Diabetes Care: Prevention and Clinical Care of Diabetic Foot Ulcers
Online Continuing Education Course

Course Description
Diabetes and wound care course on identifying and treating diabetic foot ulcers in patients. This online continuing education course is applicable for nursing, occupational therapy, and physical therapy. Course covers all aspects of diabetic foot care, including prevention, foot assessment, management of foot ulcers, and amputation. A diabetic foot ulcer picture is shown in the course.
Note: This course is also available as part of a package. See Nursing CEU Bundle - 30 ANCC Hours
Course Price: $47.00
Contact Hours: 9
Case Management Contact Hours: 6.75
Course updated on
March 4, 2025
"Excellent course! Well organized given the amount of information covered." - Renee, multi-state licensed RN
"This course was very informative. It presented many different treatment options/adjustments for the client with this diagnosis, which will help me in my field of work." - Brynn, COTA in Missouri
"This was an extremely good course on this disease, which is increasing and impacting negatively on our communities and health system." - JoAnne, RN in Ohio
"I enjoyed this course and feel like it helps to keep my mind fresh regarding the type of patients I see in the LTC setting." - Tabatha, OTA in Kentucky
Diabetes Care: Prevention and Clinical Care of Diabetic Foot Ulcers
Copyright © 2025 Wild Iris Medical Education, Inc. All Rights Reserved.
LEARNING OUTCOME AND OBJECTIVES: Upon completion of this course, you will have gained up-to-date knowledge to care for individuals at risk for developing diabetic foot ulcers and to assess and treat those with diabetic foot ulcers. Specific learning objectives to address potential knowledge gaps include:
- Identify the prevalence and impacts of diabetic foot ulcers.
- Describe the elements of an interdisciplinary approach to care.
- Discuss the importance of effective patient teaching.
- Summarize the importance of preventive measures for diabetic foot care.
- Describe the role of diabetic peripheral neuropathy and Charcot osteoarthropathy in the development of diabetic foot ulcers.
- List the steps that comprise a foot assessment in patients with diabetes.
- Summarize the management program for patients with diabetic foot ulcers.
- Describe the important components of off-loading in the prevention and treatment of diabetic foot ulcers.
- Discuss amputation as it relates to diabetic foot ulcers.
TABLE OF CONTENTS
- Introduction
- Interdisciplinary Approach to Care
- Patient Teaching
- Preventive Foot Care for Patients with Diabetes
- Diabetes Complications and Foot Ulcer Prevention
- Assessment of Patients with Diabetic Foot Ulcer
- Management of the Diabetic Foot Ulcer
- Off-Loading
- Amputation
- Conclusion
- Resources
- References
INTRODUCTION
A diabetic foot ulcer is defined as a full-thickness wound that penetrates through the skin and dermis, the most frequent sites for a diabetic foot ulcer being the plantar surface of the foot and the toes (Armstrong & Lavery, 2024; APMA, 2024).
A diabetic foot ulcer (DFU) is the most frequently occurring complication associated with diabetes and one that healthcare providers will encounter across the continuum of care. Diabetic foot ulcers are complex, chronic wounds that are often disabling and greatly impact the morbidity and mortality of patients. Patients who develop a DFU are at higher risk of early death, heart attack, and fatal stroke than people with diabetes who do not develop diabetic foot ulcers. Data show that 40% of persons with a healed DFU will develop another ulcer within a year and 60% within 3 years (Conyers & Swoboda, 2023).
Diabetic foot ulcers can occur in patients with either type 1 or type 2 diabetes.
History of Diabetes
Diabetes is one of the oldest diseases known to humanity and was first documented by an Egyptian physician around 1552 BCE. The term diabetes is Greek and means “to siphon.” It was first used around 250 BCE by the Greek physician Aretaeus, who noted how quickly diabetes drained fluid from those affected by it.
By the fifth century, the Chinese had noted that people with diabetes were more prone to infection, and in Baghdad in the fifth century, it was observed that people with diabetes developed gangrene in their extremities. In China and India, they realized that there was a difference between type 1 and type 2 diabetes, with the latter occurring more frequently in overweight, wealthy individuals compared to their less well-off counterparts. During the 18th century, diabetes was discovered to be a systemic disease.
In the Middle Ages, it was thought that diabetes was a disease of the kidneys, but it was discovered in the late 18th century that diabetes afflicted those who had pancreatic damage. It was also observed that, while diabetes was fatal in some persons, it remained a chronic disease in others, further clarifying the distinction between type 1 and type 2 diabetes (March et al., 2022).
In the early 19th century, it was found that restricted diets were beneficial to persons with diabetes and that calorie intake was important to the progress of the disease (Diabetes.co.uk, 2019). In the 20th century several discoveries and advances were made in the diagnosis and treatment of diabetes. Several of the most notable are described in the table below.
| (ADA, 2014) | |
| 1916 | Dr. Elliot Joslin published the first edition of The Treatment of Diabetes Mellitus. Dr. Joslin was known worldwide as a clinician and educator and one of the leading advocates in diabetes care. |
| 1921 | Dr. Frederick Banting and Dr. Charles Best (Banting’s student at the time) discovered insulin. |
| 1923 | Eli Lilly and Company started producing insulin commercially. |
| 1936 | Sir Harold Percival Himsworth published a research paper that differentiated between type 1 and type 2 diabetes. |
| 1940 | The American Diabetes Association was created to address the increasing prevalence of diabetes and the complications that develop as a result of the disease. |
| 1949 | Dr. Rachmiel Levine discovered that insulin transports glucose into the cells in the body. |
| 1972 | The association between elevated blood sugar levels and disease of the blood vessels was reported. |
| 1978 | The first human-based insulin was developed and named Humulin. It has the exact same structure as human insulin. |
| 1989 | The American Diabetes Association announced its first Standards of Care to better assist physicians in the treatment of diabetes. |
| 1993 | The Diabetes Control and Complications Trial demonstrated that keeping blood sugar levels as close as possible to normal slowed the onset of diabetic complications. |
| 1996 | The first short-acting insulin, Lispro, was launched in the market. |
| 1990s | External insulin pumps were invented. |
After the discovery of insulin, the treatment of diabetes was revolutionized. With further advances in refining and producing insulin commercially, diabetes evolved from being an acute condition with a limited life expectancy to being a chronic condition. Consequently, patients’ longer lifespans resulted in their developing complications related to diabetes, such as diabetic foot ulcers, that required new practices to address them.
Types of Diabetes
Diabetes is not a single disease; rather, it is a condition that includes several metabolic disorders caused by problems with insulin secretion, the manner in which insulin acts in the body, or a combination of both of these.
Diabetes has several forms. Three of the most frequently occurring forms are:
- Type 1 diabetes (previously referred to as juvenile-onset diabetes) usually occurs in children and young adults. Between 5% and 10% of people with diabetes have type 1. Type 1 diabetes occurs when the body is unable to produce insulin due to an autoimmune destruction of the insulin-producing cells (beta cells) in the pancreas.
- Type 2 diabetes (previously referred to as adult-onset diabetes) comprises approximately 90% of all people with diabetes. Type 2 diabetes results from a combination of factors, insulin resistance, and a progressive reduction in insulin secretion. Previously, this form of diabetes was typically associated with older adults, but it is now occurring more frequently in young people, adolescents, and children.
- Gestational diabetes is found in women during the second and third trimesters of pregnancy, and it can continue beyond delivery. Its symptoms resemble those of type 2 diabetes, and women who experience gestational diabetes are more prone to develop type 2 diabetes later in their lives.
(WHO, 2024)
Epidemiology of Diabetes and Diabetic Foot Ulcers
Type 2 diabetes is now the most rapidly growing chronic condition globally, with over 300 million people worldwide affected. These figures are projected to almost double by the year 2035. Since the possibility of developing diabetes increases with age, the percentage of older adults with diabetes is set to increase along with the increasingly older population. According to the World Health Organization (WHO) the number of persons worldwide with diabetes has increased from 200 million in 1990 to 830 million in 2022. In 2021, diabetes and kidney disease deaths due to diabetes were over 2 million. The prevalence of diabetes has been increasing swiftly in low- and middle-income countries (WHO, 2024).
The impact of diabetes and the complications arising from it are expected to cause greater damage and have more devastating consequences in developing countries compared to those that are affluent. Data indicate that persons with diabetes in developing countries have a higher incidence of cardiovascular disease, in part due to uncontrolled hypertension and uncontrolled dyslipidemia. Healthcare systems in these parts of the world also do not have the capacity for routine screening for complications related to diabetes, such as retinopathy and kidney disease. Diabetic foot ulcers and lower-limb amputations are also more prevalent in developing countries (Jadheea-Jutton et al., 2022).
A Global Overview of Diabetes Care
There is a wide variance in the diagnosis and treatment of diabetes and diabetic foot ulcers in different parts of the world. A prevailing factor across the developing world is the lack of diabetes education for patients and the lack of up-to-date education on diabetes care for healthcare providers. According to the International Diabetes Federation, 20% of healthcare providers worldwide don’t receive postgraduate training in diabetes care (Smokovski, 2021).
ASIA
The causes of diabetic foot ulcers are related to inadequate diagnosis, often not occurring until foot problems are advanced and serious infection is present.
- In China, research shows that there is a scarcity of foot clinics (Li et al., 2023).
- India is noted to have a significant number of persons with diabetes and a high incidence of lower-extremity amputations (Zubair et al., 2021).
AFRICA
Data show that around 24 million people in Africa have diabetes, and more than half of those with the condition remain undiagnosed. According to the World Health Organization, only 36% of countries in Africa have the necessary medications for chronic disease treatment available in public hospitals (WHO, 2023). The care of diabetic foot ulcers in Africa is complicated by several factors, including:
- There is an extended time period from the development of a foot ulcer to treatment by a healthcare professional.
- Seeking treatment from traditional healers is widely prevalent, as is self-treatment of wounds.
- Knowledge deficits of treatments among healthcare workers contribute to poor outcomes among patients with diabetic foot ulcers.
(Zubair et al., 2021)
AUSTRALIA
In Australia the yearly occurrence rate of diabetic foot ulcers in persons with diabetes is 2%. There is a rising prevalence in the numbers of those with diabetes, attributed mainly to an increase in obesity rates and an aging population. Members of the Aboriginal population who have diabetes are considered to be at the greatest risk for developing diabetic foot ulcers, with a threefold to sixfold increased probability of developing diabetes-related foot conditions (McNeil, 2023).
EUROPE
Approximately 55 million people in Europe have a diagnosis of diabetes, and 8 million of these are at risk for developing a diabetic foot ulcer. Geographic disparities relating to diabetic foot care include:
- Only a small number of countries in Eastern Europe have foot clinics and offer podiatry care.
- Regardless of the differences in healthcare across Europe, delays in referrals for specialized foot care are common.
(Zubair et al., 2021)
Diabetes in the United States
Statistics show that 11.6% of the population in the United States had diabetes in 2021, with the figures for prediabetes at 97.6 million. About 8.7 million people with diabetes remained undiagnosed. Diabetes was found to be the eighth leading cause of mortality (ADA, 2023b).
Neuropathy is one of the most prevalent risk factors for diabetic foot ulcers (Baranoski & Ayello, 2020). Statistics indicate that approximately 15% of individuals with diabetes that is complicated with neuropathy will develop a diabetic foot ulcer at some point during their lives. Between 14% and 24% of those who develop a diabetic foot ulcer will progress to amputation (WOCN, 2022).
U.S. research data also indicate that approximately 85% of lower extremity amputations are a consequence of diabetes and begin with a diabetic foot ulcer. After an amputation, the mortality rate rises according to the level of amputation (the higher up on the extremity the amputation occurs, the greater the mortality rate) and varies from 40%–70% at the five-year mark postamputation. This places the mortality rate associated with amputations in the same category as that from several malignancies (WOCN, 2022).
DIABETES AMONG YOUTH
Approximately 352,000 youth under the age of 20 are assessed to have a diagnosis of diabetes (ADA, 2023b). When type 2 diabetes occurs in children and adolescents, it is a much more aggressive disease than in adults. Sizeable cohort findings demonstrate that the rate of diabetes-related complications in children and youth with type 2 diabetes (T2D) is higher compared to the rate of complications in adults and compared to youth with type 1 diabetes (T1D) (Pinhas-Hamiel & Zeitler, 2023). Thus, clinicians will be treating much younger patients for the complications of diabetes, including diabetic foot ulcers.
DISPARITIES AMONG ADULTS WITH DIABETES
The CDC has set up the Division of Diabetes Translation (DDT) as a nationwide effort to assist people to prevent and manage diabetes and to tackle health inequalities and disparities in treatment for those with diabetes. Studies show that those living in rural areas have a higher incidence of diabetes when compared to those living in urban communities, even as 62% of rural counties have restricted access to diabetes self-management education and support services (DSMES) (CDC, 2024a).
Data for 2021 demonstrate that identified diabetes for both men and women had the highest prevalence rates among American Indian and Alaska Native adults (13.6%). The prevalence of diabetes was also shown to be high among non-Hispanic Black adults (12.1%), followed by adults of Hispanic origin (11.7%), non-Hispanic Asian adults (9.1%), and non-Hispanic White adults (6.9%) (CDC, 2024b).
Socioeconomic status also plays a significant role in the prevalence of type 2 diabetes. Research reveals that living in poverty is a factor that can double or triple the risk of developing diabetes. The risk for uncontrolled diabetes increases when patients barely have the resources to pay for their basic needs of housing, food, and utilities in addition to difficulties in paying for medications and supplies to monitor blood sugar levels. Living in poverty also increases the likelihood of developing complications related to diabetes (Hill-Briggs et al., 2021).
Foot ulcers are the most serious, disabling, and costly complication of diabetes. They most frequently occur in older adults who have had diabetes for several years. Patients aged 65 years and over with diabetes have a twofold increase in the risk of developing a foot wound and also have the highest number of major lower-extremity amputations resulting from diabetes (Zubair et al., 2021; WOCN, 2022).
ECONOMIC IMPACT
Diabetic foot ulcers are costly to treat. Studies show that patients with diabetic foot ulcers have more emergency room visits, spend more days in the hospital, and require more home healthcare services than patients with diabetes but without diabetic foot ulcers (WOCN, 2022; Baranoski & Ayello, 2020).
The overall estimated cost of diabetes care in the United States for 2022 was $412.9 billion. Cost analysis shows that care for those diagnosed with diabetes accounts for 1 in 4 healthcare dollars spent in this country (ADA, 2023a).
INTERDISCIPLINARY APPROACH TO CARE
Diabetic foot care at all stages, both preventive and in treating a diabetic foot ulcer, is based on a multidisciplinary team approach. At different stages of care the members of this team may vary. Various healthcare professionals have many overlapping roles. According to their training, practitioners from each discipline can make important contributions to the care of the patient.
In some cases, patients may be reluctant to agree to a team approach due to the time involved in attending multiple appointments. As far as reasonably possible, patient appointments with multiple providers should be scheduled for the same day at the same location.
Primary Care
Primary care providers, including nurse practitioners, are familiar with the complications that can arise from diabetic foot ulcers and the evidence-based treatments that will prevent their development. Many patients with diabetes will be treated by a primary care provider, while others may be referred to an endocrinologist.
The primary care provider plays a key role in ensuring that all members of the patient’s healthcare team are aware of medical decision-making and the patient’s current status. For example, if the primary care provider believes that the time is drawing close to switch the patient from oral medication to insulin to maintain adequate control of their diabetes, then this information will be shared with the other team members so that each clinician can help prepare the patient for the transition.
Nursing
Nurses play a pivotal role in caring for patients with diabetes. In virtually every practice setting, nurses encounter patients who have diabetes and must be equipped with the knowledge and skills to care for these patients. As well as providing hands-on care, nurses function as educators, patient advocates, and coordinators of patient care. They also provide the patient with emotional support and recognize that the complexity of diabetic treatment, self-management, and the multiple goals and lifestyle changes patients have to work toward demand a tremendous amount of both physical and psychological adjustment and require consistent, empathetic support.
The nursing role in diabetic foot care includes:
- Patient assessment and screening
- Education
- Advocacy
- Coordination of patient care
- Community outreach
Physical Therapy
Physical therapy professionals assist patients with diabetes through all stages of care, starting with preventive foot care. In diabetic foot care, physical therapists play a major role in the following areas:
- Joint mobility
- Strengthening
- Assessment and management of skin integrity
- Management of swelling (edema)
A physical therapist can create an individualized exercise program with the patient, including aerobic and resistance exercises to help the patient meet weight-loss and activity goals. Other interventions used by physical therapists to treat diabetic neuropathy and/or ulcers may include the following:
- Thermotherapy (infrared, global heat, ultrasound)
- Electrotherapy (e-stim, shockwave therapy, laser treatment, magnetic field treatment, galvanic current treatment)
- Therapeutic exercise (joint range of motion, stretching, Buerger-Allen exercise, proprioception/balance exercises)
- Shoe/footwear modification
- Prophylactic patient education (skin inspection, foot/toenail care, etc.)
- Wound care and/or debridement (within state-specific scope of practice)
- Patient/caregiver education
- Home/environmental modification recommendations
(APTA, 2021)
Musculoskeletal complications, which affect the foot in many patients with long-standing diabetes, put patients at elevated risk for developing skin ulcers. Alterations in the structure of the foot, including loss of flexibility and limited joint mobility, impede the foot’s ability to absorb and redistribute forces related to impact while walking. Foot deformities play a major role in increasing plantar pedal pressure, particularly at the metatarsophalangeal and subtalar joints (Byrant & Nix, 2023).
Physical therapists bring a wide range of skills beneficial in the care of patients with diabetes-related foot conditions. Physical therapists can identify early structural changes in the foot, evaluate a patient’s gait, and assess for signs of ulcerations. Physical therapists work closely with patients who have diabetes to minimize balance dysfunction that can occur with peripheral neuropathy. They can advise patients on footwear, inserts into existing shoes, and techniques for weight distribution along the surface of the foot. They also provide functional mobility training (Bryant & Nix, 2023).
Problems with balance are a frequent finding among patients with diabetic peripheral neuropathy (DPN). Diminished ankle, foot, and plantar muscle ability and impaired range of motion are related to compromised balance and movement in patients with DPN. Studies show that strength exercises improve proprioception and muscle strength in this population, resulting in positive changes in standing balance. Studies have also shown that exercise therapy led to substantial improvement in functional balance for patients with peripheral neuropathy (Akbari & Naimi, 2022).
Occupational Therapy
Occupational therapists are actively involved in diabetic foot care in a variety of clinical settings: acute care, long-term care, clinics, and community care. Apart from the functions that occupational therapists share with other members of the diabetic healthcare team, they address important areas of everyday life for patients with diabetes, including physical, cognitive, psychosocial, and sensory aspects.
Occupational therapists are instrumental in assisting patients with diabetes to integrate self-care activities into their existing routines. Occupational therapists also help patients overcome barriers to community participation, which is especially important to patients with limited financial resources. Studies show that occupational therapy interventions enhance the quality of life for patients with diabetes and play a positive role in patient adherence to treatment.
Occupational therapy interventions in diabetic foot care include:
- Assessing the patient’s skills and competencies
- Assessing the patient’s environment and need for adaptation
- Educating the patient on pacing and energy conservation techniques
- Teaching the patient how to use adaptive equipment
- Assisting the patient with work and community participation
- Teaching stress management techniques
- Advising the patient on meal planning and preparation
(Hill, 2023)
One successful occupational therapy program used with patients who have diabetes is called Resilient, Empowered, Active Living with Diabetes (REAL). The aim of the REAL program is to aid patients who are facing challenges in performing diabetes self-management activities by developing individualized interventions, particularly in young adults with T1D (Pyatak et al., 2023).
Case Management
A case manager or social worker—although not directly involved in hands-on care of the patient—is an essential member of the diabetic foot care team. Case management in diabetic foot care provides:
- Patient assessment
- Input into the development of the patient plan of care
- Facilitation of services
- Patient advocacy
The case manager has clinical knowledge regarding the management of the diabetic foot and the treatment and management of diabetic foot ulcers should they develop.
The case manager or social worker helps the patient in navigating the healthcare system and identifying what resources are available, for example, insurance or Medicare benefits to purchase diabetic shoes or adaptive equipment.
For patients concerned about how their diagnosis and treatment will affect their employment, the case manager or social worker educates the patient on their rights under the Family Medical Leave Act (FMLA) and how to apply for FMLA leave through the human resources department at their place of employment. The case manager can also be instrumental in advocating for workplace modifications with an employer.
Diabetes Educator
Clinicians who specialize in diabetes care and education can become Certified Diabetic Educators (CDE) through the Certification Board for Diabetes Care and Education (formerly the National Certification Board for Diabetes Educators), founded in 1986. This qualification is available to nurses, occupational therapists, physical therapists, and other professionals who meet the examination requirements. Clinicians are expected to be already working in the area of diabetes education and providing diabetes self-management education to patients. The exam to become a CDE is not an entry-level exam, and clinicians taking this exam must be knowledgeable and experienced in diabetes care.
The CDE functions as an educator and advocate for patients of all ages with diabetes and across the continuum of care. The CDE has expertise in teaching diabetes self-management skills to patients with diabetes and is a valuable resource for other clinicians who work with patients who have diabetes (CBDCE, 2024).
(See also “Resources” at the end of this course.)
Podiatry Care
All patients with diabetes should have a podiatry consult as part of preventive care. A podiatrist will diagnose and treat conditions of the foot, ankle, and related structures of the leg, including providing diabetic foot care. The podiatrist works in close association with other members of the patient’s healthcare team to ensure that recommendations for care are integrated into the treatment plan for the patient.
A podiatrist provides the patient with diabetes with:
- Specialized foot assessment
- Identification of bone and joint deformities that can lead to areas of high pressure on the feet
- Recommendations for care, including prescriptions for inserts or diabetic shoes
- Education regarding foot care
- Early detection of complications
(CDC, 2024c)
ANSWERING PATIENT QUESTIONS
Q: I have diabetes but no problems with my feet. Do I need to see a podiatrist?
A: Yes, it is advisable to see a podiatrist. The goal is to prevent foot problems from developing in the first place. The podiatrist will be able to determine your risk of developing foot problems and make suggestions that will help prevent them from happening.
Orthotic Care
An orthotist can play a part in the care of the patient with diabetes by evaluating the patient for an orthosis, which can serve the following purposes:
- Assist with movement of the extremity
- Restrict movement in a particular direction, as needed, to help reduce inflammation and improve stability and function
- Reduce weight-bearing and relieve areas of excessive pressure to the feet
- Reduce pain in the affected extremity
Orthotic devices used in diabetic foot care include ankle braces, shoe inserts, and footpads. Removable insoles in shoes provide pressure and shock absorption. When a patient is prescribed a shoe insert, such as removable insoles, they are advised to wear their regular shoes to the fitting so that the foot care specialist can fit the appropriate insert for that shoe.
For patients with diabetes who have lost even partial sensation in their feet, it is recommended that they be fitted for individually modified shoes by a pedorthist or other trained foot care specialist. A pedorthist has special training in the properties of footwear and the interactions between the patient’s feet and the shoes they wear. Customized shoes for patients with diabetes are provided by the pedorthist. The goal of care is to provide nonsurgical intervention either to relieve a foot problem or to prevent it from becoming worse (CDC, 2024a).
(See also “Appropriate Footwear,” “Healing Shoes,” and “Resources” later in this course.)
Additional Team Members
Dietitians commonly see the patient once a diagnosis of diabetes is established or when weight management is identified as part of the patient plan of care. Since many patients with diabetes also have concurrent problems with arterial circulation to their feet, a consult to a vascular surgeon will frequently be ordered. A neurologist may be consulted to perform nerve testing and assess the severity of peripheral neuropathy present.
PATIENT TEACHING
Patient education is an internationally accepted basis for diabetes management and patient empowerment. Diabetes education enables patients to help identify their own needs, requirements for lifestyle adjustment, and means to prevent or delay complications (WOCN, 2022).
While certain clinicians hold specialized qualifications in diabetes education, every healthcare provider must also be knowledgeable in basic diabetes education, especially when it comes to care of the diabetic foot. For instance, several studies have shown the important role nurses play in diabetes education and providing psychological support to patients with diabetes (Dailah, 2024).
The goal of diabetes self-management education is to provide the patient with the necessary knowledge and skills to incorporate practices into their daily life that will allow them to remain healthy and prevent complications. If the patient’s foot care is being performed by someone else, such as a family member, that individual, with the patient’s permission, is also included in the teaching sessions.
Teaching Newly Diagnosed Diabetes Patients
When a patient is first diagnosed with diabetes, teaching focuses on what is immediately necessary to keep the patient safe, such as learning how to check blood sugars, administer insulin, and make dietary changes. To clinicians, these actions may all seem straightforward and easy to accomplish. However, to a patient who has never held a needle or syringe or who is accustomed to eating only “junk food,” dealing with even the essentials of diabetes care can be unnerving. Clinicians must therefore see education as an ongoing process and not limited to a few sessions with a diabetes educator or a dietitian.
A frequent complaint from many patients with diabetes, particularly after they have been newly diagnosed with diabetes, is that “there is too much to remember.” Clinicians have to be mindful of this and summarize teaching into two or three major points, such as:
- A healthier diet
- More physical activity
- Smoking cessation
Patients are reminded that even small advancements in these three areas will improve their general health and decrease the likelihood of developing foot problems.
Individualizing the Teaching
Although the steps in preventive diabetic foot care are basically the same for all patients, teaching is individualized to meet the particular needs of each patient. Various factors are taken into consideration when devising a teaching program, such as:
Age of the patient. Cognitive impairment can be a significant problem for older adults. Unfortunately, it often goes undetected. One study found that doctors were not aware of cognitive deficits in >40% of their cognitively compromised patients (NIA, 2023).
Language. Does the patient speak English, is it their first language, and is it their preferred language for health education? Will the clinician need an interpreter present for teaching?
Literacy. Recent data indicate that adult literacy in the United States has remained stationary. Even though there are variations in literacy rates across the country, the most current studies, from 2012, 2014, and 2017, indicated that the standard result “was not measurably different” for adults over the years surveyed, with 21% of adults demonstrating low literacy skills (USA Facts, 2023).
Clinicians must also ensure that the teaching materials are appropriate for the patient. Handing a male in his late 20s a pamphlet on daily foot inspections with pictures of an older male will not convey the correct message; the young patient’s unspoken response may be, “I can wait to check my feet until I’m old.” It may also be ineffective to give a middle-aged African American woman living in a rural area educational materials that portray a young White female living in an urban area. Patients must be able to identify with the examples and the instructions in the educational materials in order for meaningful learning to take place.
Clinicians must be aware of the cultural influences and health beliefs of the patient. It is important to explore with the patient what they already know about diabetes, diabetic foot care, and how diabetes is perceived in their culture. For instance, some cultures have a negative view of insulin; they believe that taking insulin will only worsen their condition or that the use of insulin could be an obstacle to their religious practices. It is important for the clinician to respect the patient’s beliefs and provide them with facts (Cervoni, 2024). If there is a concern about the effects of diabetes care on religious practices, it may be prudent to refer the patient to a religious scholar or someone in their faith community who can provide them with accurate knowledge and insights.
The role of socioeconomic factors is also part of formulating education for patients. The clinician must consider whether any of the recommended interventions will be financially burdensome on the patient. It can be easy to label a patient as “noncompliant” when the real reason they are not participating in their care is their inability to purchase the long-handled mirror, insoles, or shoes recommended by the clinician. What alternatives and suggestions can the clinician offer to help offset these costs?
Listening and Speaking Effectively
Effective listening and speaking are vital to establishing the partnership needed between the clinician, patient, and other caregivers to achieve the common goal of preventing foot ulceration.
When people are talking, there is no need to do anything but receive them. When clinicians practice empathetic listening with a patient in an environment in which the patient feels safe to talk, they usually hear the real reasons why self-care is not being done, either directly or indirectly (e.g., “I can’t stand my wife fussing over me to check my feet; it makes me feel like an invalid”).
The clinician’s language when teaching is very important. Staying away from medical terminology and jargon as much as possible is vital for successful communication. Sometimes patients can be too stressed and too weary to request a clarification of information they may not understand. For this reason, it is helpful if a family member or other caregiver is part of the education process, with the patient’s permission.
Clinicians can probably speak volumes about the terms risk and benefit, but to many patients, these terms are vague and not easily conceptualized. When teaching preventive care to a patient with diabetes, benefits and risks must be clearly explained in concrete terms.
Consistency in terminology is imperative. If during the first encounter with the patient, the clinician talks about a diabetic “foot ulcer,” but at a subsequent visit refers to a diabetic “wound,” this may convey different meanings to the patient. Many people see a wound as something that is cleaned up and heals quickly, whereas an ulcer will be there for a long time.
PREVENTIVE FOOT CARE FOR PATIENTS WITH DIABETES
More and more emphasis is now being put on preventive measures rather than exclusively on treatment of diabetes and its complications. Preventing diabetic foot ulcers is of prime importance and must be the focus of every clinician at every encounter with the patient. Prevention of diabetic foot ulcers focuses on two specific factors: foot care and control of the underlying diabetes.
Health and Family History
The first step in preventive care is getting to know as much as possible about the patient’s health history, including family history. Taking time to get to know the patient is critical to successful treatment outcomes. Not only does the clinician glean useful information, most importantly they lay the basis for good rapport with the patient. Questions that the clinician asks are:
- How long since the patient was first diagnosed with diabetes?
- What was the patient’s initial reaction to the diagnosis? Many patients will state that they got “very serious” about their healthcare immediately, while others will describe minimizing the diagnosis, stating that they were “feeling fine” at the time and did not see any need to make changes in their routine or lifestyle.
- What comorbidities does the patient have, such as heart disease, respiratory conditions, or musculoskeletal impairments (WOCN, 2022)?
- What is the patient’s level of cognitive function and the presence of depression? A British study reported that up to 40% of individuals with diabetes were affected by depression (Diabetes.co.uk, 2019). A report from the CDC (2024d) indicates that persons with diabetes are 2 to 3 times more likely to have depression than persons without diabetes. However, only 25%–50% of those with diabetes and depression receive a diagnosis and treatment.
- Does the patient have a family history of diabetes? If so, how did the family member(s) deal with diabetes, and what complications did they have? These are details that can greatly influence how a patient views their own condition. If, for example, a patient’s mother had diabetes and died from heart disease in her mid-50s, the patient may see diabetes as a fatal condition that will greatly shorten their own life.
Limb Assessment
Being aware of the condition of the patient’s lower extremities helps the clinician to identify the patient’s level of risk for developing diabetic foot ulcers, and this will help guide the interventions needed at this stage to prevent ulcers from occurring.
The patient is asked or assisted to remove their shoes and socks, and a careful assessment is made of each leg and foot. Each limb is compared with the other and any differences between the two of them noted. While assessing the general appearance of the limb, the clinician observes for the following:
- Color. Paleness may indicate poor circulation, while redness could be a sign of inflammation or the early stages of Charcot osteoarthropathy (degeneration and destruction of weight-bearing joints) (discussed later in this course).
- Dryness. Skin surfaces that are dry, flaking, or cracked may be an indication of impaired circulation.
- Hair distribution on each leg. A lack of hair growth can be an indication of poor circulation, in particular arterial circulation.
- Edema. This is determined by pressing firmly but gently over the areas of swelling using the index finger. Edema is also a finding in problematic circulation.
- Fissures. These are dry, deep cracks in the skin, particularly on the heels, and can be a sign of neuropathy.
- Callus formation. This may indicate of an area of excessively high pressure.
- Temperature. A dermal temperature at a plantar foot location that is 2 °C (3.6 °F) or more higher than the same area on the opposite foot or than nonaffected areas on the same foot is regarded as a positive finding for inflammation. Statistics show that 82% of diabetic foot ulcers are preceded by callus formation and inflammation (WOCN, 2022).
- Fungal infection, especially between the toes, can be attributed to poor foot hygiene.
- Bunions at the base of the great toes can indicate footwear that is too tight.
- Corns may indicate rubbing or pressure from ill-fitting shoes.
- Bony deformities can be a finding in ill-fitting footwear.
During the hands-on assessment, the clinician also determines whether the patient can reach and see their own feet. Data show that 49% of patients with diabetic foot ulcers were unable to position their feet or see the complete surfaces of their feet. It was also found that 15% of persons with diabetic foot ulcers were legally blind in at least one eye (Baranoski & Ayello, 2020). In the event that the patient has such visual difficulties—for example, arthritic joints prevent them from seeing the bottoms of their feet—then the clinician identifies assistive devices that will allow the patient to perform proper foot checks on themself.
Over the course of one’s life, the anatomy and function of the feet change. Normal age-related changes include the fat pads on the bottom of the feet gradually becoming thinner, the arch of the foot frequently becoming flatter, and the foot becoming longer and wider. When examining the feet of patients with diabetes, the clinician distinguishes between age-related changes and changes related to diabetes.
Foot Care
Daily foot care practices for the patient with diabetes are a vital part in preventing diabetic foot ulcers from developing and an important self-management goal. For everyone involved—patient, families, and clinicians—it is important to keep in mind that even small changes can make a big difference.
When patients understand why foot care is important, they are more likely to do it. A generalized statement that “diabetes can damage your feet” is not sufficient. The clinician does not need to go into lengthy explanations regarding the pathophysiology of diabetes. Instead, they explain that diabetes can cause two important changes that affect the feet:
- There may be a gradual loss of feeling to the feet, which the patient will not always be aware of. This can be explained to the patient in practical terms: “When you lose feeling in your feet, you may not feel a small stone or even a nail inside your shoes. You may not know that you have developed a blister or a cut on the sole of your foot. A diabetic foot ulcer can begin with something as seemingly insignificant as a small cut (nick) that happens when trimming toenails.”
- Diabetes can cause decreased blood flow to the feet. Again, the practical implications of this can be explained to the patient by stating, “Poor blood flow to your feet makes it much harder for any injury to your foot to heal, even a small wound. This can cause a small cut to turn into an ulcer.”
ELEMENTS OF GOOD FOOT CARE
- Preventing skin breakdown. The skin covering the feet provides a physiologic barrier to bacterial and fungal invasion of the tissues and bones of the feet. Skin integrity is therefore of major importance to all patients but especially to patients with diabetes, who have compromised healing (WOCN, 2022).
- Maintaining the normal structure and function of the feet. This is crucial to the patient’s mobility.
The clinician teaches the following elements of good foot care with every patient who has diabetes and reinforces them at every follow-up visit:
- Conduct daily foot checks. Ask the patient to remove their shoes and socks and show them how to do a foot check. Every part of the foot—the tips of the toes, between the toes, the back of the heels, and the ankle areas—is to be inspected. Instruct the patient to look for red spots, blisters, cuts, swelling, and infected toenails. Many patients are unable to see the bottoms of their feet, which make foot checks difficult and can result in areas of redness being missed. In this instance, patients are instructed in the use of a long-handled mirror to visualize all areas of their feet (Healthline, 2023).
- Wash the feet every day. Instruct the patient to wash their feet with mild soap and water. Remind patients who have a lack of sensation in their feet to check the temperature of the water with a hand before immersing their feet in order to avoid the possibility of burns. After washing, dry the feet carefully, rubbing gently to avoid chafing. Particular attention must be paid to drying between the toes, since moisture left in the web spaces can contribute to a yeast infection. Washing the feet does not mean soaking them in water; foot soaks have been shown to break down the natural skin barrier and predispose the patient to ulcer formation (Healthline, 2023).
- Perform skincare. Advise the patient to massage a thin coat of skin lotion onto the tops and bottoms of their feet after drying. Explain that lotion should not be put between the toes; instead, a light dusting of talcum powder or cornstarch can be used to keep the skin between the toes dry. However, the patient must be cautioned against putting excessive powder between the toes, since this will lead to clumping and possible excoriation.
- Never walk barefoot. Most people believe that it is safe to walk in their bare feet in their own homes. For a patient newly diagnosed with diabetes, it may seem nonsensical to suddenly have to wear socks and shoes (or sturdy slippers) at all times, even in their own living room. The clinician explains that inadvertently stepping on everything from a sewing needle to a sesame seed has been found to cause a diabetic foot ulcer (Healthline, 2023).
- Check shoes before putting them on. Instruct the patient to check for objects inside each shoe and to ensure that the lining of the shoe is smooth and wrinkle free prior to putting on their shoes. Patients with small children or grandchildren, for example, have found Lego pieces and small toys in their shoes.
- Protect feet from extremes of temperature. Properly fitting shoes worn at the beach provide protection against hot sand. Also, caution the patient against using hot water bottles, electric blankets, or heating pads. Advise the patient to wear socks at night if they have cold feet. If a patient does wear sandals during the summer, recommend putting sunscreen on the top of the feet to protect against sunburn.
- Improve circulation to the feet. Good circulation is fundamental to maintaining healthy feet. Teach the patient to put their feet up whenever they are sitting, not to sit for long periods of time with their feet in a dependent (dangling) position, and not to sit with their legs crossed for an extended time. Patients are instructed in simple home exercises for the feet, such as wiggling their toes and flexing their ankles for five minutes a few times each day (Copper Fit, 2023). Caution the patient against wearing tight socks or elastic/rubber bands or garters around their legs to hold up stockings, as all of these can interfere with circulation.
- Monitor foot temperature. This is a simple intervention that the patient performs at home using a temperature-sensitive mat. Teach the patient to stand on the mat for 20 seconds each day while it measures the temperature in the soles of their feet. An increase in foot temperature can be a precursor to ulcer formation, and early detection allows for better proactive care and prevention (Lutes, 2023).
ANSWERING PATIENT QUESTIONS
Q: When should I check my feet, and how long is it going to take?
A: The best way to respond to this question is to have the patient demonstrate the foot check process without instructions or guidance from the clinician. This is a better indicator of the time required than simply telling the patient, “It will only take a few minutes.”
As to how frequently foot checks should be done, the clinician reinforces with the patient the need to make this part of a daily routine. A foot check can be compared to brushing one’s teeth; it is a good practice to do a foot check in the morning before putting on any footwear and again at night when the patient takes off their shoes and socks.
INDICATIONS FOR CONTACTING A HEALTHCARE PROVIDER
Patients are given clear instructions on when to contact their healthcare provider as a result of their regular foot care. For example, “Contact your doctor’s office as soon as possible if you notice any of the following changes”:
- You start to feel pain or cramping in the legs or buttocks while you are walking or doing other physical activity
- Your feet are burning, hurting, or tingling
- You cannot feel when someone touches your foot
- You cannot tell very well whether something is hot or cold when it touches your foot
- You notice a change to the shape of your foot
- The color of your feet starts to change, and they feel warmer than normal
- You begin to lose any former hair growth on your legs, feet, and toes
- The skin on your feet is dry and cracking
- The areas between your toes become red, damp, and itchy
- Toenails that were healthy and normal turn yellow and thick
- You find blisters, cuts, calluses, infected corns, or ingrown toenails on your feet
(WOCN, 2022)
ANSWERING PATIENT QUESTIONS
Q: I have a hard callus around a small wound on the bottom of my foot. Should I cut it off myself?
A: No. Wound and foot care for a patient with diabetes should only be done by a healthcare professional. Home surgery can often cause further damage to the wound and to the foot. It should be avoided.
BARRIERS TO PROPER FOOT CARE
Clinicians must remain aware of how difficult it can be for patients to make and maintain substantial changes to their lives, especially after receiving a diagnosis of diabetes. The emotional response and shock of being diagnosed with diabetes can be a major barrier for learning and self-management. This new diagnosis is something that can impact every facet of a patient’s life. Under such overwhelming circumstances, looking at one’s feet every day can seem trivial and unimportant.
It is the responsibility of the clinician to create a shame-free environment and to show respect and caring, even when it is difficult for the clinician to understand why a patient may not perform the care that they know to be important to their own well-being.
Appropriate Footwear
Proper footwear is incorporated into diabetes care from the onset of diagnosis, since it is a crucial part of preventing diabetic foot ulcers. Poorly fitting shoes are frequently cited as one of the foremost causes leading to the development of diabetic foot ulcers and may contribute to around half of all diabetes-associated amputations (Armstrong & Lavery, 2024).
The clinician assesses the patient’s footwear at the first visit and then at regular intervals, since changes may occur in the size or shape of the patient’s foot due to edema or Charcot foot (discussed later in this course), necessitating additional adjustments and modifications to shoes (Baranoski & Ayello, 2020; WOCN, 2022).
Most everyone wants to wear nice shoes or sandals, and one of the first complaints from patients with diabetes is that specialized diabetic footwear is ungainly and ugly. At first this may seem like a trivial complaint when compared to the seriousness of developing a diabetic foot ulcer and the real potential for amputation. However, the clinician realizes that the patient is probably not thinking along those lines. Instead, they may see the loss of “normal” footwear as another unwanted indicator that a chronic disease is putting limitations on their life. Coming from this standpoint, the clinician works with the patient in finding footwear that protects their feet but is still aesthetically pleasing to the patient. This ensures better treatment adherence on the part of the patient and better outcomes.
MEDICARE THERAPEUTIC SHOE BENEFIT
Individuals with diabetes who are at risk for foot complications and who have Medicare Part B are eligible for Medicare’s therapeutic shoe benefit. To qualify for this benefit, the person must have a diagnosis of diabetes and at least one of the following:
- History of amputation of part or all of either foot
- A previous foot ulcer or preulcerative calluses
- Peripheral neuropathy with callus development
- Diminished circulation
Each calendar year, this Medicare Part B benefit pays for:
- One pair of custom-molded shoes and inserts or one pair of extra-depth shoes
- Two extra pairs of inserts for custom-molded shoes
- Three pairs of inserts for extra-depth shoes
- Shoe modifications instead of inserts
(See also “Healing Shoes” later in this course.)
(Baranoski & Ayello, 2020)
ELEMENTS OF PROPER SHOE FIT
The clinician educates the patient on the importance of choosing the appropriate shoe. It is important that shoes are practical and useful in all circumstances the patient may encounter. They must not contribute to new concerns such as compromised stability, potential for falls, and sweating (Armstrong & Lavery, 2024).
The shape and size of the shoe must be assessed, and the patient advised to, as far as possible, match the shape of the shoe to the shape of the foot, in both width and length. A properly fitting shoe will have sufficient room in the toe area, over the instep, and across the ball of the foot. The shoe should fit snugly around the heel area.
A shoe with laces is a better choice for a patient with diabetes than a slip-on shoe because it provides better support and allows for adjustments needed for swelling, deformities, and different sock thicknesses.
For patients with diabetes who have intact sensation in their feet and no evidence of foot deformities, an ideal choice is a correctly fitting pair of shoes produced from soft materials (such as soft leather) with the capacity to stretch.
Shock absorption is an important consideration for the patient with diabetes, and a cushioned sole rather than fine leather soles will provide for better shock absorption.
It is essential that shoes provide good support and protection for the feet, and the backs of the shoes need to be strong enough that they will not collapse downward or to either side, leaving the heels exposed to injury.
Ideally, patients with diabetes should have more than one pair of comfortably fitting shoes, and it is a good practice to alternate shoes on an every-other-day basis. This will help to extend the life of both pairs.
ANSWERING PATIENT QUESTIONS
Q: Will the shoes I am wearing now prevent damage to my feet?
A: This is a question that a patient will commonly ask. To answer, the clinician assesses the patient’s shoes, observing their overall condition, size, fit, and design in relation to the shape of the patient’s foot. The clinician notes the wear stress along the soles and heels and looks for thinning areas along the bottom of the shoes and the surface of the shoe lining. Are there bulges on the outside of the shoes? Excessive wearing down of the heels? Are the heels too high and too narrow?
While it may be apparent to the clinician that a patient’s shoes are not a good fit and are placing the patient at risk for developing a diabetic foot ulcer, the patient may be reluctant to accept the clinician’s findings. For instance, perhaps the shoes are relatively new and were expensive to purchase, the shoes are a favorite pair, or “they’ve never caused any problems in the past.”
Another concern is uneven weight distribution and the creation of high–pressure point areas. A quick and inexpensive way to check for this is by using a Harris mat, which is a foot imprint system. One side of the mat is permeated with ink, and the other is clear. The patient is requested to remove their shoes but to leave their socks or stockings in place. The patient then takes a normal step onto the uninked side of the mat, leaving an impression of the foot on the mat that highlights areas of uneven weight distribution and high pressure (Armstrong & Lavery, 2024). This then allows the clinician to recommend appropriate orthotic devices to relieve pressure or to refer the patient to an orthotist or podiatrist for further evaluation.
BUYING NEW SHOES
Patients with diabetes need to be aware of the condition of their shoes and when shoes require replacing. Signs that shoes should be replaced include:
- Heel shape that no longer provides support and collapses when the patient puts the shoes on
- Holes in the lining of the shoe
- Worn, uneven heels, which can produce an unsteady gait and/or increase the risk for excessive pressure points
- Thin, worn soles
Cost and fondness for a “worn-in” pair of shoes can make patients reluctant to purchase a new pair. The clinician must stay vigilant and point out to the patient when a new purchase is required to maintain healthy feet. The clinician offers patients these instructions regarding purchasing shoes:
- Have your feet and proper size measured each time because feet change over time, especially with aging.
- Shop in the afternoon rather than the morning because feet swell during the day, especially if you have concomitant heart and kidney disease.
- Wear the socks (or type of socks) that you normally wear when you go to purchase new shoes; this will help to ensure that the shoes will fit properly for everyday wear.
- Measure the distance between your longest toe and the tip of the shoe. This is best done with the shoes on, pressing down on the empty space at the front of the shoe. This empty space should be one half of your thumb’s width to allow for an adequate fit.
- Break in new shoes gradually before wearing them for extended lengths of time. Wear new shoes for 1–2 hours, remove them, and check the feet for cuts, blisters, spots of redness, or bruising. If none of these are present, wear the shoes for 3–4 hours the following day, and each day gradually increase the wear time. If shoes are causing problems, do not “tough it out”; return the shoes to the store if the store’s return policy allows and start again with a different type of shoe.
(Byrant & Nix, 2024; Armstrong & Lavery, 2024)
Sometimes it is hard for a patient to find the type of shoe they need “off the rack.” Clinicians can also remain aware of local shoe stores that carry footwear supplies especially for patients with diabetes and knowledgeable store staff who are able to assist a patient in choosing appropriate footwear.
COMMON FEATURES OF INAPPROPRIATE FOOTWEAR
- Seams. Seams can cause repeated shear and friction as well as pressure to the areas of the foot that they come in contact with, which are mainly the dorsal forefoot and the toes. Depending on the type of shoe, more than one seam may be present. Patients with diabetes are advised to shop for shoes that are seamless.
- Narrow toe box. A lack of space in the toe box can result in diminished circulation and increased pressure to the toes. This lack of space can result in callus and blister formation. It is important for the clinician to remind the patient with diabetic peripheral neuropathy that just because a shoe does not feel tight does not mean that it is not. The patient must carefully examine the shoe and use good visual judgment in deciding whether or not the shoe is a good fit.
- High heels. High heels can cause the development of pressure points on the balls and heels of the feet that can result in calluses and subsequent development of diabetic foot ulcers. It is recommended to wear shoes with broad, square heels that are less than 1–2 inches in height (DiLonardo, 2023).
- Insufficient length. The length of a shoe is just as important a consideration as the width. The length of the shoe should allow a 3/8- to 1/2-inch space between the patient’s longest toe and the end of the shoe (WOCN, 2022).
- Thong-style sandals and flip-flops. This type of footwear poses risks for patients with diabetes due to increased exposure of the foot and toes to possible injury.
SOCK SELECTION
Socks must also be considered when reviewing footwear with a patient who has diabetes. It is possible to purchase socks that are specially made for patients with diabetes in stores and from websites that specialize in diabetic supplies. For instance, compression socks, which typically indicate the degree of compression on the packaging, may be needed.
However, if the patient prefers to buy socks in “regular” stores, they are advised to avoid socks with seams that could possibly cause pressure and to look for socks that wick moisture away from the skin. Controlling moisture is very important for patients with diabetes in order to reduce the risk of weakening the natural skin barrier and the possibility of fungal infection.
Whether the patient is buying special diabetic socks or regular socks, the features to look for include:
- Nonelastic cuffs
- No prominent seams (turn socks inside out and run the fingers along the seams to determine how soft or coarse they are)
- Socks that keep the feet warm, especially for winter use
- Socks that allow the feet to “breathe” and sweat and that wick moisture away from the skin surfaces, particularly during the summer months
- Size (neither too tight nor sagging)
- Built-in cushioning along the bottoms for extra relief from pressure
- Light color, to alert the patient to drainage
Cotton socks absorb moisture, but they do not wick moisture away from the skin. Wool socks are usually lightweight and give good insulation; they also allow the skin to breathe and absorb moisture away from the skin. Socks that are made from a mix of high-quality fibers usually provide the best wear time, comfort, and protection.
Patients are reminded to always wear socks when they have shoes on and to change their socks every day.
Patients with diabetes often experience swelling in their lower extremities, but since most of these patients also have peripheral arterial disease, high levels of compression therapy cannot be used. In patients with lower extremity swelling, the clinician discusses the value of wearing socks with mild compression (Greenstein, 2023; Byrant & Nix, 2024).
Foot Biomechanics
Biomechanics refers to how the body moves and what impacts that movement. Two primary goals of biomechanics interventions are injury prevention and rehabilitation. Biomechanical problems can lead to an array of negative consequences on ambulation and pursuit of recreational activities as well as result in an increase in musculoskeletal comorbidities. Thus, knowledge of the biomechanics of the foot is an essential part of the assessment of the diabetic foot and an important component in preventing diabetic foot ulcers.
The gait cycle refers to one full step, beginning with either the right or left foot, from heel contact of the first foot to heel contact of the second foot. The gait cycle is further divided into the stance phase (60%), which occurs when the foot is in contact with the ground, and the swing phase (40%), which occurs when the foot is free-floating.
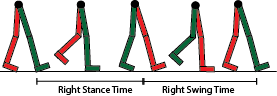
Stance and swing phases of the gate cycle. (Source: Rlawson9. Creative Commons Attribution-Share Alike 3.0.)
Proprioception refers to an awareness of the body’s position in context with the surrounding environment. Through proprioception, one knows one’s location in space and how to navigate through it. This functioning is accommodated by specialized nerve endings known as proprioceptors, which are found in the soft tissue of the musculoskeletal system. Next to the spinal cord, the foot is the area of the body with the most proprioceptive sensory receptors.
Damage to the feet will result when there is reduced proprioception. A lack of, or inadequate, information provided by proprioceptors has a negative impact on movement, postural coordination, and pressure (weight) distribution. In a diabetic foot with advanced neuropathy with loss of protective sensation (LOPS), diminished proprioception response is evident by unsteady gait, loss of balance, and unequal stress and weight-bearing on the feet.
When excessive weight is applied to a section of the foot, this overloading causes an overpowering, intense force that leaves no time for the skin to shield itself from injury. At this stage, blistering occurs. When there is continuous unrelieved pressure, calluses form as a protective barrier, which will eventually lead to ulcer formation.
The goal of biomechanics assessment and intervention is to assist the patient to live a healthy and active lifestyle by providing them with the education and necessary equipment to prevent injury, deformity, and the development of a diabetic foot ulcer. While being physically active plays a vital role in managing diabetes, problems with proprioception may make it difficult for patients with diabetes to be safely active. Clinicians should therefore be constantly aware of the need to maintain the patient’s activity levels while also compensating for existing deficits with ambulation and proprioception.
The first step is to ensure that the patient has good pedal support and that biomechanical issues are being addressed. This is achieved by preventing postural collapse of the foot. Research shows that custom orthotic supports also help to correct balance, gait, and structural alignment; prevent the occurrence of diabetic foot ulcers; and support treatment for ulcers already present (Zubair et al., 2021).
The clinician who sees the diabetic patient on a routine basis must be aware of the interaction between biomechanics and preserving the health of the diabetic foot. A consult with a podiatrist should happen early on in the plan of care and at least yearly thereafter. Any new problems related to ambulation and proprioception should also be referred immediately to a podiatrist (Armstrong & Lavery, 2024)
Addressing Foot Deformities
BUNIONS
A bunion is a protrusion, varying in size, that results from an abnormally prominent fifth metatarsal joint. Bunions can result from wearing tight-fitting, narrow shoes as well as from an inherited structural defect. During a foot exam, the clinician assesses for the presence of a bunion as a medial prominence on the side of the foot. The main treatment is to accommodate the deformity in an expanded shoe and to relieve pressure and shear that could result in wound formation (WOCN, 2022).
HAMMERTOE
Hammertoe is a common flexion deformity of the proximal interphalangeal joint of the second, third, fourth, or fifth toes. Hammertoe deformity is also known as claw toe, since the affected toes look to be bent in the shape of a claw. It is frequently found in the second toe. Hammertoe deformity is painful and increases the risk of skin breakdown and ulcer formation.
Properly fitting footwear is the most appropriate treatment for this deformity. The clinician ensures that the front of the shoe is sufficiently high to relieve pressure from the hammertoe joints; this may necessitate a consult with the podiatrist. The clinician keeps in mind that when the toes move upward, they draw the soft tissue of the foot pad along with them, which denies the areas at the base of the toes of proper cushioning and increases the risk of callus and ulcer formation in these areas (WOCN, 2022).
CALLUSES
A callus is a thickening of the epidermis that occurs at the site of pressure on the foot. Shear and friction can also lead to callus formation. The development of a callus is seen as a protective reaction to continued stress on the affected area. Calluses should not be allowed to accumulate, as they are a common precursor to the development of diabetic foot ulcers in patients who have diabetic neuropathy. Excessive callus buildup can increase pressure to that area of the foot by 25%–35%, and this leads to the development of a diabetic ulcer beneath the callus that the clinician typically cannot see or palpate on examination (WOCN, 2022).
Discoloration of a callus or bleeding into the callus is the primary indicator of the presence of an ulcer. When examining an area of callus formation, the clinician looks closely for flecks of blood or the presence of a deeper layer of macerated, white softer tissue under the outer covering of callus. This shows the presence of an evolving ulcer and the need to immediately remove the callus tissue (Baranoski & Ayello, 2020).
The initial treatment for callus is to reduce the bulk with a scalpel while maintaining tension on the skin. Callus removal is done by a podiatrist or a clinician who has experience and training in foot care. Patchy removal of callus tissue can result in focal points of excessively high pressure and increased risk for ulcer development.
Patient education includes stressing that the patient should not attempt to remove the callus themselves (i.e., no “bathroom surgery”) and that the use of over-the-counter callus removers is not recommended, since these contain strong acids that may permit infection to gain access to the foot (WOCN, 2022).
Callus formation frequently reoccurs and requires close monitoring by the clinician. The biomechanical problem leading to the callus growth must also be addressed to obtain a permanent remedy.
Tight Glycemic Control
The Diabetes Control and Complications Trial provided strong evidence that tight glycemic control (keeping blood sugar levels at or near normal levels) substantially reduces the chances of developing microvascular complications of diabetes, including diabetic foot ulcers.
Poorly controlled blood sugars are a major contributing factor in the development of neuropathy in the extremities of patients with diabetes. High blood sugars also cause impaired leukocyte function, inhibit lymphocytes, and lead to an impaired immune response, which prevents the patient from mounting an adequate response to wound infection (WOCN, 2022).
The A1C test (also called the hemoglobin A1C or the glycohemoglobin test) is one of the most important tests used in diabetes management and diabetes research. The A1C test examines the binding of glucose to hemoglobin in the red blood cells. Red blood cells normally live for around three months, and by using the A1C test, it is possible to obtain an average of a patient’s blood glucose levels over the previous three months.
The A1C blood test result is stated as a percentage. The higher that percentage, the more a patient’s blood glucose levels have risen. An A1C test can be done at any time of the day and does not require the patient to have been fasting prior to the blood draw. American Diabetes Association treatment guidelines recommend that the A1C level be maintained below 7% to prevent the risk of microvascular damage in type 1 and type 2 diabetes (ADA, 2024a; WOCN, 2022).
Primary care providers help patients to set reasonable goals for blood sugar levels, and this is often done in progressive increments. For a patient whose blood sugar levels have consistently remained high, getting down to the stated normal levels may seem unreasonable. However, it is essential for clinicians to provide encouragement and reiterate that any decrease in blood sugar levels helps to lessen the risk of microvascular complications occurring.
A patient may need to see their primary care provider on a monthly basis when diabetes is first diagnosed and blood sugar levels are being regulated. This also provides an excellent opportunity for ongoing education on foot care. The clinician helps the patient make the connection between blood sugar levels and foot care by explaining that high blood sugar levels cause narrowing and hardening of the arteries, which will decrease the amount of blood reaching the feet. Reinforcement of teaching, reassessment of the patient’s ability and commitment to foot care, and encouragement by the clinician are key constituents of successful outcomes.
Weight Loss
Encouraging patients to maintain a healthy weight is another facet of preventive care. Being overweight plays a significant role in insulin resistance. According to the CDC, obesity prevalence among U.S. adults did not increase during the past 10 years, but severe obesity prevalence did increase. From August 2021 to August 2023, greater than 4 in 10 adults were obese, and nearly 1 in every 10 adults were considered to be severely obese (CDC, 2024e).
Patients are often doubtful about weight loss and voice fears such as, “I don’t think I can lose a whole lot.” The clinician reassures them that losing even 10 pounds can produce a considerable improvement in blood sugar levels. Most patients will agree that a 10-pound weight loss is doable, and it is then up to the clinician to help them set realistic, specific goals for a weight-loss program.
The patient is typically referred to a registered dietitian for a dietary consultation and to develop a diet program. To achieve maximum success, the diet plan takes into consideration the patient’s lifestyle, food preferences, and the fact that the patient may feel discouraged by previous failed attempts to lose weight. It is important for patients with diabetes to maintain the pleasure of eating, and this is something that the dietitian takes into consideration when assisting the patient to develop meal plans.
Follow-up with the patient is necessary to evaluate progress in meeting goals and to help resolve problems they have encountered.
Smoking Cessation
Smoking places a patient with diabetes at a very high risk for peripheral vascular diseases (in particular, peripheral arterial disease) and the development of diabetic foot ulcers. Cigarette smoking and diabetes are factors in the increased incidence of macrovascular and microvascular disease, in which both the large and small blood vessels are adversely affected. Therefore, as a preventive measure for diabetes complications, patients who smoke must clearly understand the risks of smoking and be encouraged to take part in a smoking cessation program (WOCN, 2022).
In plain language, the clinician explains to the patient that nicotine narrows the blood vessels. When there is poor circulation to the feet, the skin then becomes dry and can crack easily, and any break in the surface of the skin will potentially allow bacteria to enter and cause an ulcer to develop. Similarly, decreased circulation and oxygen delivery make it harder for an ulcer to heal.
Patients may not find it easy to give up smoking and can be highly resistant to the idea, especially after receiving a new diagnosis of diabetes, when there is a constant demand for lifestyle changes and adjustments. Many times, patients will state that they “need” to continue smoking to cope with the stress of having diabetes. The clinician recognizes this is a subject that may have to be addressed on an ongoing basis over a course of weeks or months.
Once a patient is ready, the clinician helps them decide on a quitting strategy, such as stopping all at once, tapering off by cutting down the number of cigarettes smoked each day, using a nicotine patch, joining a smoking cessation program, or receiving acupuncture. Any of these strategies can be used singly or in combination.
Giving up smoking is not easy, and there can be frequent setbacks. Patients may be irritable, especially during the first few weeks without cigarettes. They require support and encouragement from family, friends, and the clinicians who work with them.
ANSWERING PATIENT QUESTIONS
Q: How does smoking cigarettes affect a diabetic foot ulcer?
A: Nicotine in cigarettes causes narrowing of small blood vessels. A diabetic foot ulcer requires a good blood supply to heal. Due to the narrowing of blood vessels in people who smoke, there is a diminished blood supply to the wound.
Hypertension Control
It has been found that the incidence of hypertension increases considerably when diabetes is present, and hypertension is one of the main risk factors for the development of microvascular and macrovascular problems in patients with diabetes (Bell, 2023).
The patient’s blood pressure is checked with each visit, with the patient seated and having rested for approximately five minutes. Interventions used to reach target blood pressures first include lifestyle changes. Many patients are also prescribed oral medications, with the first-line recommended treatment being an ACE inhibitor taken daily (WOCN, 2022).
Recommended lifestyle modifications include:
- Weight loss
- A diet low in saturated fats and total fat content
- Restricted dietary sodium intake (no more than 2,400 mg/day)
- Regular physical exercise
- Moderate consumption of alcohol (no more than two drinks daily for males and one daily for females)
- Sufficient sleep
(Mayo Clinic, 2024)
CASE
Mr. Hernandez is a 45-year-old Hispanic man diagnosed with type 2 diabetes one month ago during a routine health check at a local clinic. At that time, he did not have a primary care provider, and he is being seen today for the first time by a nurse practitioner, Celia. Prior to the appointment, she reviews the patient’s chart and notes that Mr. Hernandez was started on metformin and also has a consult for diabetes education. However, the record indicates that the patient has only attended one session with the certified diabetes educator and missed the subsequent two appointments.
Mr. Hernandez arrives for his appointment accompanied by his wife, who does not speak English. He is polite but reluctant to engage with Celia. He states that he has “no time for diabetes education” and that he is taking his medication as prescribed. He says, “My blood sugars were high because I drink too much soda.” When asked about his feet, he replies that they are fine and refuses a foot exam. Celia notes that he is wearing strong work boots and that he has no noticeable problems with his gait.
Celia acknowledges that it’s good Mr. Hernandez is having no problems with his feet and explains that this is the best time to start doing routine foot exams. She tells him that diabetes can interfere with the way the nerves in his feet work and that he can slowly lose feeling to areas of his feet without knowing it. This puts him at greater risk for injuring his feet and developing an ulcer. To compensate for the lack of feeling, daily foot checks are critical for protecting his feet from damage. Since Mr. Hernandez has refused a foot exam today, she gives him a handout on diabetic foot care and makes a note to discuss this again at his next visit.
In response to Mr. Hernandez’s statement that he has “no time for diabetes education,” Celia asks for more information about his work schedule and acknowledges that it can be hard to get time off from work to attend medical appointments. She explains that receiving diabetes education now will help lay out the best course for Mr. Hernandez to follow to control his diabetes and stay healthy, reducing the need for even more future medical appointments. She also offers to look into what other resources are available for diabetes education during the evenings or weekends.
Since Mr. Hernandez already recognizes the link between drinking too much soda and high blood sugars, Celia reinforces the need to monitor blood sugar levels and to reduce his overall sugar intake. They agree on a specific goal of reducing his current soda intake of three cans a day to one can a day, beginning the process of goal setting and gradually making the necessary lifestyle changes.
After Mr. Hernandez and his wife leave, Celia discusses his case with the physician and case manager. She explains that Mr. Hernandez does not seem to have come to terms with the fact that he has diabetes. The physician is concerned that Mr. Hernandez’s blood sugars are too high and that he is hypertensive and overweight. He requests the patient be scheduled for a follow-up appointment in four weeks. The case manager also has concerns about Mrs. Hernandez, who has previously conveyed her concern about her husband and that she doesn’t understand what is going on or what she needs to do to help him.
Together, Celia, the physician, and the case manager agree that Mr. Hernandez’s next appointment will focus on the following:
- Exploring with Mr. Hernandez his feelings about diabetes
- Including a consult with a dietitian
- Recommending a consult with a physical therapist for development of an individualized exercise program
The case manager also states that she will call Mr. Hernandez prior to the visit and get his permission to have a professional interpreter present so that his wife will know what is happening and can share her concerns.
(continues)DIABETES COMPLICATIONS AND FOOT ULCER PREVENTION
The term diabetic foot syndrome (DFS) refers to a cluster of comorbidities that result from diabetes or are made worse by having diabetes. DFS impacts the development of diabetic foot ulcers, infections, and amputations. This syndrome includes Charcot osteoarthropathy, peripheral neuropathy, and peripheral arterial disease as well as limited joint mobility and poor nutrition (Baranoski & Ayello, 2020). Research studies are also exploring a link between DFS and a rising prevalence of cancer (Goretti et al., 2024).
Peripheral neuropathy and Charcot osteoarthropathy (Charcot foot) are two conditions that play a critical role in the development of diabetic foot ulcers, and clinicians must be watchful for the presence of these conditions when working with patients who have diabetes. The early symptoms of Charcot foot are frequently subtle and can easily be missed, with devastating consequences. Therefore, early diagnosis and treatment of these complications is critical in the prevention of DFUs.
Peripheral Neuropathy
Peripheral neuropathy is one of the most frequent complications of diabetes and reported to occur in about 50% of patients with diabetes. It is also the most common predisposing factor to the development of diabetic foot ulcers. Diabetic peripheral neuropathy (DPN) is a serious condition that can greatly diminish the quality of life for those who have it (WOCN, 2022).
Peripheral neuropathy is a condition in which there is damage to the peripheral nervous system, the complex communication network between the central nervous system and the other areas of the body, including the feet. This damage inhibits the regular neurologic activity of the lower extremities. The exact cause of peripheral neuropathy has not yet been clearly defined, but ongoing studies show that it is related to abnormal metabolic actions that can lead to cell injury and nerve ischemia (Baranoski & Ayello, 2020; Byrant & Nix, 2024; Armstrong & Lavery, 2024).
Data indicate that DPN is frequently not sufficiently treated, and the role of enhanced glycemic control as part of the treatment protocol, especially in type 2 diabetes, is not yet fully understood (Wang et al., 2021). Some studies demonstrate a relationship between painful DPN and severe vitamin D deficiency. Vitamin D is believed to preserve the balance between inflammation and immune suppression. Research indicates that increased inflammation in chronic conditions such as DPN is linked to insufficient vitamin D (Xiaohua et al., 2021; Dupuis et al., 2021).
DPN normally progresses slowly. It often remains asymptomatic and is detected only with detailed examination. The majority of those affected can be symptom free, and most patients with diabetes who have DPN are not aware of the condition. It can also be the first presenting symptom of previously undiagnosed diabetes, with tingling and a feeling of numbness being the two major presenting symptoms (WOCN, 2022).
Spinal cord stimulation is a relatively new treatment for the management of DPN. In this therapy, a device inserted under the patient’s skin provides electrical stimulation to the spinal cord in order to deter pain signals reaching the brain. In one study of patients with implanted spinal cord stimulation, 90% reported a 50% pain improvement after 24 months of therapy. These patients also had improvements in motor function, reflexes, and sensation as well as a 66% overall decrease in sleep problems related to pain (Peterson, 2023).
EVALUATING FOOT SENSATION AND REFLEXES
One of the first assessments a clinician performs for every patient with diabetes is to evaluate sensation in the feet. Even if the patient does not present with any of the signs and symptoms of neuropathy, loss of protective sensation may still exist. The clinician explains to the patient that simple, noninvasive testing for foot sensation requires no special preparation on the part of the patient and can be performed in the primary care provider’s office or clinic as well as at home.
The American Diabetes Association recommends that all individuals with diabetes have a yearly foot exam that includes screening for diabetic neuropathy. Neuropathy screening should include a 10-monofilament test and at least one additional screening procedure (ADA, 2024b).
The Semmes-Weinstein monofilament test is one of the easiest methods for evaluating loss of sensation in patients with DPN. The testing instrument is made up of a set of monofilaments ranging in thickness and diameter. Several studies have validated the efficacy of 10-g monofilament use and have shown that the loss of pressure sensation confirmed by using the 10-g monofilament is highly prognostic of later ulceration (Baranoski & Ayello, 2020; Armstrong & Lavery, 2024). It is recommended to obtain the monofilaments from suppliers who sell calibrated instruments, since some brands of monofilaments have been found to bend at 8 g of force instead of at the intended 10 g of force.
Clinician examining nerve response with a monofilament instrument. (Source: www.bigstock.com, © kckate16.)
SEMMES-WEINSTEIN MONOFILAMENT TESTING PROCEDURE
Monofilament testing sites on the bottom of the foot. (Source: U.S. DHHS.)
- Choose a quiet and relaxed setting to carry out the testing.
- Help the patient to assume a comfortable supine position, with shoes and socks removed.
- Explain the procedure to the patient and address any questions.
- Show the patient the monofilament and touch their arm or the back of their hand with it, demonstrating a “C” shape. (The Semmes-Weinstein 5.07 monofilament exerts 10 g of force when bowed into this “C” shape and placed against the patient’s skin for one second.) This demonstration reassures the patient that the test will not be painful and also allows them to experience the normal sensation they should feel when the monofilament touches different points on their toes and feet.
- Instruct the patient to say “yes” each time they feel the monofilament touch their toes or feet. (Do not ask the patient, “Do you feel that?” since this question may lead the patient to believe that the expected response is yes.)
- Instruct the patient to close their eyes during testing, explaining that this ensures they are feeling the filament rather than seeing it touch their feet. Also instruct the patient to keep their toes pointed straight up during the test.
- Hold the monofilament perpendicular to the patient’s skin and, using a smooth motion, press down until the monofilament bends into a “C” shape, holding that position for 1–2 seconds before lifting off the filament from the skin surface. The patient should sense the monofilament by the time it bends. If the patient responds “yes” (that they feel the monofilament), ask the patient whether they feel the pressure on the right or left foot.
- Do not slide the monofilament across the patient’s foot or touch either of the patient’s feet with one’s hands during the testing, so that the only touch that the patient is exposed to is that of the monofilament.
- Apply the monofilament three times to each test site. Also simulate one test without applying the monofilament. Avoid repetitive testing at the same site.
- Do not test over areas of callus formation, since it is impossible for the patient to accurately determine feeling in those areas.
- For a patient who may already have a diabetic foot ulcer present, test along the margins of the wound and not directly over it.
(Baranoski & Ayello, 2020; WOCN, 2022)
The Ipswich Touch Test (also referred to as the touch-the-toes test) is another simple, fast, and easy-to-teach method to detect loss of protective sensation in the diabetic foot. The value of this test is that the clinician can instruct the patient or family members to use it at home in order to monitor the presence or absence of sensation. Studies show that when compared to monofilament testing, the Ipswich Touch Test has good predictive value in detecting loss of protective sensation.
The test is done by lightly and briefly touching the tips of the first and fifth toes of the right foot, followed by the same light, brief touch to the first and fifth toes of the left foot, and finally the third toes of the right and left feet. If the patient does not detect the touch at two or more toes, protective sensation has been lost to a large degree. However, one Ipswich Touch Test is usually not enough to determine the percentage loss of protective sensation (Kanza Kazmi et al., 2021; Armstrong & Lavery, 2024).
Other methods to detect loss of protective sensation include reflex testing, pinprick testing, and vibration perception threshold (VPT) testing.
Reflex testing concentrates on evaluating ankle reflexes, since they are regarded as the most sensitive of the lower extremity reflexes when it comes to screening for neuropathy. To perform reflex testing, the clinician ensures that the ankle is in the neutral position and then strikes the Achilles tendon with the examination hammer. An abnormal finding is present if the ankle plantar flexion response cannot be elicited. Abnormal ankle reflexes, combined with an abnormal vibratory or sensory response, is considered a decisive sign of peripheral neuropathy (WOCN, 2022).
Vibratory sense testing can also determine a patient’s sensory perception, with a patient’s inability to sense vibration from a standard tuning fork being indicative of sensory neuropathy. Studies propose that such loss of vibratory sense often happens prior to a patient’s loss of protective sensation and that testing for vibratory sense at the onset of care allows the clinician to discover neuropathy at an earlier stage, when interventions may prove more effective.
The mechanism for vibratory sense testing involves a straightforward procedure using a 128-Hz tuning fork. The clinician strikes the tuning fork on an object or their own hand and then places the tip of the vibrating fork at the patient’s first metatarsal bone at the base of each great toe, instructing the patient to indicate when they can no longer feel the vibration. Test results are regarded as abnormal if the patient can no longer feel the vibrations but the clinician can (WOCN, 2022).
Vibration perception threshold testing uses a handheld device that provides quantifiable results and is comparable to testing with a tuning fork. After explaining the procedure to the patient, the clinician places the oscillator flat against the patient’s foot, applying only enough force so that the surface of the oscillator makes contact with the skin. The amount of vibration is gradually increased until the patient is aware of it, and at this point the reading on the meter is noted. Testing is not done over areas of calluses or scars but can be done along the margins of existing wounds and necrotic tissue if present (Baranoski & Ayello, 2020; Armstrong & Lavery, 2024).
TYPES OF NEUROPATHY
Neuropathy has been broadly classified into three types—sensory, motor, and autonomic—with corresponding characteristics and interventions.
Sensory Neuropathy
Sensory neuropathy leads to the loss of protective sensation, the warning mechanism that lets individuals know that corrective action is needed. When normal sensation is present, feet are immediately removed from danger, but with sensory neuropathy, the awareness of danger is no longer present. For example, a patient may not notice having stepped on a sharp object that has become lodged in the foot or having sustained severe burns by submerging the feet in scalding hot water.
Sensory nerves also conduct messages from the central nervous system to the extremities relating to position. Thus, clinicians may find that patients with DPN have difficulty managing certain movements such as keeping their balance or walking with their eyes closed, which creates a great fall risk for these patients (WOCN, 2022).
Sensory neuropathy frequently begins with numbness and paresthesia (an abnormal sensation of burning or tingling) in the toes and feet and progresses up the patient’s leg in a “stocking” pattern of sensory loss. Eventually, the fingers and hands become affected also, with a loss of sensation moving up the arms in a “glove” pattern.
When the clinician examines the patient’s feet, they also assess for loss of feeling or sensations of “pins and needles” in the patient’s fingers and hands in order to better determine how far the sensory neuropathy has advanced.
Prior to a loss of protective sensation, the patient often experiences several years (in many instances up to 10 years) of painful neuropathy. In its mildest form the patient will refer to these sensations as “pins and needles” or “tingling.” As DPN progresses, the patient often describes “burning,” “stabbing pain,” or “electric shock” feelings. The patient may also complain of itching, heightened sensitivity to generally painless stimuli such as bedsheets on their feet, or an excessive reaction to painful stimuli (WOCN, 2022).
Relief from neuropathy pain comes from movement, and so pain is usually worse at night when the patient is lying down. Patients will complain of interrupted sleep related to neuropathy pain, cramping, restless legs, and the need to move their legs to get relief. The combination of pain and a lack of sleep frequently precipitates exhaustion and depression.
When determining the origin of lower extremity pain in a patient with diabetes, one of the first questions the clinician asks is, What relieves the pain? Ischemic leg pain due to poor arterial circulation is relieved by rest and lowering the extremities (dependent position), whereas neuropathy pain is relieved by the patient getting up and walking around.
Motor Neuropathy
Motor neuropathy is found in the muscles responsible for normal movement of the feet. Distal motor nerves in the foot are most frequently involved, resulting in muscle atrophy. Changes in muscle bulk alter the shape of the patient’s foot, causing deformities that affect the patient’s ability to walk and to bear weight. Over a period of time, patients with motor neuropathy can lose up to half of the muscle volume of their feet (WOCN, 2022).
The visible signs of motor neuropathy are an abnormal gait pattern and irregular weight-bearing that causes areas of excessively high pressure on the soles of the feet. Other signs of motor neuropathy are rigidity in the patient’s ankles and toes, which is also related to atrophy of the intrinsic muscles.
The structural deformities that result from motor neuropathy have a major impact on the patient with diabetes, including balance, ambulation, and the heightened risk for ulceration. Some of the main structural deformities that occur are:
- Hammertoe (“claw toe”): A flexion deformity resulting in a contracture of the proximal joint of one or more of the smaller toes
- Mallet toe: Similar to a hammertoe, but the flexion contracture occurs at a distal joint in the toe
When examining the foot of a patient with motor neuropathy, the clinician often finds unusually prominent metatarsal heads and claw toes. These deformities are due to shortening of the Achilles tendon. The disproportionate prominence of the metatarsal heads causes two problems for the patient: abnormal weight-bearing and a high possibility of shear and pressure injury to the metatarsal heads. This can lead to callus formation and the development of foot ulcers. Foot drop is another complication of motor neuropathy (Zubair et al., 2021 Armstrong & Lavery, 2024).
Autonomic Neuropathy
Autonomic neuropathy is a condition of the autonomic (involuntary) nervous system, which is responsible for the regulation of many body functions such as temperature control, sweating, and the width and tone of blood vessels. Problems with the autonomic nervous system can affect many parts of the body. It is a condition that is usually associated with long-standing diabetes.
A patient with diabetes who develops autonomic loss of control of the sweat glands will present with extremely dry feet, progressing to the development of fissures (either partial- or full-thickness, narrow cracks or openings in the skin). These openings are difficult to treat and keep clean and provide a portal of entry for bacteria, which can result in serious infection. Usually, the loss of sweating spreads from the feet up to the patient’s knees, and the clinician will notice dry, flaking skin on the lower legs (Zubair et al., 2021; Armstrong & Lavery, 2024).
The deregulation of blood vessels common in autonomic neuropathy leads to continuing dilation of the arteries in the feet because there is a lack of sympathetic innervation, which allows for partial constriction of blood vessels when the feet are at rest. During examination, the clinician looks for distended veins over the dorsum of the foot and in the ankle areas. These are a result of arteriovenous shunting, which is a definitive sign of autonomic neuropathy in the feet (WOCN, 2022).
ANSWERING PATIENT QUESTIONS
Q: If I develop a problem with my feet related to my diabetes, won’t I notice some pain?
A: Not necessarily. If a person has diabetic sensory neuropathy, they may have lost a great deal of, or even all, feeling in one or both feet. People with diabetes are not always aware of this, and injury can happen due to poorly fitting shoes or a foreign object in the shoe without the person noticing any pain or discomfort.
TREATING DIABETIC NEUROPATHY
Patients with neuropathy are referred to a neurologist for a full evaluation. Currently, there is no treatment that will prevent or reverse DPN. Interventions to treat DPN include medications, devices, exercise, medical foods, genetic/biologic therapies, revascularization, hyperbaric oxygen therapy, acupuncture, massage, and lifestyle modifications.
MEDICAL FOODS
The term medical food is defined as “a food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for a specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation” (U.S. FDA, 2023).
Drug treatment is by far the most common intervention and includes antidepressants, anticonvulsants, topical preparations, opioids, and nonopioid pain medications. Within drug therapy, the most widely used drugs in the treatment of DPN are the anticonvulsant medications pregabalin, gabapentin, and carbamazepine (Wang et al., 2021).
Some studies have found that treatment with pregabalin and duloxetine have resulted in an improved quality of life for patients with painful diabetic neuropathy. Pregabalin is the drug that has received the most attention, and studies show that it has a positive impact on neuropathic pain in approximately 30%–50% of patients, with the therapeutic range for the drug between 150 and 600 mg/day (Rakusa et al., 2023). In patients 65 years and older, doses are started lower and titrated more slowly.
However, a recent study sounded a warning of an increased risk of adverse health outcomes when gabapentin and pregabalin are used to treat diabetic neuropathy. Study patients were found to be at greater risk for heart failure, myocardial infarction, peripheral vascular disease, stroke, deep vein thrombosis, and pulmonary embolism when there was long-term treatment of diabetic neuropathy with gabapentin and pregabalin (Pan et al., 2022).
In its position statement on diabetic neuropathy, the ADA emphasizes the importance of screening and early recognition and intervention. The most important interventions in combating all types of neuropathy are tight glycemic control, patient education, and close monitoring for progression of symptoms. In the presence of DPN, daily foot care and inspections become more important. The clinician explains to the patient that once protective sensation is lost, their eyes now become the barrier to injury (ADA, 2024).
Treatment of diabetic neuropathies also requires the skills of all members of the diabetic foot care team. Neuropathy can lead to a progressive loss of proprioception, weakness, and impaired balance. Unsteady gait and pain can make it increasingly difficult for patients to perform activities of daily living (ADLs).
Physical therapists are instrumental in training patients in safe ambulation and/or gait training (with an appropriate assistive device, if indicated) as well as dynamic balance and proprioceptive training. Occupational therapists will provide education in the use of any equipment recommended to assist with ADLs. Studies have found that for patients who have limited capacity for exercise, low-intensity aerobic therapy can lead to better sensation in the feet and decrease pain and tingling sensations in the lower extremities (Hill, 2023).
Exercise has been shown to decrease pain related to motor neuropathy and includes a comprehensive program of strength training, exercises to improve balance, flexibility exercises, and aerobic exercises. The physical therapist individualizes a therapeutic exercise program to match patient needs and may also recommend splints or braces, if appropriate, to improve patient balance and posture (Foundation for Peripheral Neuropathy, 2023).
Charcot Osteoarthropathy
Charcot osteoarthropathy is one of the most serious complications of diabetes. Early recognition and prompt and aggressive treatment are necessary to prevent progression of the condition and possible limb amputation.
Charcot osteoarthropathy, frequently referred to as Charcot foot, has been described in nonmedical terms as a condition in which the bone crumbles. Charcot causes bone destruction in the affected foot that has been compared to what ensues in osteoporosis. Dr. Jean-Martin Charcot, a neurologist, first diagnosed the condition in 1868 in patients with tertiary syphilis. In 1936 the condition was also recognized in patients with diabetes (Zubair et al., 2021; Armstrong & Lavery, 2024).
Research shows that almost 13% of persons with diabetes who have a loss of peripheral sensation and automatic neuropathy are likely to develop Charcot foot disease (WOCN, 2022). The five-year comparative mortality rate can reach to as high 45% for patients with diabetes-associated Charcot foot, with a mean decrease in life expectancy of 14 years and an average life expectancy of 7.9 years post Charcot foot diagnosis (Armstrong & Lavery, 2024). Fortunately, greater awareness of the signs and symptoms of Charcot foot has resulted in earlier intervention.
Charcot foot many times begins with a slight injury to the joint that may not be noticed by the patient, such as stepping off the curb the “wrong” way, resulting in a minor ankle sprain.
Because the presenting symptoms are similar, Charcot foot is often misdiagnosed as cellulitis and treated with antibiotics, which does not address the underlying condition. This may happen with clinicians who are not familiar with Charcot foot when they observe an extremity that has the classic signs of infection (swelling, redness, pain, and tenderness). However, if the patient has diabetes, these signs should arouse a high suspicion of Charcot foot.
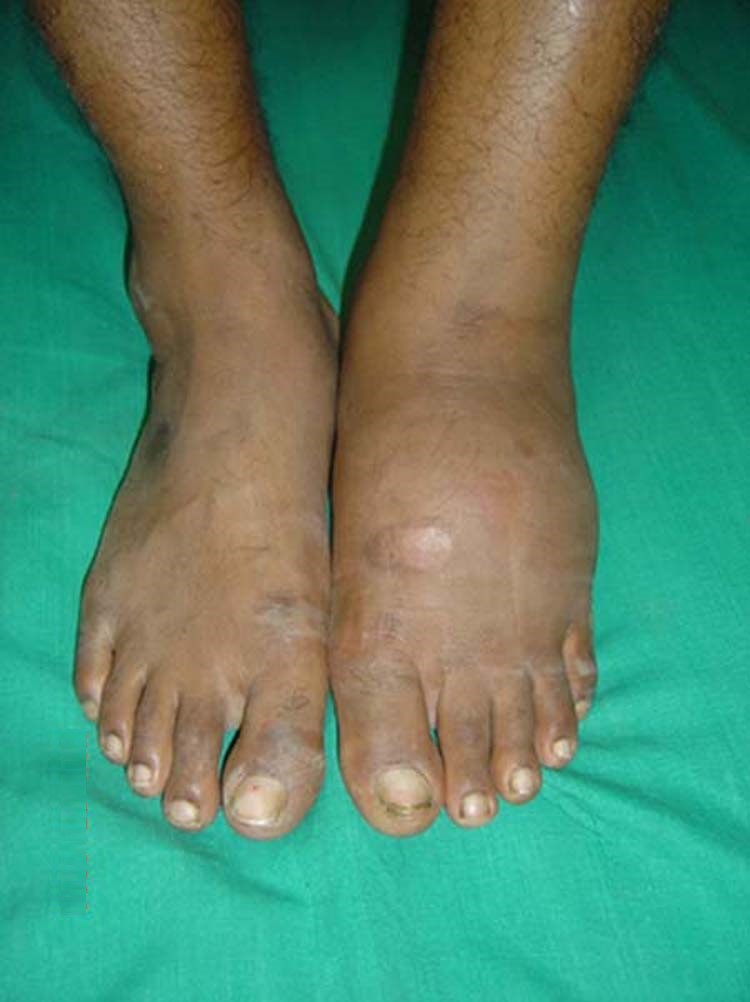
Typical Charcot foot presentation, with diffuse and nonpainful swelling in the left foot. (Source: J. Terrence Jose Jerome. Creative Commons Attribution 3.0.)
STAGES OF CHARCOT OSTEOARTHROPATHY
Charcot osteoarthropathy has been divided into three stages:
- Acute or developmental
- Subacute, coalescence
- Chronic, reconstruction-consolidation
(AOFAS, 2024; Armstrong & Lavery, 2024)
In the acute phase of Charcot foot, the patient presents with a foot that is red, swollen, and warm to the touch. There can be a difference in temperature between the affected limb and the nonaffected limb of >2 °C (3.6 °F). It should be noted that pain with Charcot foot is usually less than that experienced with cellulitis (Zubair et al., 2021; Armstrong & Lavery, 2024).
Foot X-rays may show bone crumbling, but there is also a possibility that X-rays will be normal at this early stage of the condition. A bone scan can be useful in discovering focal areas of bone damage. An important sign that the clinician looks for while examining the patient’s foot is a decrease or resolution of redness when the foot is elevated (Zubair et al., 2021; Armstrong & Lavery, 2024). Other signs that the clinician observes in the early presentation of Charcot disease are:
- Swelling of the foot and ankle on the affected side
- Palpable, bounding pedal pulses
- Diabetic peripheral neuropathy with loss of protective sensation
Laboratory studies typically used to diagnose the presence of infection or an inflammatory process (such as a white blood cell count, C-reactive protein, and serum uric acid) are all normal in the acute stage of Charcot foot. Pulses in the extremity affected with Charcot osteoarthropathy are frequently strong and bounding due to the shunting of the circulation in the foot.
There may be several weeks or months between the onset of symptoms and diagnosis. However, if diagnosis is missed at this stage, it can result in further bone destruction and irreversible damage to the affected extremity (AOFAS, 2024; WOCN, 2022; Armstrong & Lavery, 2024).
As Charcot foot progresses, pathophysiologic changes are more readily seen on X-ray. These changes include deformity, dislocation, partial dislocation of joints, and bone fractures.
The coalescence or subacute stage is noticeable for repair of the extremity, which involves a decreased swelling of soft tissue, the production of bone callus, and the healing of fractures.
Remodeling of bones takes place in the chronic reconstruction-consolidation phase. This final phase is signified by the occurrence of ankylosis of joints and hypertrophy of involved bones. Clinical findings include the development of a foot deformity, unsteadiness, and abnormal function of the affected joint. The arch of the foot collapses, with the development of what looks like a “rocker bottom” (AOFAS, 2024; Zubair et al., 2021; Armstrong & Lavery, 2024).
Charcot foot can lead to severe deformities that not only alter the patient’s gait but also create areas of high pressure on the surface of the foot, prompting the development of a diabetic foot ulcer. During examination of the patient’s foot, the clinician looks for significant deformity signifying collapse of the structures of the midfoot and the development of rocker-bottom foot (WOCN, 2022; Armstrong & Lavery, 2024).
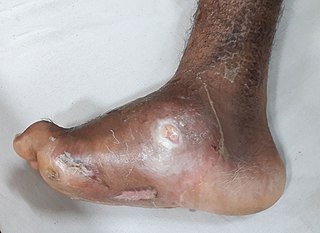
Charcot foot deformity with an ulcerated abscess. (Source: Wikimedia Commons, Creative Commons Attribution-Share Alike 4.0.)
TREATMENT OF CHARCOT FOOT
The treatments for Charcot foot include surgical and nonsurgical interventions. Nonsurgical treatments include the use of protective splints, walking brace, orthosis (the Charcot restraint orthotic walker [CROW] boot), or a cast. Weight-bearing is usually dependent on the stage of the disease; patients with stage 1 or 2 may be permitted to bear weight on the affected extremity (AOFAS, 2024a).
The appropriate treatment for the patient depends on how far the condition has progressed, the degree of foot deformity present, and which joints are involved. Off-loading and redistributing pressure over the surface of the foot is the cornerstone in the treatment of Charcot foot, preventing the occurrence of diabetic foot ulcers, and healing an existing ulcer (AOFAS, 2024; Armstrong & Lavery, 2024).
The goal of care at this stage is to decrease inflammation and edema and to limit further bone and joint destruction. Non-weight-bearing is the best way to achieve this. This involves immobilization of the extremity. Total contact casting is considered the gold standard for off-loading Charcot foot (Zubair et al., 2021; Armstrong & Lavery, 2024).
Total Contact Casting (TCC) for Charcot Foot
The total contact cast is applied according to the manufacturer’s instructions by a clinician who has been trained in the technique, since an improperly applied or removed cast can cause unforeseen pressure and lead to ulcer formation. TCC is applied to the whole surface of the foot and usually extends to just below the patient’s knee (Zubair et al., 2021; Armstrong & Lavery, 2024). (See also “Total Contact Casting” later in this course under “Off-loading.”)
Typically, the first cast is removed one week after placement to allow inspection of the foot. Thereafter, the cast is usually changed every two weeks. Casting and limited weight-bearing will continue until the inflammation has resolved.
During each cast removal the clinician carefully examines the affected foot for signs that indicate a decrease in inflammation, including decreased swelling, redness, and temperature. If a skin thermometer is available, a skin temperature in the affected foot that is within 2 °C (3.6 2 °C) of the contralateral limb is regarded as being significant for resolution of inflammation. X-rays of the affected foot should be done on a monthly basis until bone callus consolidation is evident. It can take 12–16 weeks to reach this stage of recovery. The average treatment period, which includes TCC followed by guarded weight-bearing in a removable cast walker and subsequent return to therapeutic footwear, can take 4–6 months (Bryant & Nix, 2016).
Prefabricated Pneumatic Walking Brace (PPWB)
Not all patients are candidates for total contact casting. The most important consideration is adequate circulation to the extremity. An alternative to TCC is a prefabricated pneumatic walking brace fitted with a custom insole (WOCN, 2022).
PPWBs have the advantage that they are easy to remove, have a high satisfaction rating with patients, and have not been associated with any major complications. They are made from a lightweight, partially rigid casing with an inner lining that supports the patient’s lower leg on the affected side to a few inches below the knee. They are usually secured in place with buckles and Velcro straps. However, they have limited use in patients with severe foot deformity. A further potential drawback is patient compliance, since this is a device that can be removed at home (WOCN, 2022).
Pneumatic walking brace/boot. (Source: www.bigstock.com, © Karel Bock.)
Postacute Interventions
When the stage of acute inflammation has resolved, total contact casting can be discontinued and the patient fitted for a special brace or footwear to protect the foot. A referral to an orthotist and possibly a pedorthist will be necessary. The goal at this stage is to provide continued stabilization to the foot and ankle while the patient returns to full ambulation. Options for limb protection include a patellar tendon–bearing brace, specialized shoes with modified ankle-foot orthosis, or a Charcot restraint orthotic walker (CROW) boot.
The CROW boot was created for patients who have significant foot and ankle deformity due to Charcot disease. It is fully lined, has a custom foot insert, and includes a rocker-bottom sole. The CROW boot can be modified to address changes in the patient’s foot; this is usually achieved by adding or trimming padding where and when appropriate (AOFAS, 2024a).
Surgical intervention is related to staging. In the early stages of Charcot disease, some patients may be candidates for the surgical procedure of open reduction and internal fixation and fusion. In more advanced stages of the condition, surgeries include alteration of the deformity using realignment osteotomy and fusion or surgical extraction of the bony prominence at risk for causing ulceration via ostectomy (AOFAS, 2024b).
Assistance from various members of the diabetic foot care team is critical at this stage, especially from the physical therapist and the occupational therapist. The patient will require modified gait training with the new device, as well as instruction in how to correctly don, doff, and care for it. Once the fitting specialists have given the patient instructions, it will be up to other clinicians to reinforce the teaching and to assess for any subsequent problems the patient may be encountering with the device.
Ongoing care includes lifetime monitoring of the affected limb, optimum glycemic control, and patient education on the importance of preventing further injury. Once Charcot deformity is present, patients must be made aware of the increased risk for the development of a diabetic foot ulcer. As well as performing the regular components of their routine foot checks, the patient is also taught to check for “hot spots” along their feet and ankles and to inform the clinician immediately if any are detected (WOCN, 2022; Baranoski & Ayello, 2020).
CASE (continued)
Mr. Hernandez is having his monthly follow-up appointment at the clinic. Since his last appointment, the case manager has spoken with Mr. Hernandez, who agreed to having a translator present so his wife will be fully aware of what is going on. Today, Mr. Hernandez will meet with the nurse practitioner (Celia) and physical therapist (Royce).
Mr. Hernandez is happy to share his good news with the team: he has lost 6 pounds since his last visit, which he attributes to the fact that he has stopped drinking sodas. The team congratulates him on his success as well as on the slight drop in his daily blood sugar readings. It is obvious from his demeanor that losing weight has had a positive impact on Mr. Hernandez’s attitude toward his condition, and Mrs. Hernandez asks what she can do to help her husband lose even more weight.
Celia reviews his family history with Mr. Hernandez and asks for more detail about his mother’s kidney disease. As Celia suspected, his mother had been a diabetic for many years prior to developing kidney disease. She explores with Mr. Hernandez what this meant to him, and he admitted that he had a deep fear the same would happen to him. His mother’s diabetes was poorly controlled, and he believed this was the same for all patients with diabetes. Both Celia and Royce, the physical therapist, spend time explaining to Mr. Hernandez and his wife (via the translator) that diabetes can be controlled.
Mr. Hernandez agrees to a foot exam. A Weinstein-Semmes monofilament exam indicates that Mr. Hernandez has diabetic peripheral neuropathy in both feet, with his left foot more severely affected. There is also a noticeable deformity of his left foot, with increased temperature around the dorsal surface and ankle areas compared to the contralateral limb and increased redness on the plantar surface of the left foot along the base of the metatarsal heads. Mr. Hernandez states that it’s “impossible” for him to take time off from work and that, “although my left foot is a bit red, it feels fine.”
An X-ray ordered by Celia is normal, but the team is concerned that Mr. Hernandez is developing early-onset Charcot osteoarthropathy. When Mr. Hernandez entered the exam room, Royce noted a slight deviation in his gait that propels him more to his left side. Royce examines the patient’s footwear, which are strong leather work boots with a reinforced toe area. The toe box is sufficiently deep to accommodate the foot, but the insole is hard and rigid from excessive wear. As a roofer, Mr. Hernandez states that this is the only type of footwear he is allowed to wear at work.
Mr. Hernandez has signs of early Charcot foot, which is a serious complication. Given that he cannot take time off from work, the team must develop suitable strategies to protect his feet from further damage. Ideally, he should be non-weight-bearing to his left foot, but since this is not a practical solution, the next best alternative is to schedule an immediate consultation with an orthotist. The physical therapist believes there is sufficient room in Mr. Hernandez’s boots for a specially fitted insole to help relieve pressure. The physical therapist also gives Mr. Hernandez a written list of recommendations to use when he is not at work, including wearing seamless socks and cushioned slippers and resting with his feet elevated.
His nurse practitioner and physical therapist must also respond to Mr. Hernandez’s statement that his left foot “feels fine.” Like many other patients with diabetes, Mr. Hernandez finds it hard to believe there could be anything seriously wrong with his foot since he feels no pain. The results of the monofilament testing are explained carefully to the patient. Celia tells him that he feels no pain because he has lost sensation to several parts of his left foot, but he is probably not aware of this because other areas of his foot still have sensation. Techniques on preventing injury to his feet, including a daily foot exam, need to be taught to Mr. Hernandez and his wife (if he agrees) and reinforced at every follow-up visit. They will need written educational materials in both English and Spanish.
In response to Mrs. Hernandez’s question about helping her husband continue to lose weight, the team believes a good place for her to start is with a review of their food intake. They reinforce the positive results she has noticed from her husband’s lower weight and his willingness to continue this effort. The case manager is consulted, who indicates that she will identify community resources where Mrs. Hernandez can learn about meal planning and healthy cooking for a person with diabetes.
As the appointment ends, Celia arranges to see Mr. Hernandez back in the clinic in two weeks. During that time, Royce will work closely with the orthotist on securing pressure-relieving customizations to Mr. Hernandez’s existing footwear.
(continues)ASSESSMENT OF PATIENTS WITH DIABETIC FOOT ULCERS
Patients who present with an existing diabetic foot ulcer require holistic assessment that includes:
- Physical assessment of the wound
- Wound history
- Neuropathy assessment (discussed earlier in this course)
- Peripheral arterial disease assessment
- Ankle-brachial assessment
- Infection assessment
- Classification of the wound
One of the main goals of assessment is to identify the underlying cause of the diabetic foot ulcer and to make every effort to either correct it or remove it.
Physical Assessment of the Wound
Physical assessment begins with inspecting and comparing both feet for structural changes, pressure points, wound location, and other wound characteristics.
STRUCTURAL CHANGE
Structural changes such as collapse of the arches of the foot and a rocker-bottom appearance on the plantar surface of the foot are frequently found on examination (see also “Diabetes Complications” earlier in this course).
PRESSURE POINTS
Areas of increased redness may be observed in comparison to surrounding tissue.
WOUND LOCATION
The location of an ulcer helps to determine the cause and the most appropriate method of relieving pressure. Diabetic ulcers can occur on any part of the foot. The most common locations are over the dorsal aspects of the toes and on the plantar surface of prominent metatarsal heads. Ulcers also frequently occur along the margins of the foot over bony prominences (Armstrong & Lavery, 2024). Other sites for the development of diabetic foot ulcers are:
- Toe webs, often the result of increased moisture, footwear that is too narrow, toe crowding, and toe deformities
- Over bunions, where there is increased pressure from shoes that do not fit correctly
- Distal ends of the toes, which indicate that there is poor arterial circulation
- Plantar surface of the midfoot area, a common site for diabetic foot ulcers when a Charcot foot deformity is present
- Heels, often starting as deep, narrow fissures
(Armstrong & Lavery, 2024)
SHAPE
Diabetic foot ulcers are frequently round or oblong in shape.
SIZE
Initially, it may be hard to determine the size of the ulcer. In many cases, foot ulcers are covered with a layer of callus, and the true size cannot be determined until that is removed. The size of the wound is measured in centimeters using a plastic or paper disposable ruler. Methods for obtaining measurements include:
- Measure the longest part of the wound and the widest part of the wound perpendicular to the length.
- Use the face of a clock to represent the patient’s body and describe the wound’s orientation, with the patient’s head being at the 12 o’clock position and feet at 6 o’clock. The width of the wound is then stated as the distance from 3 o’clock to 9 o’clock.
DEPTH
Wound depth can range from partial thickness, where only the upper layers of the epidermis and dermis are involved, to full thickness, extending farther to deep bone structures. At initial view, the wound may appear to be a small, shallow surface wound; however, on further examination and probing of the wound, hidden depths may be found, along with sinus tracts extending down to bone. The depth of the wound is assessed by gently inserting a sterile cotton-tipped applicator into the wound until it reaches the bottom; a mark is made on the applicator parallel to the wound surface; and the applicator is then gently removed and the depth measured with a disposable centimeter ruler.
WOUND BASE
Depending on its depth, the wound base can vary from pink to pale red for wounds that are shallow. Sometimes, it may not be possible to visualize the base of the wound, especially in the case of deep wounds that extend down to bone or where necrotic tissue is covering the base.
UNDERMINING/TUNNELING
Undermining is the loss of tissue under intact skin surfaces, frequently extending a short distance from the wound. Tunneling is a tract that continues from the surface of the wound down through the underlying tissues; it is also referred to as a sinus tract. These wound characteristics can be measured and documented using the clock-face method (see above), i.e., “undermining for 2 cm from 8 o’clock to 12 o’clock, with the greatest depth of 3 cm.” Tunneling is measured by gently inserting a sterile cotton-tipped applicator to measure the depth of the wound (see above).
WOUND EDGES
In diabetic foot ulcers, the edges of the wound are usually well defined. Undermining may or may not be present. When assessing the wound edges, the clinician observes whether the wound edges are open and proliferative; such edges indicate a potential to assist with wound healing by facilitating cell migration across the wound bed, whereas edges that are closed and rolled under prevent the wound from closing. With diabetic foot ulcers, it is not unusual to find hyperkeratosis, a callus-like tissue that forms around the edges of the wound and causes them to become hard and thickened.
PERIWOUND AREA
Callus formation frequently extends into the periwound area in diabetic foot ulcers. Other findings in the periwound area may be erythema, induration, and maceration, which indicate possible infection.
EXUDATE
Exudate (drainage) can range from small to large amounts. The amount of drainage in diabetic foot ulcers is usually small to moderate. However, where infection or other disease conditions are present, such as heart disease, renal disease, or venous insufficiency, the amount of drainage can be large. Drainage from diabetic foot ulcers is usually clear or serous. The presence of purulent drainage with a foul odor is indicative of infection.
TYPE OF TISSUE PRESENT
The type of tissue found in diabetic foot ulcers can vary from pink nonviable tissue to slough and necrotic tissue. Slough is a thin, stringy, or mucus-like substance that is yellow or tan in color and either loosely or firmly attached to the wound. Necrotic tissue is dead, devitalized tissue that is black or brown in color and usually firmly attached to the wound base. It is common for diabetic foot ulcers to have more than one type of tissue present in the wound. Exposed bone is also a critical finding in a diabetic ulcer and requires immediate attention.
(Armstrong & Lavery, 2024; Bryant& Nix, 2024; WOCN, 2022)
(See also “Osteomyelitis” later in this course.)
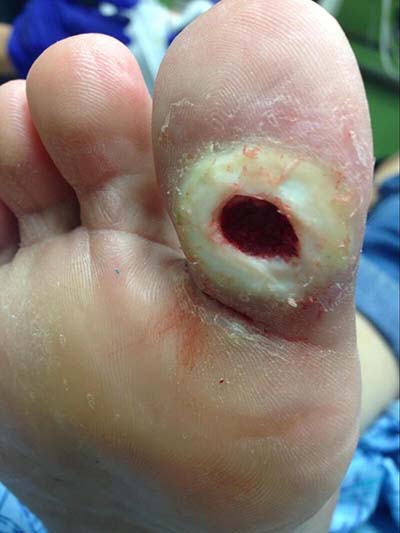
Diabetic foot ulcer with periwound callus formation. (Source: Mark A. Dreyer, Creative Commons Attribution 4.0.)
Wound History
Another essential step in assessing a foot ulcer is obtaining a complete history, especially if this is the first time the clinician has seen the patient. (See also “Health and Family History” earlier in this course.)
Questions at this stage include:
- How long has the ulcer been there?
- Has the patient had previous foot ulcers and in what locations?
- Is the patient aware of precipitating circumstances that led to the ulcer development?
- Is there pain associated with the ulcer?
- What tests and treatments has the patient had in the past, and what is the current status of their wound care? How successful does the patient rate the treatments received?
- What is the patient’s perception of the seriousness of the ulcer?
- How has the ulcer affected the patient’s activities (i.e., work, recreation, hobbies)? Does it affect the amount and quality of sleep? How has it impacted relationships with loved ones, friends, and coworkers?
- What are the patient’s goals for treatment? Is the patient able and willing to commit to the time and effort necessary to achieve healing?
- What support does the patient have? Who can provide help with wound care, keeping appointments, and travel, if needed? What type of health coverage does the patient have? Is the patient aware of community resources and has the patient used them in the past?
- Does the patient have coexistent health conditions?
- Is the patient taking any medications, including prescription, over-the-counter, and herbal/naturopathic substances?
- Does the patient smoke, and if so, how much? Does the patient drink alcohol and how much? Use any illicit drugs?
- What other healthcare providers does the patient see, and how frequently? The clinician ensures that the patient’s diabetes care team has access to all of the patient’s records from other providers so that these can be fully reviewed. This may necessitate getting the patient’s written consent for release of records from other providers.
Infection Assessment
It has been estimated that around 59% of diabetic foot ulcers are already infected when the ulcer is first evaluated by a healthcare provider (Zubair et al., 2021). Another source places the percentage of patients with diabetic foot infections at 58%, with around 20% of these patients developing osteomyelitis (Baranoski & Ayello, 2020; Armstrong & Lavery, 2024).
It is essential that infection in a diabetic foot ulcer is recognized early and treated. Every patient with diabetes who presents with a foot ulcer must be thoroughly assessed for infection. This can prevent a mild infection from progressing to a more critical stage.
Detecting infection in a diabetic foot ulcer can be challenging. The patient’s temperature and white blood cell count are often normal. Studies show that no more than 50% of severe infections in diabetic foot ulcers result in elevation of white blood cell count. An elevated temperature is usually an indication that the infection has spread into the deep spaces of the foot.
Patients with diabetes who have an undiagnosed ulcer infection may complain of feeling generally unwell, and there is frequently a sudden spike in blood sugar levels even though the patient has not made any changes to diet or activity level.
In patients with diabetic neuropathy, pain is often not a finding, and so the patient will not complain of pain in the wound or surrounding tissues.
The benchmark for diagnosing infection in a diabetic foot wound is the existence of at least two or more of the following:
- Localized swelling or tissue induration
- Erythema extending from wound margins >0.5 cm
- Localized tenderness or pain
- Localized warmth
- Purulent drainage
(Armstrong & Lavery, 2024)
Cellulitis is a common finding. Other more subtle signs of infection are friable (disintegrating easily) granulation tissue in the wound, undermining in the wound, large amounts of wound drainage, and a malodor that persists after the wound is cleaned (WOCN, 2022).
Taking cultures from a diabetic foot ulcer to check for infection is recommended if slough and necrotic tissue remain present after the wound has been surgically debrided. All open wounds are colonized with organisms, and this colonization will intensify if devitalized tissue is present.
The technique used for obtaining cultures is important to distinguish between colonization and infection. To get the most accurate results, the culture is obtained either by biopsy from the debrided wound base or by aspirating tissue fluids. Tissue biopsy is done by removing a small piece of tissue from the ulcer base using a sterile scalpel and forceps. To obtain a fluid sample, a sterile needle is inserted into the tissues adjacent to the ulcer, and fluids are then aspirated. This procedure is done by a physician or other appropriately trained and licensed clinician (Baranoski & Ayello, 2020; Bryant & Nix, 2024).
The presence of widespread inflammation and crepitus (a grating sound heard when the area adjacent to the wound bed is palpitated) are indicators of deep wound infection. Crepitus is caused by the presence of air in the subcutaneous tissues. Gangrene is another sign that is highly suggestive of a severe infection in the affected foot.
Osteomyelitis (infection in the bone) is a common finding in diabetic foot ulcers, especially in patients with moderately to severely infected diabetic ulcers. Clinical findings that suggest osteomyelitis include a diabetic foot wound >2 cm2 that has been present for six months, an elevated erythrocyte sedimentation rate, elevated white blood cell (WBC) count, fever, and a positive probe-to-bone test. The recommendation from the Infectious Diseases Society of America is that a probe-to-bone test be done on any infected diabetic foot ulcer (IDSA, 2023; WOCN, 2022).
(See also “Osteomyelitis” and “Treating Infection” later in this course.)
LIMB-THREATENING INFECTION?
Diabetic foot infections are seen as either non-limb-threatening or limb-threatening. The characteristics of non-limb-threatening infection are:
- Cellulitis that is <2 cm circumferentially
- No signs of systemic infection
- No deep abscess, osteomyelitis, or gangrene present
The characteristics of a limb-threatening infection are:
- Cellulitis around the wound that extends beyond 2 cm
- Signs of systemic infection (a temperature >98.6 °F [37 °C] is an important finding in a patient with a diabetic foot ulcer)
- Presence of a deep abscess, osteomyelitis, or gangrene
(Armstrong & Lavery, 2024)
Classification of Diabetic Foot Ulcers
Classification systems for diabetic foot ulcers have been developed to aid clinicians in organizing and relating assessment data regarding diabetic foot ulcers. Referring to the wound simply as a diabetic foot ulcer was considered too vague and did not provide concrete information from which to develop a treatment plan or determine a prognosis. Classification systems have helped to provide a consistent approach to treatment and a common language that facilitates better communication among providers. For any classification system to be meaningful, it must be used consistently by all members of the wound care team, with appropriate documentation in the patient’s records (WOCN, 2022).
Classification systems grade ulcers according to the presence and extent of several physical traits, namely, the location, size, depth, and appearance of the ulcer. The two mostly widely used are the Wagner System and the University of Texas Diabetic Foot Classification System.
WAGNER SYSTEM
The Wagner system was initially developed in the 1970s as a guideline for the level of surgical intervention. However, it is now a widely used system to determine the severity of the diabetic foot ulcer and to guide overall treatment. The Wagner system is centered on three components of the diabetic foot ulcer:
- Ulceration
- Infection
- Ischemia
It then divides the diabetic foot ulcer into six different grades, 0 to 5.
| Grade | Description |
|---|---|
| (Zubair et al., 2021) | |
| 0 | No open areas, or a history of a previous healed ulcer |
| 1 | Superficial ulcer |
| 2 | Exposed deep structures such as tendon or bone |
| 3 | Abscess or osteomyelitis detected in deep tissues |
| 4 | Wet or dry gangrene present on the toes or part of the forefoot; infection present or not present |
| 5 | Gangrene encompassing the entire foot |
UNIVERSITY OF TEXAS CLASSIFICATION SYSTEM
The University of Texas diabetic foot ulcer classification system uses grades and staging to classify diabetic foot ulcers. This system is seen as an improvement on the Wagner system, has been well validated, and is regarded as an accurate predictor of patient outcomes. Grades denote the depth of the wound, and staging signifies complications that impede healing, in particular infection and ischemia (WOCN, 2022). The depth grade ranges from 0 to 3 as described in the table below.
| Grade | Depth |
|---|---|
| (Zubair et al., 2021) | |
| 0 | No open area present |
| 1 | Presence of a superficial full-thickness or partial-thickness ulcer |
| 2 | Presence of a deep wound that includes tendon or joint capsule |
| 3 | A wound that is down to bone |
| Stage | Infection/Ischemia |
| A | No infection present |
| B | Wound infection present, but not ischemic |
| C | Ischemia present, but no infection |
| D | Ischemia and infection both present |
PEDIS SYSTEM
The PEDIS system was developed by the International Working Group on Diabetic Foot Ulcers as a user-friendly classification system for clinicians who are new to diabetic foot ulcer management. It looks at four different factors to assess the severity of the ulcer:
- Perfusion status
- Extent (size of the wound)
- Depth (amount of tissue lost)
- Infection and Sensation (presence of neuropathy)
(IWGDF, 2023)
MANAGEMENT OF THE DIABETIC FOOT ULCER
The development of a diabetic foot ulcer is a significant progression for the patient with diabetes. Many times, the patient is not seen by the wound care team until a diabetic foot ulcer is already present, and patient education must be provided in the midst of a great deal of other interventions. The patient may feel overwhelmed and lack the capacity to grasp all the new information. In such a case, the clinician focuses on creating a supportive relationship with the patient. Treatment goals, management strategies, and obstacles that the patient encounters are explored, and solutions that are workable for the patient are put in place.
Once a diabetic foot ulcer has developed, there are two important goals: 1) healing the existing ulcer and 2) preventing further ulcers from developing. A history of a prior diabetic foot ulcer is the greatest risk factor for developing further ulceration. The occurrence of an ulcer ranges from 30% to 50% in persons who have a history of a previous ulcer (Baranoski & Ayello, 2020; Bryant & Nix, 2024).
In order to achieve the goal of wound healing and complete wound closure, interventions must be started early and applied aggressively. Several interventions take place simultaneously, for example, infection control, assessing for adequate blood supply to the affected extremity, wound care, and off-loading.
It is a highly stressful time for patients and families, who can have a hard time keeping up with all that is going on, meeting new providers, the possibility of information overload, and having to make rapid decisions, plus the very real fear of losing a limb. The support of the wound care team is of paramount importance at this time, and patients will often rely on clinicians whom they are most familiar with to help them understand what is happening and guide them in making decisions. Therefore, it is important that in the frenzy of activity, the clinician makes quiet time to be with the patient as an individual and to simply ask, “How are you doing?”
Diabetes Management
To achieve healing of the diabetic foot ulcer, the wound care team must concurrently address optimal diabetes control, with the primary focus on tight glycemic control and managing risk factors such as hypertension, hyperlipidemia, and smoking. A nutritional evaluation should also be performed and any nutritional deficiencies corrected.
If the patient’s footwear has not been examined previously, a footwear assessment is done at this stage. If possible, the clinician exams the footwear that the patient wears to work and when going out as well as the slippers or other footwear worn while at home. If the patient is not yet in the habit of checking their footwear, the clinician instructs them in this important step, stressing the importance of examining all footwear for the presence of foreign bodies that may cause irritation and trauma to the patient’s foot (e.g., pebbles, small pieces of glass, straight pins, small needles, even pet hairs) (Armstrong & Lavery, 2024; IWGDF, 2023).
Vascularization of the Affected Limb
An ulcer will not heal without an adequate blood supply regardless of what other interventions are put in place. Studies indicate that 30% of patients with diabetes who have lower extremity neuropathic disease also have coexisting peripheral arterial disease (PAD) (WOCN, 2022). The essential elements of a vascular assessment include:
Inspection of the skin, hair, and nails of both lower extremities, to determine if atrophic changes are present. The clinician looks for skin that is pale in color, thin, and shiny. However, the clinician is aware that the feet can be red and warm to the touch even in the presence of severe ischemia; this results from a high shunt blood flow due to autonomic neuropathy (WOCN, 2022). Also notable is a lack of hair growth on the lower extremities that is not typical for the patient (i.e., the patient may state, “I used to have a lot of hair, even on my toes, but not anymore”). Ridged toenails may also be present.
Venous refill time. Prolonged venous refill time of >20 seconds and prolonged capillary refill of >3 seconds indicate decreased perfusion to the extremity.
Palpation of lower extremity pulses (the dorsalis pedis pulse, located on the dorsal surface of the foot, and the posterior tibialis pulse, found posterior and inferior to the medial malleolus). If the clinician is unable to palpate pulses, a handheld Doppler probe is used. Diminished or absent pulses require further testing and a probable consult to a vascular specialist. However, vascular insufficiency cannot be determined solely on the absence of pulses; some people lack one or both pulses and still have normal circulation, whereas many people with vascular insufficiency have normal pulses (WOCN, 2022).
Assessment of ankle-brachial index (ABI). This is a simple, indirect test of lower extremity perfusion done by the clinician in the clinic. It is one of the most frequently used noninvasive tests of lower extremity circulation. ABI is measured by comparing pressures in the upper extremities with those in the lower extremities.
OBTAINING AN ABI READING
- Have the patient rest supine in a quiet, comfortable environment for about 10 minutes before starting the test.
- Explain the procedure to the patient and begin by measuring the brachial pressures in each upper extremity using a standard blood pressure cuff that is the appropriate size for the patient, a Doppler probe, and transmission gel.
- Place transmission gel over the pulse site and inflate the cuff to about 20 to 30 mm higher than the last sound heard.
- Place the tip of the Doppler probe pointing toward the patient’s head at a 45-degree angle and slowly deflate the cuff until the first sound is detected. This is then recorded as the systolic pressure, and the procedure is then repeated in the opposite extremity.
- Place an appropriately fitting cuff around the lower leg about 3 cm above the malleolus, and follow the same procedure as above for obtaining the pulses. In this instance the tip of the Doppler probe is at a 45-degree angle pointing toward the patient’s knee.
- Measure the dorsalis pedis and the posterior tibialis pulses in both lower extremities.
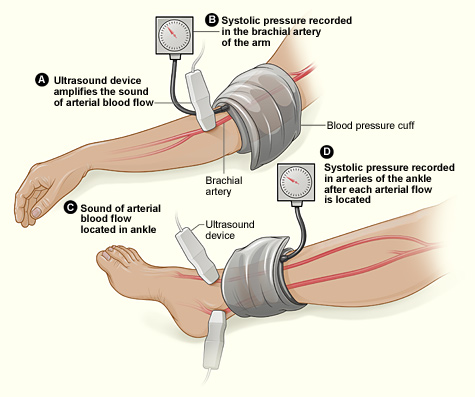
(Source: National Heart, Lung, and Blood Institute.)
The ABI is calculated by dividing the higher of the dorsalis pedis or the posterior tibialis pressure for each lower extremity by the higher of the right or left upper extremity systolic pressure. An ABI reading of 1.0 is considered normal (WOCN, 2022).
However, arteries in the lower extremities can become less compressible due to diabetes, and this can result in ABI readings of >1.1–1.3. These results are abnormal and can be found in approximately one third of all patients with diabetes. Abnormal ABI results must be followed up with more extensive testing. ABI testing is done in the imaging department, where Doppler waveform patterns will be available and provide a more accurate picture of blood flow to the lower extremities.
Indications for revascularization include not only critical limb ischemia but any situation in which there is evidence of decreased or impaired circulation. It is important for the clinician to keep in mind that adequate perfusion is essential to achieve healing and to prevent or delay a future amputation. The recommendation is that all patients who present with critical limb ischemia, an existing diabetic foot ulcer, and rest pain be referred to a vascular surgeon for evaluation of arterial reconstruction (WOCN, 2022).
Although the rate of lower extremity amputations caused by infection has remained steady, the rate of amputations related to peripheral arterial disease (PAD) has decreased 10-fold in the United States over the past 10 years. This drop is accredited to the development of endovascular surgical techniques that permit arterial inflow surgery to be extended distally with fewer complications. It is reported that up to 80% of PAD can be remedied by endovascular therapy. The existence of several areas of stenosis may need a combination of angioplasty and bypass graft. However, the drawbacks that have been noted with endovascular surgical techniques include reduced hemodynamic advantage and diminished long-term stability, compared with bypass surgery (WOCN, 2022).
The vascular status of the affected extremity must always be determined before proceeding to sharp debridement of the ulcer. Prior to performing extensive sharp debridement, a consult to the vascular surgeon may be warranted and revascularization done, if appropriate, to prevent damage to tissues that already have poor circulation (IWGDF, 2023). However, if there is wet gangrene or abscess formation in the ulcer, debridement is performed immediately, and this is usually done in surgery. Revascularization of the affected leg is attempted as soon as possible after the surgical debridement. Depending on the type of revascularization procedure, the time frame to achieve maximum blood circulation to the foot can range from a few days to up to four weeks.
Wound Care
Wound care for diabetic foot ulcers focuses on the steps listed below to create the optimum environment for wound healing:
- Radical and repeated debridement of the wound to remove all necrotic tissue
- Frequent wound inspection and assessment
- Bacterial control
- Attention to maintaining moisture balance to optimize healing
- Supporting epithelial edge advancement
- Optimal dressing selection
(WOCN, 2022)
DEBRIDEMENT
Wound and callus debridement is an essential part of diabetic foot care. For diabetic foot ulcers the gold standard for debridement is regular, local, sharp surgical debridement performed by a licensed and trained clinician using a scalpel, scissors, and/or forceps. The benefits of debridement include:
- Removing necrotic tissue and callus
- Clearing bacteria from the wound bed
- Disrupting biofilm
- Stimulating the production of growth factors
- Reducing pressure
- Allowing for full inspection of the underlying wound tissues
- Facilitating drainage of secretions from the wound
- Enhancing the effectiveness of topical preparations
- Stimulating healing
The need for debridement must be discussed with the patient, and the clinician ensures that the patient understands the procedure before obtaining their written consent to proceed. The goal of debridement is to remove all devitalized tissue, callus, and foreign bodies down to the level where viable, bleeding tissue is found. It is necessary also to debride the wound edges to permit epithelial edge advancement, which allows the epithelium to migrate across a firm, level base of granulation.
The requirement for further debridement is determined at each dressing change. Studies show that serial debridement of diabetic foot ulcers for the first four weeks of treatment has decreased the median wound area by at least 54% compared to ulcers that do not undergo debridement. Serial debridement is usually done on a weekly basis and is referred to as maintenance debridement (Bryant & Nix, 2024; Baranoski & Ayello, 2020; WOCN, 2022).
More than one type of debridement may be necessary to completely debride a diabetic foot ulcer. The type(s) of debridement used are dependent on several factors:
- The status of arterial circulation to the extremity
- Current medications (e.g., antiembolic, which would increase the risk of bleeding)
- Pain in the wound and/or surrounding tissues
- Clinical setting (outpatient, day surgery, or inpatient)
(Bryant & Nix, 2024; Baranoski & Ayello, 2020; WOCN, 2022)
| Name | Mechanism of Action | Advantages |
|---|---|---|
| (Mamou, 2022; Baranoski & Ayello, 2020; WOCN, 2022) | ||
| Conservative sharp debridement | Loosely connected necrotic tissue is removed from the wound bed using sterile scissors or scalpel and forceps. | Quick and safe way to remove dead tissue |
| Enzymatic debridement (collagenase) | Collagenase dissolves the collagen bonds that secure necrotic tissue to the wound bed. | Used when surgical debridement is not feasible, i.e., a patient on anticoagulant therapy with a risk of bleeding |
| Autolytic debridement | A natural form of debridement, it utilizes the body’s own white blood cells to clear necrotic tissue from the wound. | Safe, although slow |
| Biological/bio-surgical debridement (maggot therapy) | Maggot larvae produce a mixture of enzymes and broad-spectrum antimicrobials to remove necrotic tissue from the wound bed. | Faster than autolytic or enzymatic debridement |
DRESSING SELECTION
There is no evidence to show that any one particular dressing works better for diabetic foot ulcers than other dressings. Dressing selection for diabetic foot ulcers is based on the principles of moist wound healing, management of wound drainage, ensuring a moist wound surface, and protection of the periwound area (WOCN, 2022).
When choosing a dressing, the following factors are taken into consideration:
- What are the wound characteristics (size, depth, amount of drainage, etc.)?
- What type of tissue is present in the wound bed?
- What is the condition of the periwound area?
- Can the dressing manage the amount of drainage from the wound and prevent maceration of the periwound area?
- Will the dressing be applied to the plantar surface of the foot and will it have to fit over or between toes?
- Is the dressing easy to apply?
- Does the dressing remain intact and stay in place during wear time?
- What is the frequency of dressing changes?
- Will the dressing prevent wound trauma and pain during dressing changes?
- What type of off-loading device will the patient be using, and will the bulk of the dressing accommodate the off-loading device?
- Is the dressing cost-effective?
(WOCN, 2022)
General rules for selecting dressings are as follows:
- For dry necrotic wounds, select a dressing that will rehydrate the wound and help to soften the eschar.
- For ulcers where slough and moisture are present, choose a dressing that will control moisture and help to debride the devitalized tissue.
- Where wound infection is present, consider an antimicrobial dressing to decrease the wound bioburden and manage wound drainage.
- For newly forming granulation tissue in the wound, select a dressing that will protect the new tissue growth.
Dressings designed to be left in place for more than five days are not considered a good choice for treatment since diabetic foot ulcers must be inspected and assessed frequently.
| Type (description) | Indications | Advantages |
|---|---|---|
| (Mamou, 2022; WOCN, 2022) | ||
| Alginates (absorbent, made from light seaweed) |
In moderately to heavily draining wounds |
|
| Hydrocolloids (occlusive) |
For autolytic debridement; to protect periwound area from trauma and drainage; not for use in heavily draining wounds |
|
| Hydrogels (hydrating; donate water to the wound bed) |
In shallow wounds with scant drainage |
|
| Hydrofiber (absorbent; made from carboxymethylcellulose) |
In moderately to heavily draining wounds |
|
| Foam (absorbent, can be adhesive or nonadhesive) |
In moderately to heavily draining wounds; but problematic with off-loading devices if bulky |
|
| Composite (combination) |
To provide adhesion, absorption of wound drainage, and protective barrier against bacterial infection |
|
| Contact layer (nonadherent layers placed directly onto wound bed) |
To allow drainage to pass through to absorptive dressing above |
|
| Antimicrobial (cadexomer iodine, silver, honey, hydrofera blue) |
Against a broad spectrum of microorganisms that cause wound infection and biofilm formation |
|
| Collagen (derived from type 1 bovine, avian, or type 3 porcine collagens) |
To stabilize the chemical balance in the wound by decreasing the level of proteases, which destroy the newly forming collagen fibers in the wound bed |
|
ANTIMICROBIAL TREATMENT
Topical antimicrobial agents are frequently used in the treatment of infected diabetic foot ulcers. A short course of antimicrobial treatment is recommended to reduce the bacterial load and protect the ulcer from further contamination. Wounds treated with antimicrobials for an extended period of time may develop resistant organisms (Bryant & Nix, 2024; WOCN, 2022).
An initial two-week treatment with antimicrobials is recommended. If after two weeks the wound has improved but there are still signs of continuing infection, the wound care team may consider it clinically appropriate to continue the antimicrobial for a longer period with regular reassessment. Once the signs of infection are no longer present, the antimicrobial treatment is discontinued and the wound reevaluated for the most suitable dressing to apply at this stage.
If after another two weeks of antimicrobial treatment the wound shows no signs of improvement, the antimicrobial therapy is discontinued and the current treatment plan is reevaluated. The wound care team ensures that all underlying causes that impede healing have been addressed and are being adequately dealt with. The team also closely reassesses the patient’s understanding and compliance with the treatment plan (WOCN, 2022).
Topical antimicrobials regularly used in the management of diabetic foot ulcers are listed in the following table.
| Name | Description | Comments |
|---|---|---|
| (Mamou, 2022; WOCN, 2022) | ||
| Cadexomer iodine |
|
|
| Medical-grade honey |
|
Patient must not be allergic to honey, bee products, and bee stings |
| Silver |
|
Efficiency depends on the rate of release of ionic silver into the wound |
DRESSING CHANGES AND WOUND MONITORING
Regular monitoring of the patient’s wound and dressing is essential. For wounds that are infected, the clinician assesses the wound and changes the dressing daily. Assessment includes monitoring the status of the wound for indications that the wound is progressing, which may be characterized by the following features:
- Decrease in wound dimensions
- Decrease in wound drainage and wound odor
- Formation of healthy granulation tissue
- Open wound edges
- Intact periwound area
Once the infection is under control, dressing changes are decreased to every two or three days. Changes in the type of dressing may also be required as the status of the wound changes.
DRESSING CHANGES AT HOME
Ideally, dressing changes are done in the wound clinic, but this is not feasible for all patients. Patients may still have to go to work or may have transportation issues. In these instances, the clinician may train the patient or a caretaker to change a dressing. The individual doing the dressing changes is instructed in clean technique and to observe for the signs and symptoms of wound deterioration, such as increased pain, increased redness, swelling, odor, or increased drainage. It is a good practice for the clinician to outline the area of cellulitis present with an indelible marker and to instruct the patient to contact the wound care team if the redness extends beyond this line.
Diabetic foot ulcers are cleaned at each dressing change and following debridement using either normal saline or a cleaning solution. Cleansing removes devitalized tissue and may also help to remove biofilms from the wound bed. (Biofilms are polymicrobial communities that contain several species of bacteria and fungi and adhere tightly to the wound bed.)
The following considerations are important when first applying a dressing and for subsequent dressing changes:
- If possible, avoid bandaging over toes, since this may cause a tourniquet effect. When bandaging over toes is necessary, place a layer of gauze over the toes and secure it with a bandage from the metatarsal heads to a suitable point on the foot.
- Keep dressings smooth; avoid creases and dressings that are too bulky, especially on weight-bearing surfaces of the foot.
- Ensure that bandages are not tight at the fifth toe and the fifth metatarsal head; if necessary, trim the bandage back in these areas.
- Do not put strong adhesive tapes on fragile skin.
- Use a dressing that conforms to the contours of the wound bed to prevent dead space in the wound.
- Remember that off-loading footwear needs to accommodate the dressing.
(WOCN, 2022)
PAIN MANAGEMENT
It is now more clearly understood that many patients with diabetic foot ulcers, even those with neuropathy present, can experience wound pain and pain during dressing changes. Therefore, the clinician utilizes strategies to prevent wound trauma and minimize wound-related pain during dressing changes.
The use of soft silicone dressings and low- or nonadherent dressings is known to reduce pain during dressing changes, along with gentle and careful manipulation of the wound. If the dressing becomes attached to the wound bed and is difficult to remove, the clinician must soak it with normal saline or a wound irrigation solution and wait for several minutes before attempting to remove it (WOCN, 2022).
Treating Infection
Infection in diabetic foot ulcers can be caused by Gram-positive bacteria, Gram-negative bacteria, and anaerobic bacteria, either on their own or in combination. Frequently, infections in diabetic foot ulcers are polymicrobial, meaning that several different organisms are involved.
Gram-positive bacteria associated with diabetic foot ulcer infections are:
- Staphylococcus aureus
- Streptococcus spp.
- Enterococcus spp.
- Coagulase-negative staphylococci (if this organism is positive in a superficial swab culture, it is frequently a contaminant; however, if it is obtained from a deep tissue culture or from a bone culture, it is considered an accurate finding)
Gram-negative bacteria found in diabetic foot ulcers are:
- Enterobacteriaceae
- Pseudomonas aeruginosa
Anaerobic organisms cultured from diabetic foot ulcers include:
- Bacteroides fragilis
- Peptococcus and Peptostreptococcus
Superficial infections in diabetic foot ulcers are often caused by S. aureus or beta-hemolytic streptococci. Deep soft tissue infections and osteomyelitis are usually caused by polymicrobial organisms, which include both aerobic Gram-negative bacilli and anaerobes such as anaerobic streptococci, Bacteroides fragilis group, and Clostridium. However, it has been found that Staphylococcus aureus is an often-occurring single pathogen in these wounds (Zubair et al., 2021; Armstrong & Lavery, 2024). Methicillin-resistant Staphylococcus aureus (MRSA) is also a common finding in infected diabetic foot ulcers.
OSTEOMYELITIS
Osteomyelitis (infection of the bone) is a common finding in diabetic foot ulcers, especially in patients with moderately to severely infected ulcers. Osteomyelitis can be hard to diagnose in its early stages. Determining an accurate depth of a diabetic foot ulcer is essential. Ulcers that are large, deep, or in the area of a bony prominence are at considerable risk for underlying osteomyelitis. Other indicators of possible osteomyelitis are bone visible in or protruding from the ulcer (“sausage toe”).
The probe-to-bone test is a simple test that has been found useful in detecting osteomyelitis in diabetic foot ulcers. It can be done in an outpatient clinic or bedside. The clinician gently inserts a sterile probe into the wound. If the probe touches bone, the test is considered positive (Oliver & Mutluoglu, 2023; Armstrong & Lavery, 2024). In a study of about 250 patients with diabetic foot ulcers, researchers found that the probe-to-bone test was highly sensitive in detecting osteomyelitis (WOCN, 2022).
X-rays are the first step in confirming a diagnosis of osteomyelitis; however, early in the infection, X-rays may not be that useful since it usually takes about two weeks after the infection starts for plain X-rays to pick up changes consistent with osteomyelitis.
The Infectious Disease Society of America advises that if initial X-rays do not diagnosis osteomyelitis in the presence of clinical findings that are highly suspicious, the next step to consider is magnetic resonance imaging (MRI). A bone scan can also be done, but at this time an MRI is considered the most accurate imaging technique available in diagnosing osteomyelitis. But there are increasing data to substantiate the role of single-photon emission computed tomography (SPECT)/computed tomography scans in the diagnosis and staging of infections in bone and soft tissue (WOCN, 2022; Baranoski & Ayello, 2020; Armstrong & Lavery, 2024).
Bone cultures are also important in the diagnosis of osteomyelitis. To obtain an accurate diagnosis of osteomyelitis, it is recommended to surgically obtain a bone specimen since osteomyelitis is usually concentrated in a specific location, and percutaneous biopsy can miss sampling the affected bone (WOCN, 2022).
INTERVENTIONS FOR INFECTION
With the confirmation of deep infection and/or the presence of osteomyelitis, a consult to an infection control specialist is imperative. A crucial intervention at this stage is the identification of causative organisms (if that has not already been done) and the immediate initiation of antibiotic therapy.
Another consideration is the need for surgical debridement to remove all devitalized tissue from the ulcer and the drainage of any abscess that may be present. Slough and necrotic tissue provide a fertile ground for the growth and multiplication of organisms, and infection cannot be treated effectively until these tissues have been removed.
The combination of antibiotic therapy and serial wound debridement (known as conservative treatment) is frequently successful in resolving infection and allowing the ulcer to heal.
Antibiotic Therapy
Treatment normally begins with intravenous antibiotics, either in the hospital setting, outpatient clinic, or in the patient’s home, depending on the stage of the wound and the severity of the infection. Some of the most frequently used antibiotic therapies are listed in the table below.
| Infection type | Antibiotic(s) |
|---|---|
| * This list is not all-inclusive. (Zubair et al., 2021; Armstrong & Lavery, 2024; Bronze, 2024) |
|
| Streptococcus | Amoxicillin, clindamycin |
| Staphylococcus | Flucloxacillin, clindamycin |
| Anaerobic | Metronidazole, clindamycin |
| Gram-negative | Ciprofloxacin, gentamicin, piperacillin-tazobactam |
| MRSA | Vancomycin, daptomycin, linezolid |
Broad-spectrum antibiotic treatment is started immediately before sensitivity reports are available. Potential limb-threatening and possibly life-endangering signs and symptoms include an infection that has spread throughout the patient’s foot, wet gangrene, or the development of a hot, red, swollen foot where pain may or may not be present. In addition to antibiotic therapy, surgical evaluation and intervention are indicated if these symptoms are present.
Once the appropriate antibiotic regime has been established from culture results, antibiotic therapy is continued for at least 12 weeks. With severe limb-threatening infections, the patient will be hospitalized initially until symptoms are under control and the wound care team believes it is safe for the patient to continue antibiotic therapy on an outpatient basis or at home with the assistance of a home health nurse.
Some of the challenges of systemic therapy include the presence of peripheral arterial disease, which can diminish the effectiveness of systemic antibiotics due to inadequate localized tissue perfusion. The use of local antibiotic treatment is a means of overcoming this problem. Recent studies have shown the potential benefit of local delivery of antibiotics to the wound site using biodegradable calcium sulfate beads impregnated with antibiotics (Patil et al., 2021).
Debridement
During the course of antibiotic therapy, the diabetic foot ulcer is debrided regularly, usually on a weekly basis; however, the frequency is decided by the wound care provider or surgeon. Following each debridement, the clinician assesses the ulcer and the patient’s foot for positive signs that the infection is resolving, such as decreased redness and swelling, healthy granulation tissue in the wound bed, decreased wound drainage, and decreased wound odor. (See also “Debridement” earlier in this course.)
Advanced Wound Treatment Options
When a diabetic foot ulcer fails to heal, the wound care team carefully reevaluates the plan of care to ensure that all impediments to healing have been adequately addressed.
- Has the patient been seen by an infection control specialist?
- Have cultures of wound and bone been done and antibiotic therapy started?
- Has a thorough assessment of the patient’s vascular status been completed?
- Has tight glycemic control been achieved?
- Is off-loading being consistently implemented?
If the underlying causes have been addressed and the wound is not responding to standard wound management interventions, then it is time for the wound team to look at advanced treatment options. The goal at this stage is to convert a chronic wound environment into one that supports wound healing. Therapies that are frequently used to achieve this goal are:
- Negative pressure wound therapy
- Hyperbaric oxygen therapy
- Application of growth factors
- Skin substitutes
- Protease inhibitors
- Electrical stimulation
NEGATIVE PRESSURE WOUND THERAPY (NPWT)
In NPWT, a filler dressing (commonly a porous polyurethane foam or gauze) is placed in the wound bed and secured with a cover dressing. A pump is attached to the dressing via a special seal in order to provide negative pressure, with the other end of the tubing connected to a drainage containment device. The negative pressure suction applied to the wound removes excess exudate from the wound bed, decreases edema, and decreases bioburden, all of which help to increase perfusion to the wound bed. NPWT also promotes mechanical stretch of the cells in the wound, which assists with granulation tissue formation.
Before applying NPWT, the clinician must ensure that the wound has been thoroughly debrided, that the wound is free from necrotic tissue and slough, and that the patient is on the appropriate antibiotic therapy to treat infection. Dressings are usually changed three times a week in the clinic by a clinician skilled in the use of NPWT.
NPWTi
Negative pressure wound therapy with instillation (NPWTi) allows the wound to be soaked in irrigation fluid, with saline being the preferred topical solution for the majority of wounds. The negative pressure device is programmed to facilitate cycles of fluid instillation, followed by the application of negative pressure, which removes the irrigation solution from the wound. The use of NPWTi is showing significant improvement in treating infection in a variety of chronic wounds (Baranoski & Ayello, 2020; Armstrong & Lavery, 2024).
HYPERBARIC OXYGEN THERAPY (HBOT)
HBOT has proven to reduce the number of amputations in people with diabetic foot ulcers. However, it is an expensive therapy and usually requires the patient to commit to treatments three to five days a week for six weeks or more. A significant drawback of HBOT is that many patients are unable to complete this full course of treatment, usually due to their overall poor health.
The patient is placed in a special chamber where 100% oxygen is administered under pressure. The main effect of HBOT is to increase the ability of the blood to supply oxygen to compromised tissues and thus increase wound healing. It is thought that HBOT also assists in the resolution of osteomyelitis by enhancing the oxygen supply to the bone, which increases leukocyte activity.
TOPICAL OXYGEN THERAPY
There have also been studies using topical oxygen in the treatment of DFUs. Unlike HBOT, in which the patient breathes concentrated oxygen in a high-pressure chamber, topical oxygen is applied directly to the wound bed. The most up-to-date devices utilize a miniature, lightweight, wearable oxygen delivery system that is completely noiseless and can be worn at all times.
In the 2023 Wound Intervention guidelines, the International Working Group in the Diabetic Foot (IWGDF) gave a “conditional” recommendation for the use of topical oxygen therapy. On the positive side, they point out that it can be used in the patient’s home, unlike hyperbaric oxygen therapy, and it is a therapy that patients tend to agree to. However, due to costs involved, it was considered that equal access to treatment may decrease (IWGDF, 2023). Other studies related to the benefits of this newer topical oxygen system demonstrate that this therapy does accelerate wound healing, in particular for patients with diabetic foot ulcers (Oropallo & Anderson, 2023).
GROWTH FACTORS
Platelet-derived growth factors and human epidermal growth factor have been shown to increase healing in diabetic foot ulcers. Studies demonstrate that chronic wounds such as diabetic foot ulcers have low levels of endogenous growth factors, which are essential for wound healing. Providing an external source of growth factors is believed to promote wound repair (Zubair et al., 2021; Armstrong & Lavery, 2024).
Becaplermin gel is the only growth factor currently available. It has been approved by the U.S. FDA for treatment of nonhealing diabetic foot ulcers that are free from necrotic tissue and slough and are appropriately off-loaded.
If the wound does not show indications of healing after two weeks of growth factor therapy or if the size of the wound has not been reduced by 30% in 10 weeks of treatment, growth factor therapy is discontinued. Becaplermin gel carries a “black box” warning for the increased risk of cancer with the use of three tubes or more (Baranoski & Ayello, 2020; Armstrong & Lavery, 2024).
SKIN SUBSTITUTES
Significant healing of diabetic foot ulcers has been obtained with the use of bioengineered skin substitutes (Armstrong & Lavery, 2024). Skin substitutes deliver growth factors to the wound bed, which in turn stimulates wound healing. The ulcer should be free of necrotic tissue and slough and have an adequate vascular supply. There should be no exposed tendon, bone, or muscle in the wound bed. There are dozens of commercially available bioengineered skin products on the market, and the clinician must follow the manufacturer’s instructions on the correct method of application. Usually, dressings are changed once a week with a complete assessment of the wound (Bryant & Nix,2024).
PROTEASE INHIBITORS
Collagen is the most common protein found in the body, and it is a vital constituent of wound healing. However, chronic wounds such as diabetic foot ulcers that fail to heal contain high levels of proteases called MMPs, which are enzymes that destroy collagen. Collagen dressings help to decrease the level of protease in the wound by forming a chemical bond with MMPs.
Collagen dressings come in several different formulations and are typically made from bovine, avian, or porcine collagen. They are nonadhesive, nonocclusive dressings that are applied directly to the wound bed and a secondary cover dressing. Dressing frequency depends on the product used, the amount of wound drainage, and the frequency of wound assessment (Baranoski & Ayello, 2020; WOCN, 2022).
ELECTRICAL STIMULATION
Electrical stimulation utilizes the transfer of electrical current to the wound to assist with wound healing. Studies indicate that this therapy can produce significant wound healing. It is proposed that electrical stimulation works by increasing protein synthesis in the wound, increasing cell migration, and decreasing bacterial growth (WOCN, 2022; Baranoski & Ayello, 2020; Rabbani et al., 2023).
Electrical stimulation is applied by a physical therapist or occupational therapist who has training and experience in the use of this therapy. Direct current electrical stimulation is the form used in wound care. It is a constant, one-way current in which the voltage does not change with time. It should not be used in the presence of osteomyelitis or with patients who have electric implants such as pacemakers (Bryant & Nix, 2024; Armstrong & Lavery, 2024).
SHOCK WAVE TREATMENT
Research shows that extracorporeal shock wave therapy (ESWT) improves healing in diabetic foot ulcers with a Wagner grade of 1 to 2. Blood circulation to the wounds was increased after two weeks of treatment, reducing ischemic environments. ESWT enhances tissue regeneration and the proliferation of epithelial growth factor. Recommended application is two treatment sessions per week for a minimum of two weeks (Jeong et al., 2023).
OFF-LOADING
Off-loading is the most frequently used treatment for diabetic foot ulcers, especially for plantar ulcers. It physically alleviates pressure from the wound and surrounding tissues and allows for redistribution of pressure across a greater area of the foot. Off-loading also eliminates shear, protects the bony structure of the foot, and facilitates increased circulation to the wound (WOCN, 2022).
The International Working Group on Diabetic Foot Ulcers has made the following recommendations on the role of off-loading in the treatment of uncomplicated diabetic foot ulcers:
- Pressure relief needs to be a part of the treatment plan for all diabetic foot ulcers.
- Total contact casting (TCC) and nonremovable walker casts are the preferred forms of off-loading.
- Normal footwear and standard therapeutic shoes should not be used in an attempt to provide off-loading.
- Patients should be advised to limit standing and walking and to rest with the affected extremity elevated as much as possible.
(IWGDF, 2023)
Total Contact Casting for Offloading
TCC is regarded as the gold standard for healing diabetic plantar ulcers, with reported healing rates of 72%–100% over a period of 5–7 weeks. These healing rates are achieved by consistently distributing pressure over the complete plantar surface of the foot and by ensuring patient compliance since the cast cannot be removed. However, not all patients are candidates for total contact casting. The most important consideration is adequate circulation to the extremity (IWGDF, 2023; Baranoski & Ayello, 2020).
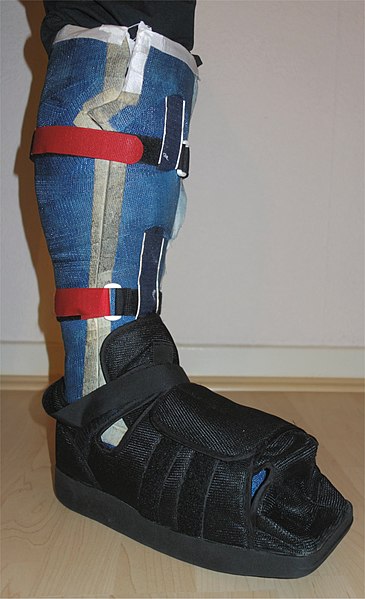
Total contact cast. (Source: Wikimedia Commons, Creative Commons Attribution-Share Alike 4.0.)
TCC APPLICATION
A total contact cast has minimal padding and is gently molded to the shape of the patient’s foot. A rocker-bottom walking plate or sole is attached to assist with walking while at the same time eliminating pressure during ambulation. The TCC is closed at the toes to prevent possible injury (WOCN, 2022).
INSTRUCTIONS FOR TOTAL CONTACT CASTING
- Explain the procedure to the patient.
- For most patients with diabetes, have them sit on the treatment table with their legs hanging down. Alternatively, have the patient lie on their stomach with the affected leg pointing up; however, many patients, especially older patients, will not find this a comfortable position.
- If a diabetic ulcer is already present, cover the ulcer before the cast is applied with a dressing that can be left in place for several days.
- Keep the patient’s ankle in a neutral position while the cast is being applied.
- Prior to discharge, instruct the patient on safe ambulation when wearing a cast. Normally, patients will need to use a cane, crutches, walker, or wheelchair for mobility safety while the cast is in place. Instruct patients who are ambulating with a cast to immediately report the onset of hip or back pain to their healthcare provider, as this may indicate musculoskeletal strain due to the cast or compensatory movement patterns.
- Instruct the patient to keep the casting material dry. A shower bag (available at most pharmacies) can be used for this purpose. Alternatively, instruct the patient to wrap the cast in plastic and secure it with tape while showering. Some patients will find it easier to use a shower chair and keep the casted leg outside the shower curtain.
DISADVANTAGES TO TCC
There are disadvantages to using TCC as an intervention for an existing diabetic foot ulcer. One of the most critical is that a TCC prevents frequent wound assessment since the cast is normally only replaced once a week. However, a skilled clinician can apply a TCC with cutout windows around the wound(s), which allows for dressing changes and wound inspection in the interval between TCC changes (Bryant & Nix, 2024).
Other disadvantages of TCC include:
- It must be applied by fully trained and experienced clinicians.
- It is time-consuming to apply and remove.
- Improper application can lead to skin irritation and further ulcer development.
- In most cases it makes it impossible for the patient to continue working.
- It can make it difficult for the patient to sleep.
- The cast must be kept dry, so showering and bathing becomes a problem.
- It can be uncomfortable to wear, especially during warm and hot weather.
INSTANT TOTAL CONTACT CAST
The instant total contact cast (iTCC) combines the concept of a removable cast walker (see below) and TCC. The patient is fitted with a removable cast walker, which is then wrapped with a layer of cohesive bandage, or casting tape, converting it into an unremovable device, or at the very minimum, a difficult-to-remove device. Studies show favorable outcomes with iTCC, and one study found no difference in healing times between TCC and iTCC. The advantages of iTCC include easier access to the wound and surrounding tissues, easier application than TCC, less time to apply, and greater cost-effectiveness (Armstrong & Lavery, 2024).
The clinician must take extra care to ensure that the cast walker is the correct fit for the size and shape of the patient’s foot and will not cause further areas of pressure. This is an important consideration in patients who have lost protective sensation and are unable to detect pain or discomfort (WOCN, 2022; IWGDF, 2023).
Removable Cast Walkers
Other options for off-loading diabetic foot ulcers are removable devices, although none of them has demonstrated the same healing rates as TCC. Such devices allow for frequent wound inspection and are often a better match for the patient’s lifestyle. They permit patients to bathe and sleep more comfortably.
The biggest drawback with removable devices is a common lack of patient compliance, and this may account for their decreased effectiveness compared to TCC. The findings from one study indicated that patients wore their removable off-loading device less than 30% of the time on a daily basis.
Another alternative to TCC is a prefabricated pneumatic walking brace (PPWB) fitted with a custom insole. PPWBs have the advantage that they are easy to remove, have a high satisfaction rating with patients, and have not been associated with any major complications. They are made from a lightweight, partially rigid casing with an inner lining that supports the patient’s lower leg on the affected side to a few inches below the knee. They are usually secured in place with buckles and Velcro straps. However, they have limited use in patients with severe foot deformity. A further drawback is patient compliance, since this is a device that can be removed at home.
Since most patients do not consistently wear removable cast walkers, patient education on this topic is vital. Walking even short distances without a cast walker is contraindicated and puts the patient at risk for a nonhealing ulcer and possible amputation (IWGDF, 2023; Bryant & Nix, 2024).
Felt Foam Dressing
Felt foam dressing (FFD) is sometimes referred to as the “football” dressing due to its final shape. It is constructed of inexpensive gauze dressings and foam. It provides off-loading and is used along with special footwear such as a surgical shoe or a walking splint. The FFD is constructed using adhesive felt cut to accommodate the shape of the patient’s foot, with a window cut out over the wound site.
The clinician applies skin prep to the patient’s foot and then places the adhesive side of the felt to the patient’s skin, ensuring that the window is directly over the wound site. The pad is next wrapped in place with a gauze bandage, secured with tape, and covered with a sock or stockinette. Finally, a surgical shoe is applied (WOCN, 2022).
Although the use of felt foam along with properly fitting footwear is likely well accepted by most patients and generally assessable in nearly all clinical settings, it is judged to be a last option for off-loading a diabetic foot ulcer and to be used only when other devices are not available (Armstrong & Lavery, 2024; IWGDF, 2023).
Healing Shoes
Inexpensive, off-the-shelf shoes are available that provide off-loading and enhance wound healing. These shoes also have the advantage of being reusable. Darco boots are one example of this type of footwear; they provide room for bulky wound dressings and closed-toe protection. They can also be fitted with cushioning insoles (Wound Source, 2024; Armstrong & Lavery, 2024).

Pressure-relieving shoes can aid in diabetic foot ulcer wound healing. (Source: Darco International, used with permission.)
An important feature of pressure-relieving shoes is a specialized insole that allows for customized pressure relief using a removable peg system. The first step is for the clinician to place a transparent film over the wound and outline the wound site with a marker. The clinician then asks the patient to step on the insole, which transfers the shape and area of the wound onto the insole. The clinician then removes the pegs from the insole in the area of the wound, effectively off-loading the wound site when the patient ambulates.
Other variations of these shoes include the Ortho-wedge forefront off-loading shoe and the heel wedge off-loading shoe. The Ortho-wedge forefront off-loading shoe provides pressure relief for the toes and metatarsal head using what is known as a rocker-bottom wedge. The construction of this shoe, which includes 10 degrees of built-in dorsiflexion, redistributes weight from the forefoot to the hindfoot, while the presence of a semirigid heel adds stability. The heel wedge off-loading shoe removes pressure from the posterior end of the patient’s foot, with pressure being transferred to the midfoot and forefoot areas (Baranoski & Ayello, 2020, Bryant & Nix, 2024).
Healing shoes have the same disadvantages as other removable pressure-relief devices, particularly patient compliance. Another concern is safe ambulation, and the wound care clinician (nurse, physical therapist, or occupational therapist) must ensure that the patient receives instruction on walking safely when wearing healing shoes. The clinician should also caution the patient that driving while wearing healing shoes is not advisable; family members and friends should be enlisted to assist with transportation.
Other Off-Loading Devices
Crutches, knee scooters, walkers, and wheelchairs can also be used to assist with off-loading.
Safe ambulation and stability are crucial for patients using these off-loading devices. The physical therapist determines the most appropriate mobility aid for the patient based on the patient’s current mobility status (i.e., wheelchair, walker, or crutches). Physical therapy interventions also include exercises for balance and coordination and gait training. Therapists instruct the patient on the type of gait that will allow for safe ambulation, usually three-point gait for patients with off-loading. Other team members also observe that the patient is performing the gait correctly and reinforce teaching.
It may also be necessary for an occupational therapist to assess the patient’s home environment, with the patient’s permission, to determine what hazards and risks are present for safe mobility. For example, for a patient on crutches who has stairs at home, negotiating stairs with crutches must be included in the patient education (Cluett, 2024).
CRUTCH WALKING
Crutch walking is a special technique and ideally includes instruction from a physical therapist, who also ensures that the crutches are a correct fit for the patient. The therapist may recommend either axillary or forearm crutches depending on the patient’s overall condition and level of trunk stability. The therapist also ensures correct sizing and positioning of the crutches. It is recommended that axillary crutches reach to 1 to 1-1/2 inches below the patient’s armpits when standing erect.
Younger patients often do better with crutches than older patients, who may not have the upper-body strength and endurance to safely use crutches. Crutches should not be prescribed for a patient with postural hypotension due to the increased risk of falling.
There are several different gait patterns that can be used with crutches. For individuals who have restricted weight-bearing on one leg, the recommended gait pattern is three-point crutch gait, which allows for partial weight-bearing. The physical therapist instructs the patient in the following step sequence:
- Move both crutches and the non-weight-bearing leg forward in one movement.
- Bear weight down on the crutches and move the unaffected leg forward.
- Repeat this pattern of movement.
While this gait pattern helps to eliminate weight from the affected leg, it also requires that the patient has good balance to be able to perform it safely.
If the patient is completely non-weight-bearing on a lower extremity, a three-point swing-through gait will be indicated for crutch use. The left and right crutches are moved forward simultaneously, then the left and right lower extremities are advanced in tandem (with the non-weight-bearing extremity not contacting the floor) (Warees et al., 2023).
KNEE SCOOTER USE
Knee scooters provide a good alternative to crutches for many patients. With the patient placing their body weight on the kneepad, the unaffected leg is in contact with the ground and provides for propulsion. The clinician will adjust the height of the knee rest so that the patient’s knee is as close to 90 degrees as possible. Although scooters are safer to use for patients than crutches, they do have limitations. Scooters cannot be used on stairs, and they can be bulky and difficult to load into a vehicle compared to crutches.
Knee scooters come in a two-, three-, or four-wheeled version. Smaller units are suitable for indoor use only, while the larger, stronger versions can be used outdoors. Before recommending a knee scooter for a patient, the clinician ensures that the patient does not exceed the weight limitations for the scooter and that the patient has the cognitive ability to safely use it.
Safety instructions provided prior to using a knee scooter may include:
- Do not use the scooter to pull up from a sitting position.
- Before placing body weight on the scooter, make sure the brakes are locked.
- Wear comfortable, nonskid footwear on the nonaffected extremity.
- Use extra caution when changing from one surface gradient to another (i.e., from the sidewalk to the street).
- Use extra care and slow down when going around corners.
- When moving from the scooter to a chair or other surface, back the scooter up to the seat and lock the brakes prior to sitting down.
(Mobility Plus, 2024)
The clinician will also look at payment sources for renting or purchasing a scooter: Is it covered by the patient’s insurance, or will the patient have to pay part or all of the cost out of pocket?
WALKER USE
Many patients may find that a walker provides more stability and comfort than either crutches or a knee scooter. There are several types of walkers, including standard, two-wheeled, three-wheeled, and four-wheeled (rollator). The clinician selects the type of walker most appropriate for the individual patient’s abilities and needs. A physical therapist may need to work with the patient to ensure safe and appropriate gait technique when using a walker.
The following instructions provide a general guideline for safe use of a standard walker:
- Begin by standing centrally behind the walker.
- Look straight ahead, not down at your feet.
- Grasp the walker grips with both hands.
- Move the walker forward to a distance that feels comfortable.
- Do not over-reach; the back legs of the walker should be even with the patient’s toes.
- Step forward into the center of the walker with the affected leg.
- Step forward with the nonaffected leg while simultaneously bearing some weight through the arms.
- Maintain a firm hold on the walker with both hands.
- Do not attempt to move until all walker legs (or wheels) are securely on the walking surface.
- Do not use the walker to pull up from a sitting position.
- Maintain upright posture while walking; avoid leaning over the walker.
- Take extra care and time when using a walker on a carpeted surface or when using an elevator.
- Never use a walker on stairs or an escalator.
- Stay away from areas covered in ice or snow, and take extra care in bad weather.
(UPMC, 2024)
WHEELCHAIR USE
Wheelchairs can provide total off-loading of non-weight-bearing extremities. Safe technique is important when training a patient to use a wheelchair and is individualized to a patient’s needs and abilities. A few general safety recommendations include:
- Ensure that the wheelchair brakes are fully locked before transferring in or out of it.
- Do not place heavy items on the back of the wheelchair; this could cause the wheelchair to tip backwards.
- Adjust the armrests and footrests to maintain optimal posture and comfort.
- When getting in or out of the wheelchair, first move the footrests (if swing-away style) to one side to prevent tripping over them.
(TOUSDA, 2021)
Assessing Off-Loading Effectiveness
Regardless of the choice of off-loading, it is essential for the clinician to continually assess its effectiveness in providing pressure relief to the area of the ulcer. Indicators of effective off-loading include:
- Progress in wound healing, demonstrated by the presence of healthy granulation tissue and a decrease in wound dimensions
- No or very little reoccurrence of callus formation around the wound
- Absence or resolution of local inflammation (decreased redness, swelling, and skin temperature)
The clinician also monitors the nonaffected extremity closely, since mechanisms used for off-loading can often cause undue pressure to the unaffected limb, increasing the risk for new ulceration (IWGDF, 2023; WOCN, 2022).
CASE (continued)
Mr. Hernandez has missed several appointments at the wound clinic since his wife was ill and he had to put in extra hours at work. When he does return to the clinic, he looks tired and ill.
On examination, the clinician finds an ulcer on the plantar surface of his left foot. The clinician performs a full wound assessment, noting greenish drainage, a malodor that persists after the wound is cleaned, and redness and swelling in the tissues surrounding the wound. When she inserts a sterile applicator into the wound, she is able to probe down to bone.
The physician takes a deep-tissue biopsy from the wound, orders an immediate X-ray, and starts Mr. Hernandez on a broad-spectrum intravenous antibiotic. He also requests that Mr. Hernandez be scheduled for vascular studies to determine the circulation status to his lower extremities.
Mr. Hernandez becomes confused and angry, stating, “All these tests are not needed! It’s just a little wound. My wife is putting iodine on it at home.” The clinician explains to Mr. Hernandez that, although on the surface of his foot the wound appears to be small, it goes the whole way down to the bones in his foot and that tests are needed to ensure that there is no bone infection. Mr. Hernandez reluctantly agrees to the X-ray but states he doesn’t know whether he can come to the outpatient clinic to have IV antibiotics due to his work schedule. He insists that there is no need for vascular studies.
The wound care team also broaches the subject of off-loading with Mr. Hernandez and explains at length to him why it is so important. He responds that he has to work and has to wear his boots.
It is apparent that Mr. Hernandez is not only confused by what is happening, but he is shocked and afraid. It is hard for him to understand that a little hole in the bottom of his foot could be putting his whole limb at risk. However, the wound care team has in the past built up good rapport with Mr. Hernandez, and maintaining that level of trust is important at this stage.
Since Mr. Hernandez insists on continuing to work, the team considers what options are available for off-loading his foot. Off-loading is vital to healing, but it also has to fit in with Mr. Hernandez’s choices about his life. The team decides that a felt foam dressing is the best option. Mr. Hernandez’s work boots will accommodate the dressing without causing extra pressure to his foot. He will also be advised that when he is not working, he should rest with his feet elevated.
IV antibiotics are also determined to be essential. Once the tissue culture results are ready, Mr. Hernandez will be switched to the appropriate antibiotic to target his infection. The physician will schedule a consult with an infectious disease (ID) specialist, and the case manager will look into scheduling the patient’s IV antibiotics around his work schedule and the ID consult. The team also decides that the best course of action is to revisit the question of vascular studies and a vascular consult once Mr. Hernandez is established with the IV antibiotic therapy schedule and after he has seen the ID specialist.
AMPUTATION
Diabetic foot ulcers are the most frequent cause of amputations. Research shows that the five-year death rate in people with diabetes following a major amputation is substantial, exceeding that of several main types of cancer. Diabetes worldwide is responsible for one major extremity amputation every two seconds (Armstrong & Lavery, 2024).
Amputations are most commonly performed to prevent spread of infection and to salvage the remaining unaffected part of the limb. The types of amputations performed include amputation of metatarsal heads, ray resection (when the complete toe is removed), midfoot amputation, hindfoot amputation, and below-the-knee amputation.
Determining Amputation Type
A primary goal is to carry out the lowest level of amputation possible so that the patient can remain ambulatory, with or without a prosthesis, and avoid the need for more surgery in the future. If possible, the surgeon will opt for a partial foot amputation such as ray amputation, transmetatarsal amputation, or Syme amputation (removal of the affected foot at ankle level) (WOCN, 2022; Baranoski & Ayello, 2020).
When deciding on the level of amputation, the surgeon and the wound care team take into careful consideration the circulation status of the extremity. It is essential that there is adequate circulation to the area of amputation to ensure healing post surgery.
Ray amputation is required when tissue death has spread within the base of the toes but with limited spread to the patient’s forefoot. A ray amputation will remove the affected toe or toes along with a portion or all of the corresponding metatarsal. Ray amputations usually preserve more function than transmetatarsal (TMT) amputations, but forefoot stability is decreased when more than two rays are removed (WOCN, 2022; Baranoski & Ayello, 2020).
When extensive infection and necrosis has spread to the forefoot, a transmetatarsal amputation will be required. This surgery will leave the patient with a residual limb that can bear weight after wound healing is complete (WOCN, 2022; Baranoski & Ayello, 2020).
When infection spreads beyond the patient’s foot and there is inadequate perfusion, a below-knee amputation is typically performed. If feasible, the length of the patient’s leg remaining below the knee will be in the range of 5 to 7 inches to accommodate prosthetic leg fitting once the surgical wound has healed (AOFAS, 2024b).
AMPUTATION INCIDENCE AND MORTALITY
Those with a lower extremity amputation due to a diabetic foot ulcer are at greater risk for reulceration when compared to those who receive conservative treatment for an ulcer. The risk of a secondary amputation of the opposite limb can be as high as 44%. Recent data indicate that the overall death rate after a major extremity amputation was 48% (WOCN, 2022; Brix et al., 2024).
These figures demonstrate the importance of clinicians working aggressively to prevent wounds reaching the stage where amputation is necessary. However, even with the best care in the world, this is sometimes an unavoidable outcome for many patients.
Preoperative Care
Preoperative care unique to amputation surgery falls into two main areas: ensuring the patient is in the best physical shape possible for surgery and preparing the patient emotionally for the surgery.
Cardiorespiratory status is assessed in close consultation with the patient’s primary care physician and cardiologist, if applicable. Tight glycemic control, along with smoking cessation if the patient is a smoker, will optimize wound healing post surgery. Studies have shown a direct correlation between glycemic control and surgical outcomes. Elevated hemoglobin A1C levels are associated with serious postoperative complications, more frequent ICU admissions, and longer hospital stays (WOCN, 2022).
Patients undergoing an amputation, regardless of the level, are going to be fearful and often shocked and angry that a foot wound could lead to losing part of a limb. The clinician must be supportive, listen, and realize that the patient’s anger is not directed at them personally. The patient and their family will also be apprehensive about what to expect post surgery and how the amputation will affect their lives. The follow-up plan should be discussed in detail with the patient and family and their concerns addressed.
If possible, the patient and family should visit the rehabilitation unit prior to surgery in order to meet with the staff and tour the facility. This helps to alleviate the fear of the unknown.
Regarding the financial burden, the case manager or social worker on the team will act as a liaison between the patient and their medical insurance provider, ensuring that coverage is available for rehabilitation, prosthesis, and adaptive equipment. The patient and family are educated about out-of-pocket expenses and options about how these might be covered.
Postoperative Care
In the immediate postoperative period, monitoring the amputation site for hemorrhaging is critical. Changes in vital signs, such as an increase in pulse rate or decrease in blood pressure, along with increase in blood staining on the dressing, can be indicators of hemorrhaging under the residual limb dressing. These signs should be reported to the surgeon and closely monitored.
Some surgeons prefer to remove the first postsurgical dressing themselves or at least to be present when it is removed so that they can inspect the wound. The incision line is gently cleaned with normal saline and examined for approximation of the skin edges, amount of drainage, and residual limb swelling. Dressing type will depend on the surgeon’s preference or recommendation from the wound care team. It usually includes a noncontact layer over the incision line, absorbent gauze dressings, and stretch bandages to assist with residual limb shaping.
Residual limb positioning post surgery requires special attention to prevent the development of contractures. Patient education focuses on the importance of correct positioning and requires the expertise of physical therapy to provide the most comfortable positioning for the patient. For below-knee amputations, it is advisable to position the knee in extension to avoid contractures; elevating while sitting can help. For above-knee amputations, it is advisable to position the hip in neutral or slight extension to avoid contractures; prone lying is a good option to allowing optimal hip range of motion. Caution is especially advised when sitting upright to avoid prolonged flexion.
Patients with an amputation are at high risk for falls, and patient education focuses strongly on safety awareness and training. Patients are instructed not to attempt to get out of bed without assistance, while family members and caretakers are instructed not to assist the patient with transfers until they have received mobility training from a physical therapy professional (Johns Hopkins, 2024).
Pain management is an important consideration in the postamputation patient. Pain experienced after an amputation can be divided into phantom limb pain (see box below) and residual limb pain (WOCN, 2022).
Residual limb pain is concentrated in the remaining part of the extremity. It can be either superficial and limited to the incisional site, or penetrating pain deep into the tissues of the residual limb. Residual limb pain begins in the immediate postsurgical period and usually disappears with healing; it can also become chronic and persist for years.
Opioids, both oral and intravenous administration, can be used in the immediate postoperative period to treat pain. When using opioids, the prevention of constipation must be considered and may require the provider to prescribe a stool softener or laxative for the patient (Drugs.com, 2024). Other pharmacologic treatments include calcium channel blockers, anticonvulsants, and antidepressants (Amputee Coalition, 2024a).
In the postoperative period, clinicians perform frequent pain assessment and ensure that prescribed analgesics are administered. A patient in postoperative pain may not be able to optimally participate in self-care activities, learn new skills, or have an optimistic view of recovery.
PHANTOM LIMB PAIN
A frequent and often frightening experience for patients post amputation is phantom limb pain, in which they have the experience of pain or an unpleasant sensation in the body part that has been amputated. The mechanism that causes phantom limb pain is not clearly understood, but it is believed that factors in both the peripheral and central nervous system play a role. The descriptors that patients use to describe phantom limb pain include a burning, pricking, or shooting sensation. Patients who have phantom limb pain are found to have an increased risk for residual limb pain, which differs from phantom limb pain in that it is felt in the residual limb (Hanyu-Deutmeyer et al., 2023).
Medications used to treat phantom limb pain include tricyclic antidepressants and anticonvulsants; nonpharmacologic treatments include acupuncture and nerve stimulation. NSAIDs and acetaminophen are the most frequently used medications to treat phantom limb pain (Hanyu-Deutmeyer et al., 2023).
The use of a mirror box is a novel idea for treating phantom limb pain, based on the concept of body perception and brain mapping of integral parts of the body. Updating body perception depends a great deal on tactile information from parts of the body; however, after an amputation, this input is lost and the “brain map” is trapped in the perception that existed prior to the amputation. Research on cortical reorganization has found that the parts of the cortex that denote the amputated limb come under the control of adjacent regions of the cortex; it has also been found that there is a relationship between the expanse of cortical reorganization and the amount of pain the patient experiences (Hanyu-Deutmeyer et al., 2023).
The basis behind the mirror box is that it appears as if the amputated limb still exists. The mirror box has two openings—one for the intact limb and one for the residual limb. The mirror allows the patient to see a reflection of the intact limb, creating the visual input that both limbs still exist. The patient performs movements and observes the intact limb moving while imagining that the amputated limb is moving. The brain is tricked into believing that the missing limb is being moved into a pain-relieving position. Instructing a patient in the use of mirror box therapy is done by a physical therapist or occupational therapist who is familiar with this treatment modality. Evidence has shown that it is effective in relieving phantom limb pain; however, more robust studies are recommended to better understand the effectiveness of this therapy (IASP, 2024).
Using a mirror, the brain is “tricked” into seeing two limbs. (Source: © Sköld et al., 2011.)
Follow-Up Care
In the weeks following the amputation, the clinician continues to monitor for healing and decreased swelling in the residual limb. Edema can often present a problem. Compression residual limb shrinkers and bandaging help to provide good edema control.
During this time, the patient and family will continue to receive emotional support. Patients frequently have a difficult time looking at the amputation site and may require encouragement to participate in their own care.
Delayed healing is a problem for patients with diabetes, especially if there is associated peripheral vascular disease and decreased circulation to the amputation site. During follow-up appointments, clinicians monitor the wound closely for signs of delayed healing and infection. Dehiscence (separation of the wound edges) is another major concern post amputation.
There may be a considerable period of rehabilitation when the patient requires interventions from multiple disciplines—including nursing, physical therapy, and occupational therapy—to maintain safety, mobility, and assistance with self-care.
It is important to ensure that the patient is in the best physical condition possible for a future prosthesis fitting. Limb wrapping and using an elastic shrinker sock are essential to preparation for prosthetic fitting. Physical therapists are specialists in this area of care and will guide other team members in correct residual limb management.
Referral is made to a dietitian if there is a concern about malnutrition or poor appetite.
Prosthetic Care
The timing for prosthetic fitting depends on how quickly the wound heals. In most instances, fitting begins somewhere between eight weeks and six months post surgery (Amputee Coalition, 2024b).
Statistics indicate that 90% of those who have a partial foot amputation will be able to use a prosthesis and maintain their mobility. For those with a below-the-knee amputation, the figure drops to 75%, and only 25% of those with an above-the-knee amputation will be successful with a prosthesis and independent mobility (WOCN, 2022).
A prosthetist is involved early in the patient’s postoperative care and assists in preparing the patient’s residual limb for fitting and educating the patient in the care of their new prosthetic device. Physical therapists help the patient learn to walk safely with an artificial limb, develop a physical training program for the patient, and monitor their functional progress. Patients are also instructed on how to apply prosthetic socks and liners correctly, to change them daily (at a minimum), and to wash them following manufacturer recommendations.
Clinicians must be aware that the residual limb is at high risk for ulceration, and many times this is preceded by callus formation. All disciplines are therefore involved in careful monitoring of the residual limb to ensure that it is tolerating the new prosthesis and that there are no areas of redness that might indicate undue pressure. If redness does occur, the prosthetist is informed immediately and adjustments made to the prosthetic fit.
Since a major amputation also puts the contralateral extremity at greater risk for ulceration, suitable footwear and education to prevent ulceration must be focused on the remaining foot.
Reintegration into Normal Routine
Getting back to normal life or defining a new normal can often be difficult for a patient after an amputation. The stressors patients face include medical expenses, whether they will be able to return to work, whether they will be permanently disabled, how their family and friends will react, and how they will cope personally with an altered body image.
Losing part of one’s body damages body integrity and can have a serious negative impact on a patient’s physical and psychological condition. Those who undergo an amputation can experience feelings of grief, depression, and anxiety, but data indicate that the incidence of depression decreases as time passes after an amputation (PAM Health, 2021).
All members of the wound care team are involved in helping the patient cope with changes in body image, with clinicians’ acceptance and respect for the patient as “a whole person” providing a positive image. For patients who exhibit severe denial or depression, referral to a mental health specialist is advisable.
Physical therapists and occupational therapists provide valuable input on workplace ergonomics and assistive devices needed to maintain employment. Several studies have shown that interventions from these therapists result in:
- Decreased risk of complications post amputation
- More successful reintegration into society
- Better emotional health
- Greater patient acceptance of the amputation
- Greater protection of the residual limb
- Increased well-being as a wheelchair user
Other studies have found that patients participating in inpatient rehabilitation had higher levels of mobility one year post amputation compared to patients who did not receive inpatient therapy. Occupational therapy plays a critical role in advanced prosthetic training, instructing the client on how to incorporate the prosthetics into more complex activities such as home and work activities, driving, sports, and recreation. Occupational therapists will also be involved in community reintegration of patients (AOTA, 2021).
A case manager or social worker on the wound care team can assist the patient with insurance claims and disability benefits.
CONCLUSION
Treatment of diabetic foot ulcers in particular is both challenging and complex and requires a high level of commitment from both the patient and the clinician. The importance of diabetic self-management programs is widely recognized, but there are still situations in which access to diabetes care and preventive care is lacking.
Interdisciplinary wound care teams have been proven successful in the prevention and treatment of diabetic foot care problems. Not only do wound care teams achieve better patient outcomes, they are also cost-effective due to consolidation of services. The intervention of a wound care team improves patient quality of life and enhances physical and emotional well-being (Byrant & Nix, 2016; WOCN, 2022).
As soon as a patient is diagnosed with diabetes, intervention must begin with a foot assessment and patient education on foot care and wound prevention. Clinicians are aware that diabetic peripheral neuropathy (DPN) and Charcot osteoarthropathy may already be present, and signs and symptoms are thoroughly evaluated.
For patients who present with a diabetic foot ulcer, the goals are healing and treating infection, which often require advanced wound care modalities. If an amputation is required, the pre- and postoperative care of the patient must take into consideration both physical and psychological well-being. Prosthetic fitting and rehabilitation care aim to return the patient to active participation in society.
RESOURCES
Amputation and diabetes: How to protect your feet (Mayo Clinic)
Amputation Prevention Alliance (ADA)
Certification Board for Diabetes Care and Education (CBDCE)
Diabetes and pedorthics (Pedorthic Footcare Association)
Diabetes: Foot- and skin-related complications (Cleveland Clinic)
Diabetes foot complications (American Diabetes Association)
Diabetic foot problems (American Orthopaedic Foot and Ankle Society)
REFERENCES
NOTE: Complete URLs for references retrieved from online sources are provided in the PDF of this course.
Akbari NJ & Naimi SS. (2022) The effect of exercise therapy on balance in patients with diabetic peripheral neuropathy: A systemic review. J Diabetes Metabolic Disorder, 21(2), 1861–71. https://pmc.ncbi.nlm.nih.gov
American Diabetes Association (ADA). (2024a). 6. Glycemic goals and hypoglycemia: Standards of care in diabetes—2024. Diabetes Care, 47(Supplement 1), S111–25. https://diabetesjournals.org
American Diabetes Association (ADA). (2024b). 12. Retinopathy, neuropathy, and foot care: Standards of care in diabetes—2024. Diabetes Care, 47(Supplement 1), S231–43. https://diabetesjournals.org
American Diabetes Association (ADA). (2023a). Economic costs of diabetes in the U.S. in 2022. Diabetes Care, 47(1), 26–43. https://diabetesjournals.org
American Diabetes Association (ADA). (2023b). Statistics about diabetes. https://diabetes.org
American Diabetes Association (ADA). (2014). History of diabetes. https://fightdiabetesnow.wordpress.com
American Occupational Therapy Association (AOTA). (2021). The occupational therapy role in rehabilitation for the person with an upper-limb amputation. https://www.aota.org
American Orthopaedic Foot & Ankle Society (AOFAS). (2024a). What is Charcot arthropathy? https://www.footcaremd.org
American Orthopaedic Foot & Ankle Society (AOFAS). (2024b). What is below-knee amputation? https://www.footcaremd.org
American Physical Therapy Association (APTA). (2021). Physical therapy guide to wounds and wound care. https://www.choosept.com
American Podiatric Medical Association (APMA). (2024). Diabetic wound care. https://www.apma.org
Amputee Coalition. (2024a). Managing surgical pain. https://amputee-coalition.org
Amputee Coalition. (2024b). Management of residual limb pain. https://www.amputee-coalition.org
Armstrong DG & Lavery LA (Eds.). (2024). Clinical care of the diabetic foot (4th ed.). American Diabetes Association.
Baranoski S & Ayello EA. (2020). Wound care essentials: Practice principles (5th ed.). Wolters Kluwer.
Bell AM. (2023). The link between diabetes and hypertension. Medical News Today. https://www.medicalnewstoday.com
Brix ATH, Rubin KH, Nymark T, Schmal H, & Lindberg-Larsen M. (2024). Mortality after major lower extremity amputation and association with index level: A cohort study based on 11,205 first-time amputations from nationwide Danish databases. Acta Orthopaedica, 95, 358–63. https://doi.org/10.2340/17453674.2024.40996
Bronze MS. (2024). Diabetic foot infections treatment and management. Medscape. https://emedicine.medscape.com
Bryant RA & Nix DP. (2024). Acute and chronic wounds (6th ed.). Elsevier.
Centers for Disease Control and Prevention (CDC). (2024a). About the Division of Diabetes Translation. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2024b). National diabetes statistics report. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2024c). Diabetes: Promoting foot health. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC) (2024d). Diabetes and mental health. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2024e). National health and nutrition survey (NHANES), 2024. https://blogs.cdc.gov
Cervoni B. (2024). Cultural considerations in diabetes management. Verywell Health. https://www.verywellhealth.com
Certification Board for Diabetes Care and Education (CBDCE). (2024). Become certified. https://www.cbdce.org
Cluett J. (2024). Tips for using crutches. Verywell Health. https://www.verywellhealth.com
Conyers Y & Swoboda L. (2023). Getting ready for foot care certification. J Wound, Ostomy, Continence Nurs, 50(3), 250–2.
Copper Fit. (2023). How to improve circulation to your feet and legs. https://www.copperfitusa.com
Dailah HG. (2024). The influence of nurse-led interventions on diseases management in patients with diabetes mellitus: A narrative review. Healthcare (Basel, Switzerland), 12(3), 352. https://pmc.ncbi.nlm.nih.gov
Diabetes.co.uk. (2019). Diabetes history. https://www.diabetes.co.uk/diabetes-history.html
DiLonardo MJ. (2023). Shoes and diabetes: What’s on your feet matters. WebMD. https://www.webmd.com
Drugs.com. (2024). Which drugs cause opioid-induced constipation? https://www.drugs.com
Dupuis ML, Pagano MT, Pierdominici M, & Ortona E. (2021). The role of vitamin D in autoimmune diseases: Could sex make the difference? Biology of Sex Differences, 12, article 12. https://bsd.biomedcentral.com
Foundation for Peripheral Neuropathy. (2023). Exercise + physical therapy for neuropathy. https://www.foundationforpn.org
Goretti C, Prete A, Brocchi A, Lacopi E, Pieruzzi L, & Piaggesi A. (2024). Higher prevalence of cancer in patients with diabetic foot syndrome. J Clin Med, 13(5), 1448. https://www.mdpi.com
Greenstein E. (2023). To compress or not to compress: Practical tips for treating lower extremity ulcers. Wound Source. https://www.woundsource.com
Hanyu-Deutmeyer AA, Cascella M, & Varacallo M. (2023). Phantom limb pain. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
Harris-Hayes M, Schootman M, Schootman JC, & Hastings MK. (2020). The role of physical therapists in fighting the type 2 diabetes epidemic. Journal of Orthopaedic and Sports Physical Therapy, 50(1), 5–16. https://doi.org/10.2519/jospt.2020.9154
Healthline. (2023). Your guide to diabetes foot care. https://www.healthline.com
Hill C. (2023). OT and diabetes care. https://www.ottoolkit.com
Hill-Briggs F, Adler NE, Berkowitz SA, Chin MH, Gary-Webb TL, Navas-Acien A, Thorton PL, et al. (2021). Social determinants of health and diabetes: A scientific review. Diabetes Care, 44(1), 258–79.
Infectious Diseases Society of America (IDSA). (2023). IWGDF/IDSA guidelines on the diagnosis and treatment of diabetes-related foot infections. Clinical Infectious Diseases, ciad527. https://www.idsociety.org
International Association for the Study of Pain (IASP). (2024). Is mirror therapy all it is cracked up to be? Current evidence and future directions. https://www.iasp-pain.org
International Working Group on the Diabetic Foot (IWGDF). (2023). 2023 guidelines on the prevention and management of diabetes-related foot disease, 2023. https://iwgdfguidelines.org
Jadheea-Jutton A, Hindocha A, & Bhaw-Luximon A. (2022). Health economics of diabetic foot ulcer and recent trends to accelerate treatment. The Foot, 52, 101909. https://www.sciencedirect.com
Jeong D, Lee JH, Lee GB, Shin KH, Hwang Jang SY, Yoo J, & Jang WY. (2023). Application of extracorporeal shockwave therapy to improve microcirculation in diabetic foot ulcers: A prospective study. Medicine, 102(11), e33310. https://www.ncbi.nlm.nih.gov
Johns Hopkins. (2024). Amputation. https://www.hopkinsmedicine.org
Kanza Kazmi S, Iqbal Naviwala H, & Aziz M. (2021). Ipswich touch test—a simple yet reliable indicator of diabetic neuropathy. J Clin Transl Endocrinol, 23, 100252. https://doi.org/10.1016/j.jcte.2021.100252
Li M, Wei M, Han B, & Wu H. (2023). Stepping up foot care: Assessment over foot care knowledge and behavior among individuals with diabetic risk levels. Int J Diabetes Dev Ctries, 44, 545–53. https://link.springer.com
Lutes T. (2023). VA’s remote temperature monitoring prevents diabetic limb loss. VA News. https://news.va.gov
Mamou MK. (2022). Wound care. Wild Iris Medical Education. https://wildirismedicaleducation.com
March CA, Libman IM, Becker DJ, & Levitsky LL. (2022). From antiquity to modern times: A history of diabetes mellitus and its treatments. Horm Res Paediatr, 95(6), 593–607. https://doi.org/10.1159/000526441
Mayo Clinic. (2024). 10 ways to control high blood pressure without medication. https://www.mayoclinic.org
McNeil S, Waller K, Poy Lorenzo YS, Mateevici OS, Telianidis S, Qi S, Churilov I, MacIsaac RJ, & Galligan A. (2023). Direction, management, and prevention of diabetes-related foot disease in the Australian context. World Journal of Diabetes, 14(7), 942–57. https://www.ncbi.nlm.nih.gov
Mobility Plus. (2024). How to use a knee scooter. https://www.mobilitypluscolorado.com
National Institute on Aging (NIA). (2023). Assessing cognitive impairment in older adults. https://www.nia.nih.gov
Office of Minority Health (OMH). (2021). Diabetes and African Americans. https://minorityhealth.hhs.gov
Oliver TI & Mutluoglu M. (2023). Diabetic foot ulcer. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
Oropallo A & Anderson CA. (2023). Topical oxygen. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
Pan Y, Davis PB, Kaebler DC, Blankfield RP, & Xu R. (2022). Cardiovascular risk of gabapentin and pregabalin in patents with diabetic neuropathy. Cardiovascular Diabetology, 21(1), 170. https://doi.org/10.1186/s12933-022-01610-9
Patil P, Singh R, Agarwal A, Wadhwa R, Bal A, & Vaidya S. (2021). Diabetic foot ulcers and osteomyelitis: Use of biodegradable calcium sulfate beads impregnated with antibiotics for treatment of multidrug-resistant organisms. Wounds, 33(3), 70–6.
Peterson E. (2023). Spinal cord stimulation provides pain relief in diabetic neuropathy. Cleveland Clinic Journal of Medicine. https://www.ccjm.org
Pinhas-Hamiel O & Zeitler P. (2023). Type 2 diabetes in children and adolescents—A focus on diagnosis and treatment. Endotext [Internet]. https://www.ncbi.nlm.nih.gov
Post-Acute Medical (PAM) Health. (2024). Coping with limb loss: Tips and strategies. https://postacutemedical.com
Pyatak EA, Ali A, Khurana AR, Lee PJ, Sideris J, Fox DS, Diaz J, et al. (2023). Research design and baseline participant characteristics of resilient, empowerment, active living with diabetes–telehealth (Real-T) study: A randomized control trial for young adults with type 1 diabetes. CESR-Schaeffer Working Paper. https://papers.ssrn.com
Rabbani M, Rahman E, Powner MB, & Triantis JF. (2023). Making sense of electrical stimulation: A mega-analysis for wound healing. Ann Biomed Eng, 52, 153–77. https://link.springer.com
Rakusa M, Marolt I, Stevic S, Rebrina SV, Milenkovic T, & Stepien A. (2023). Efficacy of pregabalin and duloxetine in patients with painful diabetic neuropathy (PDPN): A multi-centre phase IV clinical trial—BLOSSOM. Pharmaceuticals (Basel, Switzerland), 16(7), 1017. https://doi.org/10.3390/ph16071017
Smokovski I. (2021). Impact of diabetes complications: Managing diabetes in low-income countries. Springer Publications.
TOUSDA. (2021). How to use a wheelchair safely. https://www.tousdamedical.com
University of Pittsburgh Medical Center (UPMC). (2024). Using a walker. https://www.upmc.com
U.S. Food and Drug Administration (U.S. FDA). (2023). Frequently asked questions about medical foods (3rd ed.). https://www.fda.gov
USA Facts. (2023). Which states have the highest and lowest adult literacy rates? https://usafacts.org
Wang M, Zhang Z, Mi J, Wang G, Tian L, Zhao Y, Li X, Li X, & Wang X. (2021). Interventional clinical trials on diabetic peripheral neuropathy: A retrospective analysis. J Pain Res, 14, 2651–64. https://pubmed.ncbi.nlm.nih.gov
Warees WM, Clayton L, & Slane M. (2023). Crutches. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
World Health Organization (WHO). (2024). Diabetes. https://www.who.int/news-room/fact-sheets/detail/diabetes
World Health Organization (WHO). (2023). Diabetes, a silent killer in Africa: Analytical fact sheet. https://files.aho.afro.who.int/afahobckpcontainer/production/files/iAHO_Diabetes_Regional_Factsheet.pdf
Wound, Ostomy, and Continence Nurses Society (WOCN). (2022). Core curriculum: Wound management (2nd ed.). Lippincott, Williams & Wilkins.
Wound Source. (2024). DARCO MedSurg DUO pressure relief shoe. https://www.woundsource.com
Xiaohua G, Dongdong L, Xiaoting N, Shuoping C, Feixia S, Huajun Y, Qi Z, & Zimiao C. (2021). Severe vitamin D deficiency is associated with increased expression of inflammatory cytokines in painful diabetic peripheral neuropathy. Frontiers in Nutrition, 8, 612068. https://doi.org/10.3389/fnut.2021.612068
Zubair M, Ahmad J, Malik A, & Talluri MR. (2021). Diabetic foot ulcer: An update. Springer Publications.





Customer Rating
4.9 / 691 ratings