug diversion by patients
Institutional measures are also an important part of addressing the opioid epidemic, such as:
- Medication formulation and abuse-deterrent formulations
- Prescription drug monitoring programs (PDMPs)
- Surveillance systems
Teaching Safe Use, Storage, and Disposal of Prescription Medications
Educating patients on safe use, storage, and disposal of medications is an essential part of addressing the opioid and drug diversion epidemic. Nurses and prescribers can address the following points with patients who have been prescribed opioids.
SAFE USE
- Since opioids come in different forms, understand what type you were prescribed and read the information that comes with your prescription.
- Only take opioids prescribed to you and as directed by your healthcare provider; never take opioids that were not prescribed to you.
- Before you are prescribed opioids, tell your healthcare provider about all other medications and supplements you are taking.
- Do not drink alcohol while you are taking οрiоiԁѕ.
- Do not take οрiоidѕ with medicines that make you sleepy unless your healthcare provider tells you to. Examples include diazepam (Valium), alprazolam (Xanax), abapentin (Neurontin), pregabalin (Lyrica), baclofen, cyclobenzaprine, zolpidem (Ambien).
- Talk to your healthcare provider about your risk for overdose. Tell your healthcare provider if you or your family has a history of alcohol or drug addiction.
- Don’t share your medications with others because they may cause harm to someone else.
- Tell your healthcare provider if you experience changes in your mood, balance, sleep, or pain level and if you find it difficult to stop or decrease opioid use.
- Talk to your healthcare provider about whether it is safe to drive.
- Discuss with your healthcare provider alternative ways to manage your pain.
(AAFP, 2023; UpToDate, 2025)
SAFE STORAGE
Opioids are controlled substances, and their possession and use is regulated by state and federal laws. More than 70% of people who misuse prescription opioids obtain them from family and friends. Therefore, it is important that patients safely store their prescription medications. The CDC also recommends that prescribers discuss risks to household members and other individuals if opioids are intentionally or unintentionally shared with others for whom they are not prescribed, including the possibility that others might experience overdose at the same or at lower dosage than prescribed for the patient.
- Store opioids in their original packaging inside a locked cabinet, a lockbox, or other secure location.
- Do not store opioids in obvious places like bathroom cabinets or on kitchen counters where others might find them.
- Note when and how much medication you take in order to keep track of the amount left.
(AAFP, 2023)
SAFE MEDICATION DISPOSAL
Prescribers and pharmacists often provide specific disposal instructions for unused or expired medicines, and patients are educated to follow those instructions. There are a variety of ways to dispose of medications. The U.S. Food and Drug Administration (U.S. FDA, 2024a) outlines three options for drug disposal according to the type of drug: a take-back site, the flush list, or household trash.
Take-Back Programs
The best way to dispose of most types of unused or expired medicines (both prescription and over the counter) is to immediately drop off the medicine at a drug take-back site, location, or program. Pharmacies, firehouses, or police departments will often “take back” unused medications, particularly opioids. Some areas have specific dates on which they offer this service; other sites will take back medications at any time. Alternately, unused medicines can be sent using a prepaid drug mail-back envelope.
The Drug Enforcement Agency (DEA) website provides a locator app where the user can search for drug drop-off points within a 10- to 100-mile radius, and each year the DEA sponsors a National Prescription Take-Back Day. Delaware currently has permanent medication collection boxes located across the state. Expired, unused, or unwanted medication can be dropped off at these locations to ensure proper disposal (DDPH, n.d.-c).
(See “Resources” at the end of this course to locate a disposal location.)
Flushing Disposal
If a drug take-back option or DEA-authorized collector is not available and a medication is on the FDA flush list (see table below), the FDA recommends safely flushing such approved medications down the toilet. The medicines on the flush list are those sought after for their misuse and abuse potential or those that can result in death from one dose if inappropriately taken. For these reasons, FDA recommends that patients flush them down the toilet to immediately and permanently remove these risks from their home.
Based on the available data, FDA believes that the known risk of harm, including toxicity and death, to humans from accidental exposure to medicines on the flush list far outweighs any potential risk to human health and the environment from flushing these unused or expired medicines (U.S. FDA, 2024b; AAFP, 2023).
| Drug Name | Examples |
|---|---|
| Drugs That Contain Opioids | |
| (U.S. FDA, 2024b) | |
| Any drug that contains the word buprenorphine | Belbuca, Buavail, Butrans, Suboxone, Subutex, Zubsolv |
| Any drug that contains the word fentanyl | Abstral, Actiq, Duragesic, Fentora, Onsolis |
| Any drug that contains the words hydrocodone or benzhydrocodone | Apadaz, Hysingla ER, Norco, Reprexain, Vicodin, Vicodin ES, Vicodin HP, Vicoprofen, Zohydro ER |
| Any drug that contains the word hydromorphone | Exalgo |
| Any drug that contains the word meperidine | Demerol |
| Any drug that contains the word methadone | Dolophine, Methadose |
| Any drug that contains the word morphine | Arymo Er, Avinza, Embeda, Kadian, Morphabond ER, MS Contin, Oramorph SR |
| Any drug that contains the word oxycodone | Codoxy, Combunox, Oxadydo (formerly Oxecta), Oxycet, Oxycontin, Percocet, Percodan, Roxicet, Roxicodone, Roxilox, Roxybond, Targiniq ER, Troxyca ER, Tylox, Xartemis XR, Xtampza ER |
| Any drug that contains the word oxymorphone | Opana, Opana ER |
| Any drug that contains the word tapentadol | Nucynta, Nucynta ER |
| Drugs That Do Not Contain Opioids | |
| Any drug that contains the term sodium oxybate or sodium oxybates | Xyrem, Xywav |
| Diazepam rectal gel | Diastat, Diastat Acudial |
| Methylphenidate transdermal system | Daytrana |
Household Trash Disposal
If a drug take-back program is not available and a medication is not on the flush list, the FDA provides the following guidance on how to dispose of drugs via household trash:
- Remove the drugs from their original containers.
- Mix medicines (liquid or pills; do not crush tablets or capsules) with an unappealing substance such as dirt, cat litter, or used coffee grounds.
- Place the mixture in a container such as a sealed plastic bag.
- Throw away the container in the trash at home.
- Scratch out all personal information on the prescription label of empty medicine bottles or medicine packaging, then trash or recycle the empty bottle or packaging.
Even after fentanyl patches have been used, some medication remains. The FDA recommends promptly disposing of used patches by folding them in half with the sticky sides together and then flushing them down a toilet. Patches should not be placed in the household trash, where children or pets can find them.
Since inhalers are dangerous if punctured or if they come in contact with fire, they must be treated with care. Local trash and recycling facilities typically provide information on how to properly dispose of inhalers in their area (U.S. FDA, 2024a).
Understanding Commonly Abused/Misused Drugs
The Controlled Substances Act regulates five classes of drugs: narcotics/opioids, depressants, hallucinogens, stimulants, and anabolic steroids. All controlled substances have abuse potential or are immediate precursors to substances with abuse potential. The nonsanctioned use of these substances is considered drug misuse. The extent to which the drug is reliably capable of producing intensely pleasurable feelings (euphoria) increases the likelihood of that substance being abused (U.S. DEA, 2024b).
Three specific classes are most commonly abused and thus most susceptible to diversion for nonmedical use:
- Pain medications/narcotics. Opioid pain relievers (narcotics) are the most commonly diverted controlled prescription drugs. Opioid medications are effective for the treatment of pain and have been used appropriately to manage pain for millions of people; however, increased rates of abuse and overdose deaths have raised concerns about proper use of these medications in the treatment of chronic pain. Fentanyl is a potent synthetic opioid drug approved by the FDA for use as an analgesic and anesthetic; it is approximately 100 times more potent than morphine and 50 times more potent than heroin. Overdoses of narcotics are not uncommon and can be fatal.
- Central nervous system (CNS) depressants/sedatives/hypnotics. CNS depressants slow brain activity and are useful for treating anxiety and sleep disorders. Since many patients with pain also experience anxiety or sleep disturbances, increased prescribing of sedative hypnotics has paralleled the increase in prescribing of opioids. Clinicians who add sedative hypnotics to the treatment plan for chronic pain patients may potentiate the risk for patients who are also prescribed opioid medication. In 2023, 3.9 million people misused a prescribed CNS stimulant in the past year, down from 4.3 million in 2022 (SAMHSA, 2024).
- Stimulants. Stimulants are prescribed primarily for treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy. They may also be used as an adjunct medication in the treatment of depression. When taken nonmedically, stimulants can induce a feeling of euphoria and thus have a high potential for abuse and diversion. They also have a cognitive enhancement effect that has contributed to non-medical use by professionals, athletes, and other individuals who rely on productivity. Nonmedical use of stimulants poses serious health consequences, including addiction, cardiovascular events, and psychosis.
(NIDA, 2023; U.S. DEA, 2024b)
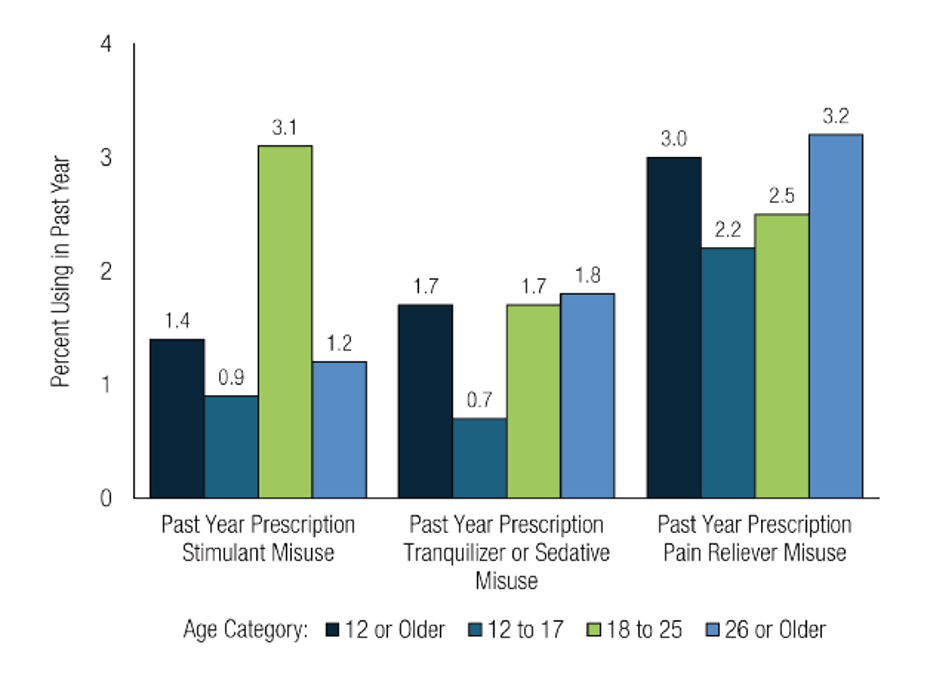
Past-year prescription stimulant misuse, past-year prescription tranquilizer or sedative misuse, or past-year prescription pain reliever misuse among people ages 12 or older, 2023. (Source: SAMHSA, 2024.)
SOURCES FOR MISUSED PAIN RELIEVERS, 2023
- 46.5%, prescription(s) from one or more providers
- 39.1%, free, bought, or taken from a friend/relative
- 8.0%, bought from drug dealer/stranger
- 0.5%, stole from doctor’s office, clinic, hospital, pharmacy
- 5.8%, some other way
(SAMHSA, 2024)
COUNTERFEIT PILLS
Criminal drug networks are mass-producing counterfeit pills and falsely marketing them as legitimate prescription pills. These counterfeit pills are easy to purchase, widely available, often contain fentanyl or methamphetamine, and can be deadly. They are easily accessible and often sold on social media and e-commerce platforms. Many are made to look like prescription opioids such as oxycodone (Oxycontin, Percocet), hydrocodone (Vicodin), and alprazolam (Xanax) or stimulants like amphetamines (Adderall).
Counterfeit pills have been seized in all 50 states and the District of Columbia. In 2024, DEA seized 5.36 million fentanyl pills and over 7,500 pounds of fentanyl powder, equivalent to more than 354 million doses. Five out of 10 pills tested in 2024 contained a potentially deadly dose of fentanyl, down from 7 out of 10 pills in 2023.
The DEA warns that the only safe medications are those obtained from licensed and accredited medical professionals and that pills purchased anywhere other than a licensed pharmacy are dangerous and potentially lethal.
(U.S. DEA, 2025)

Left: Authentic oxycodone M30 tablet. Right: Counterfeit oxycodone M30 tablet containing fentanyl. (Source: U.S. DEA.)
Recognizing Aberrant Drug-Related Behaviors
Some patients who are prescribed opioid pain medication are at increased risk for opioid misuse and diversion. These patients may demonstrate certain misuse behaviors that can provide clues to the clinician. By recognizing what are called aberrant drug-related behaviors (ADRBs), nurses can respond appropriately and help patients to remain safe.
ADRBs may occur because a patient is experiencing poor pain control or has a fear of uncontrolled pain, which can lead to hoarding of medication. The behaviors may also be attributed to elective use of opioid medication for the euphoric effect or for non-pain-related symptoms such as anxiety, depression, insomnia, and stress.
ADRBs in patients who are prescribed opioids should trigger clinicians to the possibility of addiction. Current literature suggests a range of aberrant drug-related behaviors, with some more predictive of addiction than others. Being aware of the behaviors described in the following box can help guide clinicians who are treating and monitoring patients who are receiving prescription opioid therapy for long-term pain management.
EXAMPLES OF ADRBs
- Altering the mode of administration of drug delivery
- Obtaining prescriptions from nonmedical sources
- Obtaining drugs from other prescribers without informing the clinician
- Stealing or borrowing drugs from others
- Concurrent drug/alcohol use
- Intoxicated/somnolent/sedated
- Occasional impairment
- Pattern of drug-related deterioration
- Medication misuse
- Overdose
- Repeated dose escalations even when warned
- Occasional unsanctioned dose escalation
- Unapproved use of the drug to treat other symptoms
- Unapproved use of drugs to treat nonpainful symptoms
- Repeated resistance to change despite adverse effects
- Noncompliance with therapeutic recommendations
- Increasing pain complaints
- Aggressive complaints about need for more or stronger medication
- Selling prescription drugs
- Prescription forgery
- Frequently lost prescriptions
- Inconsistent urine toxicology screen
- Unkempt appearance without other signs of impairment
- Request for early refills
- Request for specific drugs
- Request for refills instead of appointments with clinician
- Emergency department visits for pain medications
- Saving unused drugs for later use
- Canceled clinic visit
- Discharged from practice
- No show or no follow-up
(Maumus et al., 2020)
The presence of aberrant behaviors, however, may indicate a range of problems other than misuse or diversion, and the clinician must explore a differential diagnosis. Possible etiologies include addiction, pseudo-addiction, another psychiatric disorder, personality disorder, chronic boredom, mild encephalopathy, withdrawal states, and genuine undertreatment of pain. Therefore, it is important to monitor, document, and communicate any aberrant behaviors using objective means and in a team-based fashion over the patient’s entire course of care. This process will also remove any bias on the part of a single provider.
In the hospital setting, monitoring and responding to ADRBs is important in order to:
- Determine the success or failure of treatment
- Help prevent the transition of chronic pain to opioid dependence and SUD
- Help prevent psychiatric disorders such as anxiety and depression
- Identify undiagnosed SUD
- Identify patients at high risk for diversion
- Identify possible complications of opioid therapy
(Maumus et al., 2020)
RECOGNIZING DRUG SEEKING OR POTENTIAL DRUG DIVERSION BY PATIENTS
- Demands immediate attention
- Recites textbook symptoms
- Gives very vague medical history
- Exaggerates medical condition
- Wants appointments toward the end of office hours or arrives after regular business hours
- Not interested in an examination or undergoing diagnostic tests
- Unwilling to give permission to obtain past medical records
- Claims they failed to pack medication, lost it, or that it was stolen
- Claims that hospital or clinic with past medical records is out of business or burned down
- Deceives providers
- Seeks alternate providers while regular provider is out of the office
- Solicits Medicaid recipients to use Medicaid cards as payment method
- Offers to buy other patient’s pills
- Alters prescriptions
- Falsifies records
- Inflicts wounds to self, family members, and pets
- Requests specific medication due to allergies
- States they are vacationing in area, no local address
- Requests pain medications for a pet
(U.S. DEA, n.d.-a)
System Measures to Prevent Prescription Drug Misuse
System measures are also an important part of addressing the opioid epidemic. Several such measures are discussed below.
MEDICATION FORMULATION
Abuse-deterrent formulations (ADFs) have been developed to help reduce or prevent altered routes of administration—particularly chewing, crushing then smoking, or intravenous injection—used by individuals who misuse opioids. However, questions remain regarding whether prescribing ADF opioids reduces overall opioid misuse. ADFs do not prevent people from taking more than prescribed or change the drugs’ addictive properties.
Abuse-deterrent strategies currently being used include:
- Physical/chemical barriers that prevent chewing, crushing, cutting, grinding, or dissolving, or render it difficult to inhale or inject
- Agonist/antagonist combinations that cause an antagonist (which will counteract the effect of the drug) to be released if the product is manipulated
- Aversive substances that are added to create unpleasant sensations if the drug is taken in a way other than directed
- Delivery systems such as long-acting injections or implants that slowly release the drug over time
- New molecular entities or prodrugs that attach a chemical extension to a drug that renders it inactive unless taken orally
(Rosenquist, 2025)
| Compound | ADF mechanism |
|---|---|
| (Rosenquist, 2025) | |
| Oxycontin (oxycodone extended-release) and abuse-deterrent generic equivalent | Difficult to crush; if dissolved, the tablet forms a viscous gel that is difficult to inject IV. |
| Xtampza ER (oxycodone extended-release) | Capsules contain microspheres of oxycodone and inactive ingredients that hinder dosage dumping via intranasal and oral misuse; microspheres cannot be readily dissolved and will solidify within a needle to prevent injection. |
| Targin (oxycodone extended-release plus naloxone) | Contains naloxone (opioid antagonist), which is not active when taken orally but blocks opioid associated euphoria when injected or inhaled. |
| Roxybond (oxycodone immediate-release) | Difficult to crush; if dissolved, the tablet forms a viscous gel that is difficult to inject IV. |
| Hysingla ER (hydrocodone extended-release tablet) and abuse-deterrent generic equivalent | Difficult to crush; if dissolved, the tablet forms a viscous gel that is difficult to inject IV. |
PRESCRIPTION DRUG MONITORING PROGRAMS
Prescription drug monitoring programs (PDMPs) are statewide electronic databases that track controlled substance prescription. They can provide health authorities with timely information about both prescribing and patient behaviors, thereby improving opioid prescribing, informing clinical practice, and protecting patients at risk (CDC, 2024d).
Some states have implemented polices that require providers to check a state PDMP prior to prescribing certain controlled substances. When pharmacists dispense controlled substances to patients, they have to enter the prescription into the state PDMP. However, pharmacies submit this data to state PDMPs at varying intervals—ranging from monthly to daily to “real-time,” i.e., under five minutes.
The Delaware Prescription Monitoring Act (16 Del. C.§ 4798) authorizes the Office of Controlled Substances (OCS) in the Delaware Division of Professional Regulation to establish, maintain, and monitor the Delaware Prescription Monitoring Program (PMP). The purpose of the PMP is to reduce misuse of controlled substances in Delaware and to promote improved professional practice and patient care.
The PMP is a system that collects information on all controlled substance (schedules II–V) prescriptions. Using the PMP website, Delaware-licensed pharmacies and prescribers who dispense controlled substances report prescription data to the PMP daily. Prescribers and dispensers registered with the PMP can obtain immediate access to an online report of their current or prospective patient’s controlled substance prescription history. This PMP website is available 24/7 and is to be used for healthcare purposes only (DDPR, n.d.).
(See also “Resources” at the end of this course.)
PRESCRIBING AND ADMINISTERING OPIOID ANTAGONISTS (NALOXONE)
The availability of the opioid overdose-reversal drug naloxone has been shown to reduce the rate of these overdose deaths, and laws have been enacted in all U.S. states, including Delaware, to expand access to this life-saving medication.
DELAWARE LAWS ON OVERDOSE REVERSAL AGENT ACCESS
Delaware statutes related to naloxone access include:
- Title 16 § 138 (community-based access)
- Title 16 § 3002G–3006G (opioid antagonist access program)
- Title 18 § 3571X (health insurance requirements for medication-assisted treatment)
- Title 18 § 3343 (insurance for serious mental illness)
Substantive amendments to these laws include:
- July 20, 2017, amendment to title 16 § 3001G adds immunity protections for pharmacists who dispense naloxone.
- June 3, 2021, amendment to title 16 § 3001G provides that a lay individual who administers naloxone to an individual under the Community-Based Naloxone Access Program is considered to have provided emergency care.
- November 2, 2022, amendment to repealed title 16 § 3001G (eff. August 4, 2014) and replacement with title 16 §§ 3002G through 3006G.
- July 17, 2023, amendment to title 16 §§ 3003G expands authority of Director of the Division of Public Health to designate a licensed physician or registered advance-practice nurse to sign a treatment protocol or standing order for the administration of an opioid antagonist.
A statewide standing order for naloxone took effect June 27, 2018, and has been subsequently reauthorized. The standing order authorizes approved community-based training programs and participating pharmacies to distribute nasal naloxone kits to people who have completed opioid overdose responder training.
(LAPPA, 2025)
Prescribing Naloxone
Naloxone is an opioid antagonist that blocks opioid receptors. The drug comes in intravenous, intramuscular, and intranasal formulations and is FDA-approved for use in an opioid overdose and for the reversal of respiratory depression associated with opioid use. In 2023, the FDA approved over-the-counter (nonprescription) naloxone nasal spray (Narcan) (U.S. FDA, 2023). OTC Narcan is now available without a prescription in all states; Washington, DC; and Puerto Rico.
Naloxone can reverse the effects of an overdose from opioids, including:
- Heroin
- Morphine
- Oxycodone (Oxycontin)
- Methadone
- Fentanyl
- Hydrocodone (Vicodin)
- Codeine
- Hydromorphone
CDC guidelines for prescribing opioids recommend that naloxone be coprescribed to any individual who is prescribed high-dose opioid therapy (≥50 MME per day) or any combination of opioids and benzodiazepines. Recommendations also call for overdose prevention education to both patient and household members (Dowell et al., 2022).
Candidates for naloxone include individuals who are concerned about the risk of an opioid overdose, including people who use prescription or illicit opioids, their family members, friends, caregivers, and concerned members of the public. This includes patients:
- Receiving >50 mg morphine milligram equivalent (MME) of opioid treatment
- With a respiratory condition (e.g., COPD, asthma, sleep apnea)
- Who smoke marijuana, hooka, tobacco, etc.
- Being treated for opioid use disorder (DSM‐V)
- With a personal or family history of substance misuse (alcohol or drugs)
- Released after having experienced an opioid overdose
- Taking benzodiazepines, hypnotics, muscle relaxers, or other sedative use
- Being switched between opioids product formulations
- With difficult access to emergency services (e.g., rural)
- With heavy alcohol use
- By voluntary request from patient or caregiver
(SAMHSA, 2023b; WV DHHR, 2025b)
Patient Education Regarding Naloxone Administration
Patient education includes showing patients, their family members, or caregivers how to administer naloxone (see below). The medication can be given by intranasal spray or intramuscular, subcutaneous, or intravenous injection.
Individuals who are provided with an automatic injection device or nasal spray should keep the item available at all times. The medication must be replaced when the expiration date passes and if exposed to temperatures below 39 °F or above 104 °F.
Naloxone is effective if opioids are misused in combination with other sedatives or stimulants. However, it typically is not effective in treating overdoses of benzodiazepines or stimulant overdoses involving cocaine and amphetamines unless they are mixed with opioids, such as fentanyl (CDC, 2024e).
Since naloxone is a temporary treatment and its effects will wear off, medical assistance must be obtained as soon as possible after administering/receiving naloxone. Patients must be educated to immediately call 911 after recognizing a possible overdose and before administering naloxone. Individuals who have overdosed may again experience overdose symptoms once the effects of naloxone have worn off, typically after 30–90 minutes.
SIGNS OF OPIOID OVERDOSE
Recognizing the signs of opioid overdose can save a life. They include:
- Small, constricted “pinpoint pupils”
- Losing consciousness, unresponsiveness
- Slow, gargled breathing or no breathing
- Choking or gurgling sounds
- Limp body
- Cold or clammy skin
- Blue lips or nails
(CDC, 2024f)
Side effects of naloxone may include an allergic reaction from naloxone, such as hives or swelling in the face, lips, or throat, for which medical help should be sought immediately. Use of naloxone also causes symptoms of opioid withdrawal. Opioid withdrawal symptoms include:
- Feeling nervous, restless, or irritable
- Body aches
- Dizziness or weakness
- Diarrhea, stomach pain, or nausea
- Fever, chills, or goose bumps
- Sneezing or runny nose in the absence of a cold
Administering Naloxone
Following are instructions on how to administer naloxone.
Step 1. Identify an opioid overdose: Shout to see if the individual responds and gently shake their shoulder. Rub your knuckles on their upper lip or up and down the front of their rib cage.
Step 2. If the individual is unresponsive, call 911.
- State that the individual’s breathing has stopped and they are unresponsive.
- Provide the exact location of the individual.
- Indicate whether or not naloxone has already been given to the individual and if it helped.
Step 3. Perform rescue breathing.
- Place the individual on their back. Place one hand on their forehead and the other under their chin.
- Tilt their chin up gently to open the airway.
- Check to see if there is anything in their mouth blocking their airway, such as gum, a toothpick, undissolved pills, a syringe cap, a fentanyl patch, etc. If so, remove it.
- Pinch their nose with one hand and keep chin tilted up with the other hand. Create an airtight mouth-to-mouth seal and give two even, regular-sized breaths. Blow enough air into their lungs to make their chest rise. If the chest does not rise, make sure you pinch their nose and tilt their head back with each breath.
- Give one breath every five seconds.
Step 4. Give naloxone.
- Follow the instructions provided with the naloxone medication (see below).
- While getting ready to give naloxone, make sure you are not waiting too long between rescue breaths.
- After giving naloxone, continue rescue breathing with one breath every five seconds until emergency responders arrive.
- If victim is still not responding, additional doses of naloxone can be repeated at 2- to 3-minute intervals.
Step 5. Stay until help arrives: Naloxone can reverse an overdose but can also make someone enter withdrawal. After someone is given naloxone, make sure they do not take any more opioids, because they could go back into overdose after the naloxone wears off. They can also go back into overdose if they took a long-acting opioid. In these situations, repeat doses of naloxone may be needed.
(DDPH, n.d.-d)
ADMINISTRATION INSTRUCTIONS
Intranasal Naloxone
- Step 1. Pull or pry off yellow caps.
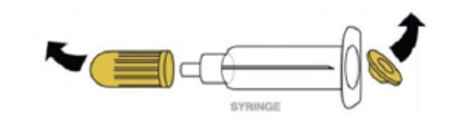
- Step 2. Pry off red cap.
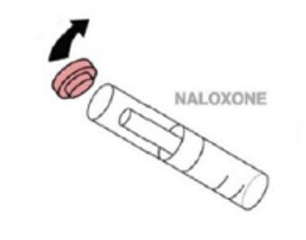
- Step 3. Grip the syringe.
- Step 4. Gently screw capsule of naloxone into barrel of syringe. Screw white cone (atomizer) onto top of syringe.

- Step 5. Insert white cone into nostril; give a short, vigorous push on end of capsule to spray naloxone into nose, one half of the capsule into each nostril. For best results, aim away from the middle of nose (toward ear).
- Step 6. If not breathing in 3 minutes, give a second dose.
Evzio Injectable Naloxone
- Step 1. Pull Evzio from outer case.
- Step 2. Follow the directions provided through the speaker in the device.
- Step 3. If not breathing in 3 minutes, repeat steps with the other device from the box.
- Note: May be injected through the person’s clothes.

Narcan Nasal Spray
- Step 1: Place nozzle in either nostril until your fingers touch the bottom of the person’s nose.
- Step 2. Point the nozzle toward the person’s ear (away from the middle of the face).
- Step 3. Press the plunger firmly to release the medication.
- Step 4. If the person is not breathing well after 3 minutes, repeat with the other device in the package.
![Narcan nasal spray.]](792/xnarcan-nasal-spray.png.pagespeed.ic.0qKAQ_4H4k.jpg)
(WV DHHR, n.d.)
HOW TO OBTAIN NALOXONE IN DELAWARE
Delaware’s Division of Public Health (DPH) has partnered with NEXT Distro, a nonprofit organization, to provide Narcan (naloxone) at no cost through the mail to Delaware residents. Naloxone is also available without a prescription at pharmacies throughout the state, at statewide distribution and training events, and through certain clinics. The goal is to remove barriers to obtaining this lifesaving medication, which can reverse an opioid overdose (HelpIsHereDE.com, 2025).
(See also “Resources” at the end of this course.)
SUBSTANCE MISUSE AND DRUG DIVERSION IN THE WORKPLACE
Nurses may be at increased risk for misuse or diversion of prescription medications due to working in environments where frequent and easy access to controlled substances is part of their daily work routine.
Drug Diversion among Healthcare Professionals
Drug diversion from the workplace is seen across all clinical disciplines and all levels of an organization, from management to frontline staff. Diversion may occur with opened or unopened vials, partially used doses of medication that are not wasted, and medication that has been disposed of and left in sharps containers. The drugs most commonly diverted from healthcare settings are opioids. While drug diversion is substantially underestimated, undetected, and unreported, an increasing number of drug diversion incidents has accompanied the rise of the opioid epidemic. For instance:
- In 2024, a nurse was sentenced to 12 months in prison for stealing fentanyl and hydromorphone intended for intensive care patients. She admitted to removing the pain medication from their vials, refilling the vials with tap water, and returning the vials to the controlled storage locker.
- In 2023, a nurse pled guilty to diverting controlled substances from her hospital for her own use, including falsely documenting she had administered medications to patients.
(Nyhus, 2021; HealthcareDiversion.org, 2025)
Practitioners, including prescribers, may also be motivated to divert drugs for several reasons:
- Money/financial gain
- Fear
- Stop blackmail
- Sexual favors
- Stay in business/codependency
- Addicted family members
- Personal use/misuse
(U.S. DEA, 2018)
RECOGNIZING WORKPLACE DIVERSION
Every nurse plays an important role in drug diversion prevention and should be able to recognize patterns, trends, and behaviors associated with drug diversion in the workplace. These may include:
- Consistently arriving early, staying late, or frequently volunteering for overtime
- Frequent breaks or trips to bathroom
- Heavy wastage of drugs
- Drugs and syringes in pockets
- Anesthesia record that does not reconcile with drug dispensed and administered to patient
- Patient with unusually significant or uncontrolled pain after anesthesia
- Patients with a higher pain score as compared to other anesthesia providers
- Times of cases that do not correlate with when provider dispenses drug from automated dispenser
- Inappropriate drug choices and doses for patients
- Missing medications or prescription pads
- Drugs, syringes, or needles improperly stored
- Signs of medication tampering, including broken vials returned to pharmacy
- Compromised product containers
- Frequent medication losses, spills, or wasting
- Controlled substances removed without a prescriber’s order
- Controlled substances removed on recently discharged or transferred patient
- Controlled substances removed for a patient not assigned to the nurse
- Medication documented as given but not administered to the patient
- Frequent reports of ineffective pain relief from patients
- Frequent unexplained disappearances from the unit
- Incorrect controlled substance counts
- Consistently documenting administration of more controlled substances than other nurses
- Numerous corrections on medication records
- Offers to medicate coworkers’ patients for pain
- Saving extra controlled substances for administration at a later time
- Altered verbal or phone medication orders
- Variations in controlled substance discrepancies among shifts or days of the week
(AANA, n.d.)
QUESTIONS TO IDENTIFY DIVERSION AMONG PRESCRIBERS
While none of the actions described below indicates definitively that a practitioner is engaged in diversion or illegal prescribing, asking the following questions may help identify possible diversion among practitioners:
- Does the practitioner follow state laws when prescribing controlled substances?
- Does the practitioner conduct cursory medical exams or any medical exam at all?
- Does the doctor perform diagnostic testing or refer patients out for diagnostic testing?
- Is the practitioner referring patients to other specialists (surgery, physical therapy, etc.)?
- Are the initial office visits or follow-up visits brief?
- Does the practitioner prescribe multiple drugs within the same drug category?
- Does the practitioner prescribe excessive quantities of controlled substances relative to the medical condition the prescription is purported to treat?
- Do patients travel a great distance to see the practitioner?
- Does the practitioner ignore signs of abuse?
- Patient appears to be under the influence
- Patient asks for the controlled substances they want
- Patient is doctor shopping in PDMP
- Practitioner is warned by family members that the patient is abusing or selling his controlled substances
- Does the practitioner ignore toxicology reports?
- Does the practitioner only treat patients with narcotic controlled substances?
- Does the practitioner start on a low-dose or low-level controlled substance and then over time work up to higher levels, or does the practitioner just start patients on a high-dose narcotic?
- Does the practitioner continue to prescribe controlled substances to patients even though it would be ineffective for treatment purposes?
- Does the practitioner allow the non-medical staff to determine the narcotic to be prescribed, and the practitioner just signs the prescription?
- Does the practitioner coach patients on what to say so that patients can get the narcotics that they want?
- Does the practitioner violate pain management policies and guidelines?
- Does the practitioner ignore warnings from insurance companies, law enforcement, other practitioners, family members, etc.?
- Does the practitioner receive other compensation for narcotic prescriptions (sex, guns, drugs, etc.)?
- Does the doctor still charge patients for visits if the patients do not receive narcotic prescriptions?
- Are patient deaths attributed to drug abuse or overdose?
- Does the practitioner use inventory for personal use?
(U.S. DEA, n.d.-a)
PREVENTING WORKPLACE DIVERSION
A comprehensive strategy to prevent the diversion of controlled substances includes efforts at the points in the medication management process where diversion may occur. Organizational prevention programs include elements across multiple levels:
- Administrative level: following all legal and regulatory requirements, providing organizational oversight and accountability
- System level: human resources management, automated dispensing technology, monitoring and surveillance, and investigation and reporting
- Individual level: chain of custody; storage and security; internal pharmacy controls; prescribing and administration; returns, waste, and disposal
(Clark et al., 2022)
Specific controls shown to be effective against diversion of controlled substances include:
- Following all policies and procedures
- Using the PDMP regularly
- Keeping complete and accurate records
- Limiting access to drug inventory
- Storing controlled substances in a securely locked cabinet
- Not sharing passwords
- Conducting audits to discover discrepancies, losses, or thefts
- Verifying destructions
- Training and updating staff
- Being vigilant of staff members
- Conducting background checks of prospective employees
- Conducting drug testing at hiring and random drug testing thereafter
- Questioning and reporting suspicious activities/transactions
- Using electronic prescriptions to reduce forged/altered/fraudulent scripts
- Never signing prescription blanks in advance
- Keeping prescription pads locked when not in use; not leaving prescription pads unsecured (e.g., in jacket pocket)
- Inspecting and numbering prescription pads
- Clearly indicating/writing the amount prescribed
(U.S. DEA, 2018)
Recognizing Impairment in the Workplace
Impairment from substance use disorder, drug diversion, or other physical or psychological causes has far-reaching impact. It not only threatens the health and safety of patients but also creates serious consequences for the impaired professional, colleagues, and the healthcare facility that employs the impaired clinician.
The Nurse Worklife and Wellness Study found that in the year prior to the study illicit drug use among nurses was 5.7% and misuse of prescription drugs was 9.9% (Trinkoff et al., 2022). The exact number of nurses afflicted is unknown, but the prevalence of addiction among nurses is believed to mirror the general population.
RISK FACTORS FOR SUBSTANCE ABUSE
Healthcare professionals may be at increased risk for abuse of prescription-type medication due to the added risk of working in environments where frequent and easy access to controlled substances is part of their daily work routine. For instance, evidence suggests the types of drugs abused by nurses may depend on what drugs are most accessible in their individual work environments. The most common prescription drugs abused by nurses are benzodiazepines and opioid painkillers such as fentanyl and hydrocodone. Nurses who abuse prescription drugs are those who have the greatest access, with the highest rates of abuse among nurse anesthetists (American Addiction Centers, 2023).
WORKPLACE RISK FACTORS
- High-stress work environment
- Low job satisfaction
- Role strain
- Long hours and irregular shifts
- Fatigue
- Periods of inactivity or boredom
- Remote or irregular supervision
- Easy access to controlled substances
- Lack of education regarding substance use disorders
- Nursing attitudes toward drugs
- Lack of pharmaceutical controls in the workplace
- “Enabling” by peers and managers
(Addictions.com, n.d.; Smith, 2021)
SIGNS OF SUBSTANCE ABUSE AND IMPAIRED PRACTICE
Impairment renders a clinician unsafe to provide patient care. Physical, psychosocial, and behavioral clues, however, can be subtle and easily overlooked. Colleagues may notice clues but seek other explanations and avoid suggesting substance abuse as a possible cause.
Generally, disruptions in family, personal health, and social life manifest long before an individual shows evidence of impairment at work. Thus, all indicators, no matter how subtle, appearing in the workplace must be taken seriously. Any of the following may be signs of impairment in the workplace, and patterns of such behavior and a combination of these signs are cause for increased suspicion.
| Type | Signs |
|---|---|
| (Nyhus, 2021; AANA, n.d.; Kopitnik & Siela, 2024) | |
| Physical |
|
| Psychosocial |
|
| Behavioral |
|
INTERVENING IN CASES OF IMPAIRED PRACTICE IN A COLLEAGUE
When planning to intervene in a case of suspected impairment, the first step is knowing Delaware state laws and rules pertaining to substance abuse and impairment in the workplace among licensed healthcare providers. It is also important to be familiar with and to follow an employer’s policies and procedures relating to substance abuse and impairment.
Healthcare professionals can follow these steps when they begin to notice possible impaired practice in a colleague:
- Observe job performance; be aware of signs and symptoms of impairment that are common in the workplace.
- Look for patterns of behavior indicating possible impairment that are consistent over a period of time.
- Document (date, time, place, witnesses) any inappropriate behavior; be concise and include objective, clear, and factual information:
- What happened?
- Who was involved?
- When did the incident occur?
- How was it discovered?
- Where did it occur?
- Were there any witnesses?
Supervisors should be involved in planning an intervention and taking steps to respond to concerns about impairment in the workplace.
- Planning and participating in an intervention are critical responsibilities of the manager, and it should never be implemented alone.
- It is important to develop a careful plan of action before implementing an intervention and also important to secure help.
- Interventions should focus on documented facts.
- The primary objective of an intervention is to request the clinician refrain from practice until a fitness-to-practice evaluation has been completed (IPN, 2023).
- To assure safety, a clinician who is impaired should never be left alone and should not be permitted to drive.
REPORTING IMPAIRED PRACTICE IN DELAWARE
Delaware Administrative Code, Title 24 §1900, Board of Nursing, describes regulations pertaining to impaired practice by nurses. Such practice is considered “unprofessional conduct” and grounds for discipline. It is defined as “practicing nursing when unfit to perform procedures and make decisions in accordance with the license held because of physical or mental impairment or dependence on alcohol or drugs.”
Under Delaware code, any licensed nurse is required to report, on a complaint form, names of subject individuals to the Board of Nursing if the nurse has reasonable cause to suspect that a nurse or an applicant has violated any of the grounds for discipline. This report must be made within 30 days of becoming aware of such information.
VOLUNTARY TREATMENT OPTION
As an alternative to discipline, the Delaware code also provides for a voluntary treatment option for licensed professionals determined to have violated the professional conduct standard related to impairment or dependence on alcohol or drugs. They may be permitted to continue to practice, subject to any limitations on practice established by the Board of Nursing and only if the nurse presents no danger to public health, welfare, or safety. The nurse must enter into and comply with all terms of a program agreement, which include but are not limited to:
- Enter and progress satisfactorily in a treatment program approved by the Board of Nursing
- Consent to progress reports from the treatment program to the Board
- Assume all costs and charges associated with the voluntary treatment option and treatment program(s)
- Comply with any terms or restrictions placed on professional practice
- Disqualification from the voluntary treatment option for failing to comply with the terms or to make satisfactory progress
(See Delaware Professionals’ Health Monitoring Program listed in the “Resources” section at the end of this course.)
RELUCTANCE TO REPORTING
There are many reasons why peers may be reluctant to report their colleagues for impaired practice, including:
- Uncertainty about reporting requirements
- Uncertainty of consequences to their peer, such as loss of license and job
- Concern about retaliation by peer
- Fear of social stigma of reporting a peer
- Reluctance to report if not 100% sure
CONCLUSION
Currently, there is an epidemic of prescription drug misuse, diversion, and overdose deaths not only in Delaware but also across the country. The complexity of this crisis creates challenges for federal, state, and local governments as well as nongovernmental partners who must confront the growing impacts on the community.
Overprescribing opioids for more than a decade has contributed to prescription opioid addiction and led to a sharp increase in opioid addiction, including overdose deaths. A multifaceted public health approach is necessary in order to effectively reduce opioid-related morbidity and mortality.
The opioid epidemic in this country has evolved and escalated along with an epidemic of chronic pain. With current evidence affirming that less-risky pain alternatives are just as effective as opioids for managing chronic pain, it is clear that there must be a cultural shift away from treating chronic pain with opioid medication.
Nurses are in a unique position to address this crisis with the right clinical skills and knowledge in assessment and management of addiction risk and best practices for safe opioid prescribing. A comprehensive approach that supports safe and effective pain management without increasing patient risk for addiction must be priority in every clinical practice setting.
Nurses are also responsible for the safety of patients, and it is every nurse’s duty to recognize and respond to impairment in the workplace. This requires that all nurses understand substance use disorder as an occupational hazard and be able to recognize signs and behaviors associated with impairment. It also requires nurses to be vigilant about their own risk and take steps to support healthy work environments that encourage education about substance use disorder and support for nurses who need help.
RESOURCES
American Society of Addiction Medicine
CDC Clinical Practice Guideline for Prescribing Opioids for Pain
Delaware Prescription Monitoring Program (PMP)
Delaware Professionals Health Monitoring Program
Get Smart about Drugs (DEA)
Medication collection sites in Delaware
Opioid safety: Veteran/patient education (Veteran’s Administration)
Overdose prevention (CDC)
Screening and assessment tools chart (National Institute on Drug Abuse)
Substance use disorder (AANA)
REFERENCES
NOTE: Complete URLs for references retrieved from online sources are provided in the PDF of this course.
Addictions.com. (n.d.). Nurses and workplace substance abuse risk factors. https://www.addictions.com
American Academy of Family Physicians (AAFP). (2023). Safe use, storage, and disposal of opioid drugs. https://familydoctor.org
American Addiction Centers. (2023). Prescription drug use among nurses. https://americanaddictioncenters.org
American Association of Nurse Anesthesiology (AANA). (n.d.). Signs & behaviors: If you see something, do something. https://www.aana.com
American Psychiatric Association (APA). (2024). Addiction and substance use disorders. https://www.psychiatry.org
Becker WC & Starrels JL. (2024). Prescription drug misuse: Epidemiology, prevention, identification, and management. UpToDate. https://www.uptodate.com
Centers for Disease Control and Prevention (CDC). (2025). Drug overdose mortality by state, 2022. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2024a). U.S. overdose deaths decrease in 2023, first time since 2018. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2024b). Overdose prevention: Commonly used terms. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2024c). Illicit drug use. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2024d). Prescription drug monitoring programs (PDMPs). https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2024e). 5 things to know about naloxone. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2024f). Lifesaving naloxone. https://www.cdc.gov
Clark J, Fera T, Fortier C, Gullickson K, Hays A, Murdaugh L, Ogden R, et al. (2022). ASHP guidelines on preventing diversion of controlled substances. Am J Health-Syst Pharm,79(24), 2279–2306. https://doi.org/10.1093/ajhp/zxac246
Crozer Health. (2022). PQRST pain assessment method. https://www.crozerhealth.org
Delaware Department of State (DDOS), Division of Professional Regulation. (2017). Uniform controlled substance act regulation. 16 Delaware code, Section 4731. https://dprfiles.delaware.gov
Delaware Division of Professional Regulation (DDPR). (n.d.). Delaware prescription monitoring program. https://dpr.delaware.gov
Delaware Health and Social Services, Division of Public Health (DDPH). (2025). Help is here: Health care providers. https://www.helpisherede.com
Delaware Health and Social Services, Division of Public Health (DDPH). (2017). Delaware prescription opioid guidelines for health care providers. https://dprfiles.delaware.gov
Delaware Health and Social Services, Division of Public Health (DDPH). (n.d.-a). Sample treatment agreement. https://ddph-materials.s3.amazonaws.com
Delaware Health and Social Services, Division of Public Health (DDPH). (n.d.-b). Sample informed consent agreement. https://ddph-materials.s3.amazonaws.com
Delaware Health and Social Services, Division of Public Health (DDPH). (n.d.-c). Medication collection sites in Delaware. https://www.dhss.delaware.gov
Delaware Health and Social Services, Division of Public Health (DDPH). (n.d.-d). Guidance for patients and caregivers: How to administer naloxone. https://www.dhss.delaware.gov
Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. (2022). CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep, 71(RR-3), 1–95. http://dx.doi.org/10.15585/mmwr.rr7103a1
Dydyk AM. (2023). West Virginia opioid prescribing for chronic pain while avoiding drug diversion. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
HealthcareDiversion.org. (2025). Prevent nurse diversion. https://healthcarediversion.org
HelpIsHereDE.com. (2025). Where can I get Narcan? https://www.helpisherede.com
Intervention Project for Nurses (IPN). (2023.). Employer information. https://www.ipnfl.org
Kopitnik NL & Siela D. (2024). Recognizing alcohol and drug impairment in the workplace in Florida. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
Legislative Analysis and Public Policy Association (LAPPA). (2025). Overdose reversal agent access: Summary of state laws. https://legislativeanalysis.org
Maumus M, Mancini R, Zumsteg DM, & Mandali DK. (2020). Aberrant drug-related behavior monitoring. The Ochsner Journal, 20(4), 358–61. https://doi.org/10.31486/toj.20.0108. https://www.ncbi.nlm.nih.gov
National Institute on Drug Abuse (NIDA). (2023). What classes of prescription drugs are commonly misused? https://nida.nih.gov
Nyhus J. (2021). Drug diversion in healthcare. https://www.myamericannurse.com
Rosenquist R. (2025). Abuse-deterrent opioids. UpToDate. https://www.uptodate.com
Smith J. (2021). The risk of substance use among nurses and nursing students. AddictionResource.net. https://www.addictionresource.net
Strain E. (2024). Opioid use disorder: Epidemiology, clinical features, health consequences, screening, and assessment. UpToDate. https://www.uptodate.com
Substance Abuse and Mental Health Services Administration (SAMHSA). (2024). Results from the 2023 national survey on drug use and health: Graphics from the key substance use and mental health indicators report. https://www.samhsa.gov
Substance Abuse and Mental Health Services Administration (SAMHSA). (2023a). Highlights for the 2023 national survey on drug use and health. https://www.samhsa.gov
Substance Abuse and Mental Health Services Administration (SAMHSA). (2023b). Non-prescription (“over-the-counter”) naloxone frequently asked questions. https://www.samhsa.gov
Toney-Butler T. (2023). Nursing admission assessment and examination. StatPearls [Internet]. https://www.statpearls.com
Trinkoff AM, Selby VL, Han K, Baek H, Steele J, Edwin HS, Yoon JM, & Storr CL. (2022). The prevalence of substance use and substance use problems in registered nurses: Estimates from the Nurse Worklife and Wellness Study. Journal of Nursing Regulation, 12(4), 35–46.
U.S. Department of Health & Human Services (U.S. DHHS). (2019). HHS guide for clinicians on the appropriate dosage reduction or discontinuation of long-term opioid analgesics. https://www.hhs.gov
U.S. Drug Enforcement Administration (U.S. DEA). (n.d.-a). Potential diversion. https://www.deadiversion.usdoj.gov
U.S. Drug Enforcement Administration (U.S. DEA). (2025). One pill can kill. https://www.dea.gov
U.S. Drug Enforcement Administration (U.S. DEA). (2024b). Drugs of abuse: A DEA resource guide. https://www.getsmartaboutdrugs.gov
U.S. Drug Enforcement Administration (U.S. DEA). (2018). Practitioner diversion awareness conference: Methods of diversion. https://www.deadiversion.usdoj.gov
U.S. Food and Drug Administration (U.S. FDA). (2024a). Disposal of unused medicines: What you should know. https://www.fda.gov
U.S. Food and Drug Administration (U.S. FDA). (2024b). Drug disposal: Dispose “non-flush list” medicine in trash. https://www.fda.gov
U.S. Food and Drug Administration (U.S. FDA). (2023). FDA approves first over-the-counter naloxone nasal spray. https://www.fda.gov
UpToDate. (2025). Patient education: Taking opioids safely (the basics). https://www.uptodate.com
West Virginia Department of Health and Human Resources (WV DHHR). (n.d.). Save a life: How to use naloxone to reverse an opioid overdose. https://dhhr.wv.gov
West Virginia Department of Health and Human Resources (WV DHHR). (2025b). Naloxone. http://sempguidelines.org






Customer Rating
4.9 / 305 ratings