Clinical Care for the Heart Failure Patient
Online Continuing Education Course

Course Description
5-contact-hour online CEU course on clinical care for patients with heart failure. Learn more about cardiac failure (formerly called congestive heart failure or CHF), including the types, causes, signs, symptoms, treatment options, and cardiac rehabilitation. Includes American Heart Association (AHA) guidelines.
Course Price: $35.00
Contact Hours: 5
Pharmacotherapeutic Hours: 0.5
Course updated on
August 2, 2024
"Great resource! Very helpful to my current practice as an inpatient acute care OT. Thank you!" - Amanda,OT in Kentucky
"I appreciated the thoroughness and the well-organized manner of the information presented. I will and have already implemented my new knowledge in my professional practice. Thank you for creating such a helpful CE course." - Nicole, OT in Washington
"This course was great professionally and also a wake-up call for me to take care of my own health better for prevention. Thanks!" - Nancy, RN in Georgia
"I loved this course! It was nicely written and included images for added visual reinforcement. Wonderful!" - Terri, RN in California
Clinical Care for the Heart Failure Patient
Copyright © 2024 Wild Iris Medical Education, Inc. All Rights Reserved.
LEARNING OUTCOME AND OBJECTIVES: Upon completion of this continuing education course, you will have a current, evidence-based understanding of the prevalence, causes, diagnostic testing, treatment, and patient care for various types of heart failure. Specific learning objectives to address potential knowledge gaps include:
- Describe heart failure.
- Summarize the epidemiology of heart failure.
- Discuss the pathophysiology and etiology of heart failure.
- Explain the comorbidities of heart failure.
- List diagnostic methods to determine presence and severity.
- Describe pharmacologic and nonpharmacologic treatment measures for heart failure.
- Explain the multidisciplinary approach to cardiac rehabilitation.
- Discuss patient education strategies to prevent recurrence and rehospitalization.
- Describe end-of-life care for the patient with heart failure.
TABLE OF CONTENTS
INTRODUCTION
Heart failure (HF) is a complex syndrome of symptoms that causes the inability of the heart to pump sufficient blood throughout the body to satisfy the oxygen needs of the organs and cells. It may also be referred to as cardiac failure and was formerly referred to as congestive heart failure.
HF may be caused by cardiac insult such as a myocardial infarction secondary to coronary artery disease or may be a natural effect of aging as the cardiac pump progressively weakens and becomes less effective. Two of the most common comorbidities are hypertension and coronary artery disease (CAD) (Harding et al., 2022).
- Systolic heart failure is caused by weakened ventricular contractions from a dilated left ventricle that fails to pump blood effectively. This results in heart failure with reduced ejection fraction (HFrEF). Cardiac ejection fraction (EF) is the amount of blood pumped into the systemic circulation by the left ventricle, expressed as a percentage.
- Diastolic heart failure is decreased cardiac output in the presence of normal EF due to ventricular stiffness.
- Right-sided heart failure refers to failure of the right ventricle to pump blood effectively to the pulmonary arteries, thus causing a backflow of blood into the right atrium and into the venous circulation.
- Left-sided heart failure, which is the most common, refers to the failure of the left ventricle to pump blood to the rest of the body. When one ventricle fails, if left untreated, the other ventricle will also inevitably fail.
(Harding et al., 2022)
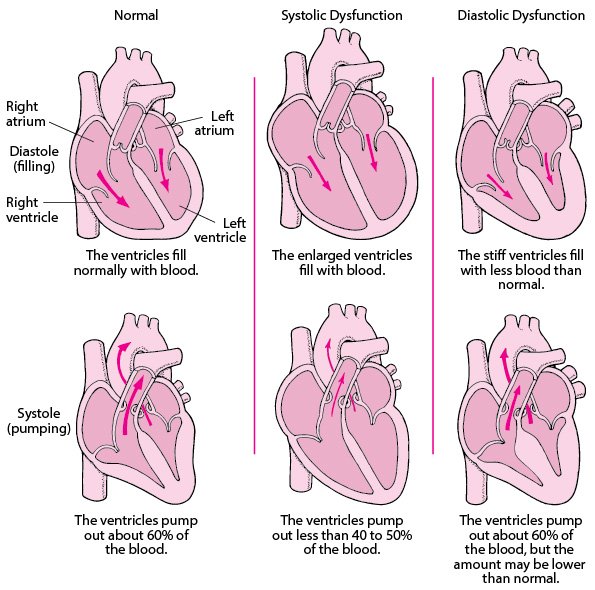
Heart failure pumping and filling problems. (Reprinted by permission from the Merck Manual Consumer Version, known as the Merck Manual in the United States and Canada and the MSD Manual in the rest of the world, edited by Robert Porter. © 2021, by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co, Inc., Kenilworth, NJ. Available at http://www.merckmanuals.com/home.)
HF can be chronic (most common) or acute, where there are recognizable symptoms before treatment is implemented successfully (as in the case of pulmonary edema) (Harding et al., 2022).
(See also “Pathophysiology and Etiology” later in this course for more detailed descriptions.)
IMPACTS AND EPIDEMIOLOGY OF HEART FAILURE
Nurses and other healthcare professionals are charged with providing culturally competent healthcare that recognizes the following epidemiologic factors.
Occurrence
Heart failure affects more than 64 million people globally. It is considered a global epidemic, particularly in countries with rapidly aging populations, such as the United States and Japan. In countries where successful treatment of cardiovascular disease has improved life expectancy in general, the aging of the population also causes an increase in the occurrence of HF (Gtif et al., 2021; Savarese et al., 2023).
HF affects approximately 6.7 million people in the United States and is projected to rise to more than 8 million, or 3% of the adult population, by 2030, with 56% being women. The median age of those diagnosed with HF is 73 years (Martin et al., 2024).
The rise in HF is attributed to three confounding factors in the population:
- People are living longer, and the elderly population is rapidly increasing, with more people in their 80s and 90s surviving with HF.
- People with acute coronary syndrome are surviving for much longer after an insult such as myocardial infarction (MI) due to earlier recognition of coronary artery disease, use of beta receptor blocking agents for patients with MIs, treatment of high cholesterol and narrowed coronary arteries, and early intervention to improve coronary artery blood flow and preserve ejection fraction.
- The more recent use of medications to treat comorbidities has helped to prolong the lives of those with HF. Antihypertensives, antidiabetics, or aldosterone antagonists for patients with an MI or diabetes and an ejection fraction of less than 35% have prolonged the life of patients with HF who would otherwise have succumbed at an earlier age to those comorbidities.
- The incidence of obesity and diabetes is increasing related to diet and exercise and affecting younger patients ages 20 to 45, who are experiencing HF in unprecedented numbers.
(Rauf, 2022)
Heart failure is the most common reason for hospital admissions in adults in the United States, at over 1 million admissions per year. Approximately 25% of those patients will be rehospitalized within 30 days and nearly 50% within 6 months. Most HF hospitalizations are caused by one of five precipitants: infection, ischemia, dysrhythmia, medications, and noncompliance with dietary recommendations.
Patients hospitalized with worsening HF are at high risk of rehospitalization. Among patients with a single identifiable precipitant, research shows that the one-year risk for HF readmission varies with the particular precipitant. The precipitant for HF rehospitalizations is more likely to be the same precipitant as for the initial admission (Diamond & Devore, 2022; Harding et al., 2022).
Cost
The annual cost of care for each patient with HF in the United States is almost $30,000, with 70%–80% of that amount spent on hospitalizations. Other expenses include healthcare services, medications to treat HF, and days lost from work. With the aging of the population, the total annual cost of HF in the United States is projected to reach at least $70 billion by 2030 when hospitalization, physician, pharmaceutical, and home healthcare costs are all considered (Vecchione, 2022).
Mortality
A frequently cited systematic review and meta-analysis showed that more than 50% of people diagnosed with HF survived for 5 years and approximately 35% survived for 10 years (Johnson, 2023). Another review showed that approximately 35% of all HF patients died within a year of their initial diagnosis (Rauf, 2022).
Despite improved technology and medications for treating HF, the mortality rate for HF patients who must be admitted or readmitted to an acute care hospital remains high. Newer medications to improve the force of contractions; implantable defibrillators, pacemakers, and support devices; and peripherally inserted central catheter (PICC) lines for home-administered medications have advanced the ability to treat HF (Huizen, 2024).
| Stage | Description | 5-Year Survival Rate |
|---|---|---|
| (Schiller, 2024) | ||
| A | High risk for heart failure, but without symptoms or structural heart disease | 97% |
| B | Structural heart disease, but without signs or symptoms of heart failure (also known as pre–heart failure) | 95.7% |
| C | Structural heart disease with prior or current symptoms of heart failure | 74.6% |
| D | Advanced heart failure characterized by recurrent hospitalizations despite attempts to optimize treatment | 20% |
| % LVEF | Mortality |
|---|---|
| (Schiller, 2024) | |
| ≤15 | 51% |
| 16–25 | 41.7% |
| 26–35 | 31.4% |
| 35–45 | 25.6% |
Geographically, the preponderance of deaths due to HF are in the southern and midwestern states (CDC, 2023).
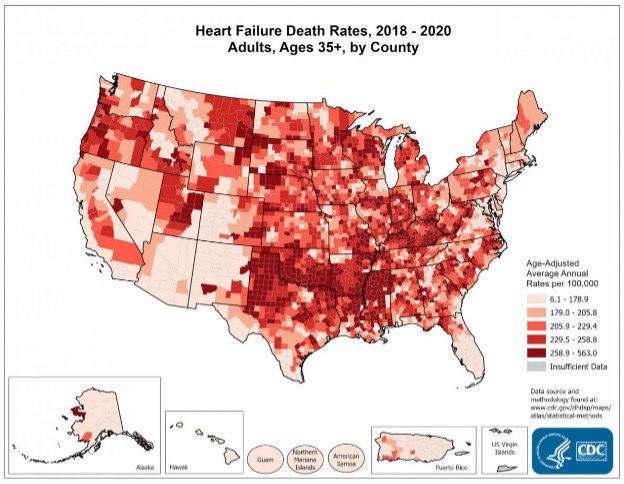
(Source: CDC, 2023.)
By Age
HF is the leading cause of potentially avoidable hospitalizations among Medicare and Medicaid patients, according to Centers for Medicare and Medicaid Services studies. Although mortality rates are lower for younger HF patients, they are still significant. After hospitalization, 3.9% of patients under 44 are likely to die after 30 days; after one year, 12.4% are likely to die, and after 5 years, 27.7% are likely to die (Schiller, 2024).
Racial and Ethnic Disparities
African American and Latinx people are usually diagnosed with HF at an earlier age. African Americans have the highest risk of developing HF, followed by Latinx, White, and Chinese Americans, in that order.
The higher risk in African Americans reflects differences in the prevalence of hypertension, diabetes, and socioeconomic status. African Americans have a higher incidence of HF, develop symptoms at an earlier age, and experience mortality at a younger age than Whites with the same disease. African Americans with HF ages 35–64 years have more frequent hospitalizations and an age-adjusted mortality rate that is 2.97-fold higher and 2.6-fold higher than White women and men, respectively.
The Coronary Artery Risk Development in Young Adults (CARDIA) study showed a 20-fold higher incidence of HF in young Black women and men under age 50 (1.1% and 0.9%, respectively) versus White women and men (0.08% and 0%, respectively) within this cohort (Lewsey & Breathett, 2021). Disparities in access to healthcare may also contribute to earlier morbidity and mortality.
African American patients are also more likely to be readmitted to the hospital within 30 days of admission than White or Hispanic patients. Two or more hospitalizations for HF within a year increases the probability of mortality by 30%. The presence of structural cardiac changes such as asymptomatic left ventricular (LV) hypertrophy and LV systolic and diastolic dysfunction was higher in African American patients. Structural cardiac changes are associated with increased clinical HF and underrecognized in African American and Hispanic patients. More African American patients are also likely to have HF from nonischemic causes (Lewsey & Breathett, 2021).
Sex
Men are diagnosed more frequently with systolic HF or HF with reduced ejection fraction (HFrEF), and women are diagnosed more frequently with diastolic HF. Men with HF benefit more from angiotensin-converting enzyme (ACE) inhibitors, and women with HF are more likely to have the ACE inhibitor–related cough as a persistent side effect.
Men are diagnosed more frequently with systolic HF due to higher prevalence of coronary artery disease (CAD). Women develop HF at an older age than men. Women are more likely to be diagnosed with diastolic HF or HF with preserved ejection fraction (HFpEF) due to a higher prevalence of hypertension. Women with HF are also more likely to have CAD, diabetes, and valvular disease (Harding et al., 2022).
PATHOPHYSIOLOGY AND ETIOLOGY
There are several classifications of heart failure, which depend on the causes and duration of the illness. HF can be any combination of these three classifications:
- Systolic vs. diastolic
- Left-sided vs. right-sided
- Acute vs. chronic
Systolic vs. diastolic HF refers to whether the cause of HF is impaired pumping of the ventricles (systolic) or impaired filling of the ventricles (diastolic), based upon the underlying problem as either one of force of the contractions or altered relaxation. This is significant in that treatments vary depending upon the cause.
Left-sided HF refers to inefficient pumping of the left ventricle, leading to decreased cardiac output and therefore compromised perfusion. The volume of blood remaining in the left ventricle increases with each heartbeat, causing the blood to back up into the left atrium and eventually into the lungs. In right-sided HF the blood backs up to the right atrium and eventually to the periphery.
Acute heart failure refers to an episode of the illness that occurs suddenly or appears as an acute exacerbation of chronic HF (the latter may be referred to as acute-on-chronic HF). Chronic HF occurs slowly over time, with the gradual deterioration of cardiac function resulting in worsening symptoms.
Some causes and signs/symptoms of HF are shown in the table below according to the chambers involved.
| Classifications | Left-sided | Right-sided |
|---|---|---|
| (Harding et al., 2022) | ||
| Systolic HF causes |
|
|
| Diastolic HF causes |
|
|
| Signs/symptoms |
|
|
Neurohormonal Mediated Effects
HF with a reduced ventricular function results in a reduced cardiac output. Reduction of cardiac output can result in activation of the sympathetic nervous system (SNS) and the renin-angiotensin-aldosterone system (RAAS) as a compensatory mechanism. These neurohormonal responses cause physical changes in the body, particularly in the heart, kidneys, and blood vessels, as the body attempts to return to a state of homeostasis. Neurohormonal activation is one of the most common causes of the progression of HF.
These compensatory systems attempt to maintain a normal cardiac output by promoting retention of sodium and water, causing peripheral arterial vasoconstriction and inflammatory moderators that promote cardiac repair and remodeling. Neurohormonal activation will cause increases in cardiac preload, afterload, and left ventricular (LV) overfilling. These can lead to LV remodeling (changes in the LV shape, mass, and volume) and decreased LV pump function.
Beta-adrenergic blocking agents (beta blockers), angiotensin-converting enzyme (ACE) inhibitors, aldosterone agonists, and angiotensin receptor blockers (ARBs) are prescribed for patients with HF to improve cardiac cell contractility in the failing heart caused by the activation of the neurohormonal systems in HF. The progression of HF may be reduced by beta blockers. ACE inhibitors and aldosterone agonists help prevent cardiac fibrosis in HF. ARBs reduce vasoconstriction, reducing the resistance against which a failing heart must pump (Harding et al., 2022; Merck & Co, 2024).
Natriuretic peptides are hormones synthesized by the heart, brain, and other organs. Atrial natriuretic peptide (ANP) is released by the atria in response to atrial distension and so becomes elevated in a hypervolemic state such as in HF. B-type (also called brain-type) natriuretic peptide (BNP) is synthesized by the ventricles and the brain. Serum BNP levels are used to diagnose the presence and severity of HF. They work in response to the SNS and RAAS compensatory mechanism in HF; the levels indicate necessary treatment to promote the excretion of sodium and water (Harding et al., 2022).
Systolic Heart Failure
Systolic HF is a defect in ventricular pumping resulting in a reduced ejection fraction (EF) and referred to as heart failure with reduced EF (HFrEF). Normal EF is 55%–60%, but with systolic heart failure, EF is abnormally low at <40% and in severe cases as low as 5%–10%. A new classification of midrange EF (HFmEF) has an EF of 41%–49%.
With systolic HF, the left ventricle is unable to exert enough pressure to pump sufficient blood through the aorta and out to the rest of the body. Eventually, the left ventricle becomes dilated and hypertrophied from overwork, weakening the cardiac muscle. This results in the blood backing up into the left atrium and then into the lungs, causing pulmonary congestion and edema.
Systolic HF may be caused by increased afterload (as in hypertension), impaired contractile function (as in myocardial infarction), or mechanical abnormalities such as valvular disease. Preload represents the volume of blood in the ventricles. Afterload represents the arterial resistance against which the ventricles pump blood. Abnormalities in the preload and afterload may also affect the cardiac output (Harding et al., 2022).
Systolic HF is also caused by various effects of aging. Aging causes a loss of elastin in the blood vessels, producing stiffness in the blood vessels. The myocardium also becomes stiffer due to a change in the effect of calcium in the cells and an abnormal growth in connective tissue. This causes ventricular hypertrophy, contributing to cardiomegaly (an increase in the size or silhouette of the heart). A hypertrophied ventricle pumps less efficiently, resulting in HF. Stiffness is further aggravated by interstitial collagen deposits in the cardiac tissue. The smooth muscle layers in the arteries become thicker, making them less elastic (Merck & Co, 2024).
Diastolic Heart Failure
Diastolic HF occurs in the presence of normal EF and is referred to as heart failure with preserved ejection fraction (HFpEF). In diastolic HF, the left ventricle is stiff or noncompliant, possibly due to hypertension or aging, causing decreased filling and high filling pressures and resulting in a reduction in cardiac output. Approximately 50% of patients with HF have HFpEF.
The reduced preload (ventricular volume) results in a diminished stroke volume (SV). As stroke volume is part of the cardiac output (CO) equation (SV × HR = CO), there is a diminished amount of blood pumped by the left ventricle into the systemic circulation.
The noncompliance of the ventricle is usually caused by hypertension. An imbalance in normal cardiac contraction and relaxation can also be due to the change in calcium function that occurs when there is an insufficient reuptake of calcium ions into the cardiac cells. HF interferes with the normal reabsorption of calcium into the heart muscle. This causes the tissue to become weaker, creating a vicious cycle as the weak tissue is less able to reabsorb calcium (Harding et al., 2022; Merck & Co, 2024).
The characteristics of the patients with HFpEF are older age; greater likelihood of being female; and greater prevalence of hypertension, obesity, and anemia than those with HFrEF (Harding et al., 2022).
HEART FAILURE AND DECREASED CIRCULATION
Any form of HF may result in decreased blood flow. Whether HF is secondary to poor ventricular filling (diastolic) or a weak pump (systolic), the end result is less systemic perfusion and increased congestion at the cellular level. Poor perfusion to major organs may result in progressive organ failure. Poor renal perfusion may result in decreasing kidney function, as evidenced by a decreasing glomerular filtration rate (GFR). Decreased cerebrovascular circulation in the form of reduced basilar artery inflow may lead to irreversible cognitive or motor impairment, as in the case of a stroke (Harding et al., 2022).
Left-Sided Heart Failure
Left-sided HF is the most common type. It results in decreased cardiac output and increased pulmonary venous pressure as the incompletely emptied left cardiac chambers cause compromised blood flow. The left ventricle is unable to receive the blood from the left atrium, since it remains partially filled, which then makes the left atrium unable to receive the normal amount of blood from the lungs, thereby causing the lungs to become congested.
When the pulmonary artery capillary wedge pressure (PACWP) is >24 mmHg, the capillaries begin to leak into the alveoli and interstitial space, escalating the work of breathing. The resultant pulmonary edema forces deoxygenated blood through the congested alveoli, diminishing the arterial oxygen content. The ability of the lungs to process deoxygenated blood and oxygenate it is significantly compromised, resulting in excessive fluid accumulation in the lungs and dyspnea.
Congestion of the liver (higher pressures in the hepatic veins/inferior vena cava) and gastrointestinal tract (edema caused by increased intestinal permeability) is a common clinical manifestation of left-sided HF (Harding et al., 2022; Merck & Co, 2024).
(See also “Causes and Signs/Symptoms of Heart Failure” above.)
Right-Sided Heart Failure
Right-sided HF may be systolic or diastolic depending on whether the cause of failure relates to insufficiency of blood pumped out by the heart due to a weak pump or low volume. In right-sided HF, the right ventricle does not empty completely, causing the blood left in the chamber to back up into the right atrium. The elevated systemic venous pressure causes edema in dependent tissue and the abdominal viscera (ascites). This primarily affects the liver, but the stomach and intestines can also become congested. Dependent tissue edema will cause a delayed venous return of fluid and hypertension (Harding et al., 2022; Merck & Co, 2024).
(See also “Causes and Signs/Symptoms of Heart Failure” above.)
CASE
Mi-Young Kim, an 84-year-old woman, is brought by her granddaughter to the urgent care clinic complaining of swelling in her feet and ankles, infrequent urination, and difficulty walking. Angela, the family nurse practitioner, gets the patient settled in an exam room and performs a head-to-toe examination.
Angela notices that Ms. Kim is walking slowly and with great difficulty and must lean heavily on her granddaughter’s arm for support. She observes 3+ pitting edema of the feet and ankles, abdominal distention with ascites, a palpable liver, and 3+ jugular vein distention when sitting upright on the exam table. Ms. Kim’s vital signs are BP 168/94 mmHg, pulse 122, respirations 18, deep and unlabored, temperature normal, and an O2 saturation of 89% on room air. An ECG shows a sinus tachycardia with occasional unifocal premature ventricular contractions (PVCs).
Angela suspects right-sided HF, explains her findings to Ms. Kim, and arranges for EMT transport to the nearest hospital. She notifies the patient’s primary care provider. She explains to the patient and her daughter that they will be given information to help them better understand how to take care of Ms. Kim to address what is causing her symptoms and to prevent the same thing from happening again, thereby preventing a hospital stay. Angela clarifies that a plan of cardiac rehabilitation may be put into place to help her cope with her symptoms.
Acute Heart Failure
Acute HF is the sudden onset of the signs and symptoms of HF, resulting in an urgent medical condition requiring immediate intervention. It may also manifest as the pulmonary edema that results after an abnormal accumulation of fluid in the lungs. This fluid compromises oxygenation, making it difficult for the patient to breathe. The heart starts to beat faster as a compensatory mechanism to improve oxygenation. The rapid heart rate causes the heart muscle to become exhausted, forming the foundation for the blood to become backed up or congested.
Acute HF typically occurs after a sudden insult to the myocardium, such as a myocardial infarction (MI). It may result in pulmonary edema if there is insult to the left ventricle. The following are common causes of acute HF:
- MI
- Liver failure
- Kidney failure
- Hematologic conditions (such as anemias and coagulopathies that cause hemodynamic imbalance and edema)
- A sudden exacerbation of a chronic disease (referred to as acute-on-chronic HF)
The symptoms of acute HF are:
- Production of frothy, pink sputum with coughing
- Auscultation of adventitious breath sounds such as crackles
- Hypoxia that may result in panic/anxiety, tachycardia, shortness of breath, restlessness, orthopnea, or confusion
- Jugular vein distention
- Cyanosis
(Harding et al., 2022)
Chronic Heart Failure
Chronic HF is a syndrome of ongoing ventricular dysfunction characterized by a progressive worsening of symptoms in which the heart no longer supplies adequate blood volume to satisfy the body’s circulatory needs. Once there is a reason for the heart to begin failing—for instance, chronic hypertension or an acute injury such as a myocardial infarction—the heart works harder to compensate for decreased output, and the increased cardiac workload causes the heart to fail even more.
The following are general clinical manifestations of chronic HF:
- Fatigue
- Dyspnea
- Paroxysmal nocturnal dyspnea (PND)
- Tachycardia
- Edema
- Nocturia
- Skin changes
- Behavioral changes
- Chest pain
- Weight changes
(Harding et al., 2022)
CASE
Antoinette Roberts is a 75-year-old, morbidly obese African American woman who is admitted to Sunshine Coast Hospital for the fifth time for recurring symptoms of HF. It has been 18 days since her last admission for these same symptoms. She has a history of hypertension, hypercholesterolemia, type 2 diabetes, and hypothyroidism. Her physician has ordered dietary and physical therapy consultations to support her in her efforts to lose weight and control her blood sugar.
At her initial physical therapy consultation, she seems downcast and dejectedly tells the evaluating therapist, Warren, that everyone in her family has some form of heart disease and “there’s just nothing I can do about it.” Warren validates Ms. Roberts’ feelings by agreeing that genetic risk factors for heart disease cannot be helped and are a valid concern. He then goes on to discuss the fact that other modifiable risk factors, such as weight, blood pressure, and blood sugar, are more within Antoinette’s control and that her multidisciplinary healthcare team will help her to gain more control over these factors.
Ms. Roberts brightens a bit at this and tells Warren about her recent dietary consult, where the dietitian recommended a controlled-carbohydrate diabetes diet in order to help get her weight and blood pressure under better control. Warren tells Ms. Roberts that physical therapy (PT) can also help with these factors and that the two of them will work together to craft a realistic and progressive cardiopulmonary conditioning program of therapeutic exercises.
Ms. Roberts diligently follows the recommendations of her dietitian and goes to PT twice weekly for a period of eight weeks. On her last day of PT, she proudly tells Warren that she has been experimenting with a heart-healthy cookbook and now uses olive oil in place of canola oil and wheat bread instead of white. She is happy to report that her blood sugar has lowered by several points, she has lost 7 pounds, and she feels more in control of her food choices. When Ms. Roberts finishes her last PT session, she is noticeably less fatigued and less short of breath than she was at her first session. Warren takes her blood pressure and announces that it, too, has lowered significantly over the past two months.
Ms. Roberts and Warren review a long-term exercise program for her to continue independently at home, including an after-dinner walk, working in her newly planted vegetable garden, and joining a weekly aquatic exercise class at the local senior center. As she leaves the clinic, Ms. Roberts gives Warren a hug, thanks him for helping her to feel more in control of her health, and promises to bring him some tomatoes from her garden.
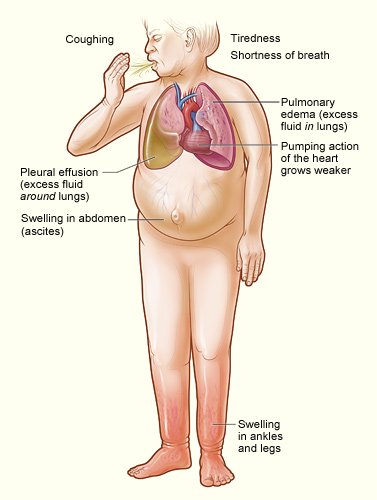
Major signs and symptoms of heart failure. (Source: NHLBI, 2022a.)
Risk Factors
Risk factors for heart failure include:
- Advanced age
- Dietary and lifestyle factors
- Obesity
- Genetic disposition
Comorbidities may also put someone at greater risk for HF and include:
- Myocardial infarction
- Hypertension
- Diabetes
- Renal failure
- Metabolic syndrome
- Untreated sleep apnea
(NHLBI, 2022b)
Advanced age (>70 years) usually results in increased afterload due to increased arterial resistance. This is caused by thickening of the smooth muscle and a loss of elastin in the blood vessels. Calcium changes and interstitial collagen deposits in the myocardium cause a loss of elasticity, affecting the ability to pump blood out of the ventricles.
Women experience a higher occurrence of diastolic failure than men. Women with diabetes are more predisposed to HF. Women have a higher incidence of obesity, which is another risk factor for HF. Women also have a higher incidence of increased left ventricular end-diastolic pressure, or preload.
Dietary and lifestyle factors also impact HF risk. HF is higher in males with hypertension, whereas healthy lifestyle factors (normal weight, not smoking, regular physical activity, lower alcohol intake, and consumption of fruits and vegetables) are related to lower risk of HF. The use of tobacco products containing nicotine causes the release of catecholamines, resulting in the vasoconstriction that causes hypertension, and hypertension causes increased vascular resistance that may cause HF over time.
Obesity may be a precipitating factor for HF. It can be associated with diabetes, hypercholesterolemia, and hypertension, all common comorbidities with HF.
Individuals may be predisposed to acquiring HF because of specific genes or gene mutations they have inherited. Cardiovascular disorders such as hypertension, coronary artery disease, and cardiomyopathy (a weakening of the heart muscle) all have a genetic link and may predispose a patient to HF. Cardiomyopathy alone may be connected to as many as 40 defective genes (Harding et al., 2022).
Cocaine causes cardiac injury through both ischemic and toxic pathways. Cocaine increases the synaptic catecholamine concentration, producing coronary vasoconstriction. Prolonged cocaine use results in ischemia, producing cell death and myocardial fibrosis. Myocardial toxicity plays a major role in cocaine-induced cardiac injury. Cardiac injury may subsequently cause myocardial infarction, stroke, acute aortic dissection, coronary artery aneurysm, myocarditis, HF, cardiac dysrhythmias, and cardiomyopathy. Marijuana-induced coronary vasospasm and tachycardia, in conjunction with excessive cocaine use, may contribute to a risk of sustaining a myocardial infarction. General treatment to alleviate symptoms and prevent serious outcomes include beta-adrenergic blocking agents and stents (Fogoros, 2023).
The American Heart Association’s “Life’s Simple 7” guidelines are associated with a lower lifetime risk of HF. They include stopping smoking, lower BMI, physical activity for at least 30 minutes at least five times per week, healthy diet, lower-range cholesterol, BP <140/90 mmHg, and glucose within advised parameters (Hasbani et al., 2022).
HEART FAILURE AND COMORBIDITIES
Comorbidities associated with HF include hypertension, myocardial infarction, diabetes, renal failure, metabolic syndrome, and respiratory insufficiencies.
Hypertension
Hypertension is the most common comorbidity of HF. Hypertension that includes a chronic BP of >160/90 mmHg increases the life risk of developing HF by 1.6 times that of a usual BP reading of <120/80 mmHg at all ages.
Hypertension reflects increased vascular resistance to the efforts of the heart, especially the left ventricle, to pump blood to the body. The harder the heart works to pump blood, the more work the muscle (myocardium) must do. As with any muscle, the more work required to pump blood, the larger the muscle grows to work effectively. This results in myocardial hypertrophy, creating a larger, less-flexible muscle that must in turn work even harder, causing ventricular remodeling. Ventricular remodeling results in large, oddly shaped contractile cells, increased oxygen consumption, increased cardiac workload, increased wall tension, and impaired contractility.
Uncontrolled hypertension is also the most common cause of an HF exacerbation. Since it is a modifiable risk factor for HF, early recognition and intervention for hypertension helps to prevent further worsening of cardiac function. Managing blood pressure is an essential part of preventive care to avoid the recurrence of aggravated HF symptoms. Blood pressure management is a multifaceted, multidisciplinary approach that includes medications, diet, exercise, and establishment and support of activities of daily living.
Cardiac dilation occurs when a sustained BP elevation causes enlargement in the cardiac chambers, usually the left ventricle. This causes the myocardial fibers to stretch in response to increased volume at the end of diastole. The degree of stretch is related to the force of the contraction during systole (the Frank-Starling law). This is a compensatory mechanism that supports BP, cardiac output, and perfusion, but eventually fails as the patient decompensates and starts to experience HF (Harding et al., 2022).
Myocardial Infarction
Myocardial infarction (MI) is a common comorbidity for HF. An MI in the area of the left ventricle (LV) may cause a dysfunctional LV, leading to a drop in cardiac output due to incomplete emptying of the ventricle. This causes the myocardium to have to exert greater effort to pump blood to the rest of the body. Over time, this increased effort causes the cardiac pump to be less effective, eventually leading to HF.
Cell injury in the infarcted area causes an inflammatory response. As cardiac output and subsequent renal blood supply drops, the renin-angiotensin-aldosterone system (RAAS) goes into effect. Additionally, the production of antidiuretic hormone (ADH) stimulates further production of the vasoconstrictor endothelin, causing arterial vasoconstriction that will increase cardiac contractility and hypertrophy, intensifying the effects of HF.
Infarcted myocardial tissue becomes akinetic (motionless), causing pump failure (systolic failure, or HFrEF). As discussed earlier in this course, poor coronary artery blood flow causes myocardial ischemia that may lead to an infarction of the myocardial tissue over time. Infarcted tissue is necrotic, losing all former abilities, including contractility. The necrotic tissue is therefore no longer capable of movement, causing a decrease in the strength of the heart to function as a pump. This pump failure affects the amount of blood emptied through force from the affected chamber(s). When the left ventricle is affected, a smaller volume of blood leaving the left ventricle to the aorta affects the ability of the other cardiac chambers to empty, causing congestion.
Diabetes
Diabetes mellitus is frequently associated with HF. The dramatic increase in the number of people diagnosed with diabetes will most likely cause a similar growth in the number of people with HF.
Insulin is a powerful sodium-retaining hormone, and patients with type 1 diabetes who are receiving insulin have a greater tendency to retain fluid, thus exacerbating the effects of HF. In patients with type 2 diabetes and HF, use of empagliflozin (a sodium-glucose transporter 2 inhibitor) acts as a diuretic, and these patients show a reduction in hospitalizations. Those with no HF had a lower incidence of developing the disease over time (McCuistion et al., 2023).
High blood glucose results in vascular scarring in the layer of the tunica intima, affecting circulation. Prolonged periods of uncontrolled blood glucose levels (relative to recommended level of <150 mg/dL) result in permanent scarring and cause significant impact as blood flow is affected. This may result in poor tissue healing, blindness, renal failure, and HF as the effect of compromised circulation takes its toll on arteries and organ systems over time.
Poor peripheral circulation causes decreased venous return, reducing ventricular filling. Reduced ventricular filling results in a lower cardiac output and reduced ejection fraction. This can cause HF as the cardiac muscle attempts to compensate for the decreased amount of blood volume exiting the left ventricle and the congestion of blood due to sluggish circulation.
Renal Failure
Renal failure is associated with adverse outcomes in patients with HF. Renal dysfunction, defined as an estimated glomerular filtration rate (eGFR) of <60 mL/minute, is highly prevalent in HF patients.
Reduced renal blood flow because of compromised circulation with a reduced ejection fraction may cause the kidneys to perform poorly. A reduced volume of blood passing through the kidneys will reduce the amount of blood to be filtered, resulting in higher levels of waste products, including nitrogenous wastes (i.e., blood urea nitrogen [BUN], creatinine).
In HF combined with renal failure, stimulation of the sympathetic nervous system and the renin-angiotensin-aldosterone system (RAAS) causes the excretion of catecholamines and angiotensin II, among other substances. The release of angiotensin II produces ventricular remodeling. Abnormal neurohormonal responses can also exacerbate the degree of HF. The RAAS acts to control blood pressure by promoting fluid retention and vasoconstriction. When cardiac output drops, decreased renal circulation causes the kidneys to release renin, initiating the RAAS response. This causes fluid sodium retention and will increase the blood pressure and the workload in an already failing heart (Harding et al., 2022).
Metabolic Syndrome
Metabolic syndrome is a group of risk factors that may predispose an individual to heart disease, including HF, diabetes, and stroke. It is characterized by a collection of health issues that includes obesity, high triglycerides, low levels of high-density lipoprotein cholesterol, hypertension, and high blood glucose. An individual is considered to have metabolic syndrome if they have at least three of these five factors (Harding et al., 2022; Purwowiyoto & Prawara, 2021).
The progression of HF correlates highly with metabolic syndrome. Minor increases in serum glucose cause a high risk for HF. Insulin resistance occurs with 60% of patients with HF. Metabolic syndrome correlates with both systolic and diastolic HF (Purwowiyoto & Prawara, 2021).
Respiratory Insufficiency
Many HF patients have respiratory comorbidities such as chronic obstructive pulmonary disease (COPD) or asthma. COPD and asthma are both characterized by respiratory limitations, often caused by inflammation in the airway. HF and respiratory patients share several of the same symptoms, such as dyspnea, fatigue, psychological disturbances, deconditioning, and exercise intolerance.
Pleural effusion can be a common complication of HF. Pleural effusion is when the natural fluid between the two layers of the pleura that exists for lubrication becomes excessive secondary to increased pressure in the pulmonary capillaries. The clinical manifestations are dyspnea, cough, and chest pain. These symptoms may imitate symptoms of acute HF and confound a clinician’s ability to diagnose the specific cause of the worsening symptoms unless a physical assessment and diagnostic tests are performed (Elgwairi et al., 2024).
COVID-19 AND HEART FAILURE
COVID-19 is an acute, sometimes severe, often fatal, respiratory disease that is caused by the novel coronavirus SARS-CoV-2. Serious diseases that may be a long-term result of a case of COVID-19 with more extreme symptoms include dysrhythmias, cardiomyopathy, acute cardiac injury, thromboembolism, pulmonary emboli, disseminated intravascular coagulation (DIC), hemorrhage, arterial clots, Guillain-Barré syndrome (rare), sepsis, shock, and multi-organ failure (Merck & Co., 2024).
HF as a preexisting condition and COVID-19 both have similar risk factors, tend to cause polypharmacy, and promote nutritional challenges. HF risk factors such as advanced age, male sex, elevated D-dimer and lactate dehydrogenase levels, and a higher NYHA (New York Heart Association) score translate to a higher percentage of mortality for patients with a concomitant COVID-19 infection. The inflammation common to both diseases potentiates a poor outcome for the patient. The presence of both diseases causes an increase in dysrhythmias: tachycardia, bradycardia, prolonged QT interval, and polymorphic ventricular tachycardia (Khan, 2021).
DIAGNOSIS OF HEART FAILURE
The diagnosis of HF is primarily made based on symptoms and backed by more precise diagnostic tests to either rule out similar disease processes or confirm a diagnosis of HF.
Health History
Taking a focused health history is an essential part of reaching a differential diagnosis of HF. This may include any medical or surgical history, list of medications taken, recent diagnostic test results, any concerning or pertinent symptoms (such as orthopnea, paroxysmal nocturnal dyspnea, weight gain, abdominal bloating, reduced appetite, and dyspnea-free activity), family cardiac history, and vital signs.
Physical Examination
A physical examination is performed to verify the subjective complaints of the patient and further determine a diagnosis of HF. Abnormal findings may support the diagnosis of HF.
Cardiovascular examination includes blood pressure, heart rate, pulses, and cardiac auscultation. Chest pain may occur from decreased coronary artery perfusion secondary to decreased cardiac output or coronary artery obstruction. The point of maximum impulse (PMI), which is the point furthest from the sternum where the cardiac impulse can be felt, may be displaced due to left ventricular hypertrophy.
Intake and output and daily weights are measured accurately using a consistent method to determine fluid balance or overload.
Inspection of the skin for edema or skin discoloration will show the presence of fluid retention. Copper-colored, shiny lower-leg and ankle discoloration accompanied by dry, flaky skin may indicate recurrence of swelling and reabsorption of fluid. Moderate or severe HF may produce visible shortness of breath. The skin may be pale, ashen, or cyanotic. Jugular veins may appear distended. The skin may be cold and clammy from vasoconstriction.
Palpation is used to determine the severity of peripheral edema and whether a depression (pitting) is produced that does not resolve immediately. The abdomen is palpated to establish hepato- or splenomegaly. Abdominal tenderness over the liver is indicative of hepatic congestion.
Auscultation of the heart and lungs may elicit adventitious breath sounds, such as crackles in the case of pulmonary edema or pleural effusion caused by left-sided HF. S3 and S4 heart sounds or a cardiac murmur may be heard. Labored respirations may indicate hypoxia, pulmonary edema, pleural effusion, and hypervolemia.
Vital signs are usually checked every 1–8 hours during hospitalization based on the severity of the HF and checked with each physician or clinical visit, where the patients bring in a log of their blood pressures that they have taken themselves. Hypertension is common due to increased venous resistance (afterload) or hypervolemia from fluid retention. Tachycardia >100 beats per minute is often an early sign of HF as a compensatory mechanism for decreased cardiac output.
Cardiac monitoring may need to be performed continuously to assess for dysrhythmias. The respiratory rate may be elevated (>14–16 breaths per minute) due to pulmonary edema or decreased cardiac output as the body attempts to increase oxygen intake to compensate for hypoxia.
Hemodynamic monitoring when a patient is in the ICU may include continuous BP monitoring via an arterial line as well as cardiac output, pulmonary artery pressure, and pulmonary artery capillary wedge pressure via a pulmonary artery or Swan-Ganz catheter. Diuretic medications and ultrafiltration or aquapheresis (fluid removal) orders are based on these readings to remove excess sodium and fluid.
Oxygen saturation is decreased (<94%) in the presence of fluid in the lungs and may need to be monitored continuously, necessitating supplemental oxygen or positive pressure to be given (Harding et al., 2022).
Electrocardiogram (ECG)
An ECG is performed to determine the presence of any dysrhythmias, including an abnormal heart rate, since damage to the ventricular myocardium following an acute myocardial infarction may result in ventricular dysrhythmias. This can also be observed on the patient’s cardiac monitor atrial fibrillation. The ECG can also reveal evidence of myocardial ischemia indicating coronary artery disease as an indirect cause of HF.
Chest X-Ray
A chest X-ray may show cardiomegaly, which is common in protracted HF. It may also display abnormalities in the cardiac chambers, pulmonary congestion, displacement of the heart, and abnormalities of the lungs and greater blood vessels.
Treadmill Stress Test
A treadmill stress test is performed to determine activity tolerance. It is used to diagnose coronary artery disease, cardiac valvular disease, and chronic HF. It is performed concurrently with ECG and BP monitoring by a physician or trained clinician. The testing is stopped if the patient experiences extreme hypertension, tachycardia, or chest pain. A treadmill stress test is preferable to assess activity tolerance, but a chemical stress test with nuclear imaging may be needed for those unable to physically walk or raise their heart rate to a target goal (Mayo Clinic, 2022).
Echocardiogram
An echocardiogram shows abnormalities of the valves, the size and structure of the heart and chambers, and the condition of the pericardial sac and the ascending aorta. The ejection fraction is also measured during an echocardiogram, signifying the strength of the myocardium as a pump. Therefore, an echocardiogram is crucial in determining whether the HF is systolic or diastolic. This is performed by a trained sonographer who uses conducting gel and a transducer to transmit information to a specialized computer via sound waves. It is evaluated by a cardiologist.
Cardiac Catheterization
Cardiac catheterization (heart cath), or coronary angiography, is an invasive procedure used to obtain heart pressures. The physician advances a thin, flexible catheter via the radial or femoral artery (for a left cardiac catheterization) and through the internal jugular, femoral, or subclavian veins (for a right cardiac catheterization). Dye is then injected into the arteries, and a radiologic video (cine) is taken to measure the degree of blockage in any of the coronary arteries. During this procedure, an angioplasty and stent placement may be performed. Each coronary artery will reveal the percentage of obstruction, guiding the need for and type of intervention. Coronary artery disease is one possible cause of HF (NIH, 2023).
Laboratory Data
BNP (B-type natriuretic peptide) testing is the most definitive diagnostic test for HF. This biomarker establishes both the presence and severity of HF. BNP levels are also frequently rechecked to measure the success or failure of treatment. Normal range for BNP is <100 pg/mL. The higher the reading, the more severe the HF.
The neurohormone BNP is produced by myocardial cells. An increase in this hormone is caused by increased atrial or ventricular diastolic wall stretch to show the degree of left ventricular failure, as in HF. The BNP level may read as normal in HF in patients with HFpEF. Patients with acute decompensated HFpEF may have a BNP that is elevated (100–500 pg/mL), but lower than that of patients with HFrEF. Obesity sometimes causes decreased BNP production and increased BNP clearance, resulting in lower BP levels, sometimes to the point of normal (Pagana et al., 2022).
Other diagnostic laboratory data for HF include:
- A complete blood count (CBC) will determine the presence of infection or anemia.
- A comprehensive metabolic panel (CMP) measures electrolytes, kidney function (BUN and creatinine), liver function, glucose, calcium, magnesium, and albumin.
- Urine sodium is measured to determine the possibility of sodium retention.
- Urine specific gravity measures urine concentration.
- Thyroid function is measured to rule out thyroid disease as a cause of HF, and for elderly patients and those with atrial fibrillation.
- Serum iron, ferritin, and total iron-binding capcity (TIBC) are measured to distinguish the possible causes of anemia.
- Serum interleukin-6 is a cytokine that signals the presence of inflammation.
- C-reactive protein (CRP) is produced by the liver and is increased in the presence of inflammation.
- Tumor necrosis factor (TNF) alpha is a cytokine that signals the presence of inflammation.
(Merck & Co, 2024)
| A widely accepted and long-established system of HF classification is the New York Heart Association (NYHA) functional classifications. This system distinguishes different levels of heart failure severity according to the patient’s ability to perform certain physical activities. | |
| Class | Patient Ability |
|---|---|
| (Harding et al., 2022) | |
| I | No physical limitations |
| II | Slight limitations; comfortable only at rest; minimum activity starts to produce the symptoms of HF |
| III | Marked limitations; comfortable only at rest; moderate activity produces HF symptoms |
| IV | Unable to perform any activity without symptoms of HF; symptoms while at rest |
| Stage | Description |
|---|---|
| (Harding et al., 2022) | |
| A | At high risk of developing heart failure, but without structural heart disease or symptoms of HF (e.g., people with hypertension, coronary artery disease, diabetes, history of drug or alcohol abuse, rheumatic heart disease, or family history of cardiomyopathy) |
| B | Structural heart disease with or without symptoms of HF (e.g., left ventricular structural changes, heart valve disease, or history of myocardial infarction) |
| C | Structural heart disease with prior or current symptoms of HF (e.g., shortness of breath, fatigue, or symptom free and receiving treatment for prior symptoms) |
| D | Refractory or end-stage HF requiring specialized interventions such as cardiac transplantation or compassionate care such as hospice |
| * American College of Cardiology/American Heart Association | |
TREATING HEART FAILURE
While there is no cure for heart failure, there are several levels of treatment. Treatment goals are:
- Symptom management
- Prevention of exacerbation
- Prevention of advancement to worse functional classifications
Collaborative management refers to the combined efforts of the various healthcare team members who combine therapies and areas of expertise to manage symptoms and prevent exacerbations. A well-coordinated, collaborative approach can also prevent advancing to a worsening level of function.
Medications may be used to improve cardiac output, reduce cardiac workload, improve symptoms, reduce mortality, and reduce the occurrences of readmission to the hospital.
Surgical interventions such as heart valve replacement or repair, internal cardiac defibrillators, transtelephonic electrocardiographic transmission devices, coronary artery bypass grafts, or heart transplants are used to treat and repair some of the underlying causes of HF, reduce dysrhythmia, improve organ perfusion, and reduce mortality.
Cardiac assist devices serve to improve organ perfusion.
Supportive devices treat symptoms and reduce the occurrence and length of hospitalizations. Human embryonic stem cell therapy can reduce cardiac tissue necrosis. Cardiac monitoring devices continually collect and analyze cardiac-related data and can alert the clinician to the presence of important physiologic events, often before symptoms occur.
Nutritional therapy helps to promote weight reduction and prevent edema.
Collaborative Management
Patients with heart failure receive treatment from a wide spectrum of healthcare professionals. Acute and chronic phases of the disease therapy, including cardiac rehabilitation, require specialists to address various aspects of care. The individual disciplines may include physicians, different levels of nurses (RN, advanced practice, vocational or practical nurse), physical therapists, occupational therapists, pharmacists, respiratory therapists, nutritionists or registered dietitians, mental health professionals, and social workers.
The use of a coordinated, multidisciplinary heart failure team results in improved outcomes for HF patients both when hospitalized and as outpatients. The introduction of such teams to provide expertise in the initiation and management of therapy decreases inpatient and one-year mortality rates, hospital length of stay, and the number of hospital readmissions for HF (Harding et al., 2022).
CASE
Miguel Sanchez is in the hospital for the fourth time for symptoms of heart failure. He is 79 years old, is moderately obese, has type 2 diabetes, and has a stage 3 pressure injury (ulcer) on his heel. He is unable to walk because of pain from the ulceration. He and his family are native Spanish speakers.
Mr. Sanchez’s physician has determined that the patient is stable and ready for discharge but also recognizes that there are some barriers to his being well cared for at home, where he lives with his daughter. The patient’s daughter has not yet had the opportunity to practice dressing changes on her father’s heel ulcer due to language barriers with the inpatient nursing staff. Mr. Sanchez’s heel ulcer has affected the stability of his gait as well as his standing balance for functional tasks, which poses a safety risk in the home setting. His daughter has also told the nurse that the food choices suggested by the inpatient dietitian did not make any mention of culturally specific foods that her father most enjoys.
Mr. Sanchez’s physician determines that he and his family need instruction in diet, wound care, and mobility, particularly ambulation and strength training. On the physician’s orders, Mr. Sanchez is seen by a certified wound care/ostomy nurse, the dietitian, a physical therapist, an occupational therapist, and a discharge planner before his discharge.
The wound care nurse makes a thorough examination of the heel ulceration. She orders a MediHoney paste, applies it to the wound, and dresses it. She is fluent in Spanish and explains to the patient’s daughter how to treat the wound when he goes home. She gives his daughter samples of the MediHoney and some dressing material and explains that she can obtain more in any pharmacy or grocery store with a pharmacy section.
The dietitian uses the services of a certified medical translator to instruct the daughter in preparing a calorie- and carbohydrate-limited diet for Mr. Sanchez. She asks for suggestions from the daughter about his favorite foods and incorporates some Mexican specialties into the diet plan, with instructions on substitutions for traditional high-carbohydrate foods like tortillas, beans, and rice.
The physical therapist evaluates Mr. Sanchez and recommends the use of a rolling walker with partial weight bearing for all ambulation while his foot wound heals. He provides gait training to instruct the patient in partial weight bearing in order to minimize pressure on his heel wound. The physical therapist also educates both Mr. Sanchez and his daughter regarding the importance of regular ambulation to improve circulation and prevent edema.
The occupational therapist evaluates Mr. Sanchez and observes his execution of activities of daily living (ADLs). She suggests that the patient may benefit from the use of a covered cup for liquid intake and large utensils to facilitate eating since his advanced age and diabetes have caused visual impairment. The occupational therapist also suggests the use of a weekly medication holder to help the daughter organize her father’s daily medications to prevent omissions or errors.
The discharge planner also uses the services of the medical interpreter to arrange for admission to a rehabilitation facility to help Mr. Sanchez with functional mobility and to prepare him to eventually be discharged home.
Pharmacologic Interventions
MEDICATIONS TO REDUCE CARDIAC WORKLOAD
In HF, many factors can increase the workload of the heart. Excess circulating volume forces the heart to work harder to pump an increase of fluid throughout the body. Stress may produce a rapid heart rate, causing the heart to work overtime. Hypertension reflects vascular resistance against which the heart must pump harder to produce cardiac output. Antihypertensives exert individual chemical properties to control physical response to sympathetic nervous system stimulation, vasoconstriction, or fluid overload secondary to sodium and fluid retention.
Aldosterone (mineralocorticoid receptor) antagonists are used in HF to promote diuresis as a means of lowering blood pressure to reduce myocardial tissue damage, cardiac myopathy, and fibrosis. These have shown to reduce mortality and hospital readmission in HF patients. Examples include spironolactone and eplerenone (McCuistion et al., 2023).
Angiotensin-converting enzyme (ACE) inhibitors reduce vascular resistance by interfering with the renin-angiotensin-aldosterone system (RAAS) to reduce the conversion of angiotensin I to angiotensin II. This decreases aldosterone excretion and sodium retention to reduce blood pressure and improve blood flow. Angiotensin II is a powerful short-term vasoconstrictor, and over the long run it affects the blood vessels’ tissue growth, which results in remodeling of the vessel walls and causes hypertension. Examples include enalapril (Vasotec), lisinopril (Zestril), and captopril (Capoten). Asian Americans have a high (up to 50%) risk for ACE inhibitor–induced cough as a side effect (Harding et al., 2022; McCuistion et al., 2023).
Angiotensin receptor blockers (ARBs) similarly affect the RAAS to reduce the pressure in the heart and may be prescribed for those who cannot take ACE inhibitors due to chronic cough or angioedema. These drugs include losartan (Cozaar) and valsartan (Diovan) (McCustion et al., 2023).
Angiotensin receptor–neprilysin inhibitors (ARNIs) are a newer classification of drugs for the treatment of HF. The FDA has approved sacubitril/valsartan (Entresto) as the first drug in this category to be used with NYHA classes II, III, or IV. This drug has two main effects: the first, from the valsartan, is a similar effect to ACE inhibitors and ARBs (see above); the second, from the sacubitril, strengthens a hormone regulated by the heart muscle itself, which releases BNP. BNP causes vasodilatation and increases sodium excretion by the kidneys and an increased diuresis. These result in a significant reduction in hospitalization and mortality. Known side effects are hypotension, dizziness, syncope, hypovolemia, hyponatremia, cough, and renal insufficiency (McCustion et al., 2023).
Vasodilators increase the internal diameter of the blood vessels to promote better blood flow and reduce blood pressure.
Nitrates cause vasodilation and increased venous capacity by relaxing the smooth muscle of blood vessel walls. When HF accompanies myocardial ischemia secondary to CAD, nitrates are particularly beneficial, causing coronary artery dilation and improving blood flow to the myocardium to relieve or prevent chest pain. Examples of nitrates are isosorbide dinitrate (Isordil) and sublingual nitroglycerin. The most common side effects of nitrates are vasodilation of peripheral arteries, resulting in reduced venous resistance and lowered blood pressure, and headache. African Americans are the only group for whom the combination drug isosorbide dinitrate/hydralazine (BiDil) is approved as treatment for HFrEF (Harding et al., 2022; McCustion et al., 2023).
Loop diuretics promote renal excretion of fluids and sodium chloride to decrease the circulating intravascular volume, which reduces blood pressure. This action takes place in the ascending loop of Henle in the renal tubules. Excretion of excess fluid reduces cardiac workload and oxygen consumption. Examples of loop diuretics are furosemide (Lasix), bumetanide (Bumex), and torsemide (Demadex).
Vasopressin-2 receptor antagonist tolvaptan (Jynarque, Samsca) in renal failure patients with HF, when added to conventional therapy, has shown improved dyspnea symptoms, lower required doses of loop diuretics, and increased urine output compared with those patients treated with loop diuretics alone. This has proven effective for renal dysfunction patients, since they are usually refractory to loop diuretics, requiring higher doses that are themselves the cause of increased renal dysfunction. Tolvaptan is a selective vasopressin-2 receptor antagonist that acts on the distal portion of the nephron and inhibits the kidney’s ability to reabsorb water (McCustion et al., 2023).
MEDICATIONS TO IMPROVE CARDIAC OUTPUT
Positive inotropic agents increase the force of cardiac contractions (inotropic effect) to improve cardiac output. Some reduce the heart rate (chronotropic effect), allowing for more complete ventricular filling to increase stroke volume. Catecholamines are positive inotropic agents given to HF patients with severe disease. They are powerful vasoconstrictors, acting to support failing blood pressure.
- Dopamine and norepinephrine are endogenous catecholamines used in severe or end-stage HF to increase contractility, but which also cause increased cardiac workload and dysrhythmias. They may be used to support a failing heart that awaits transplantation.
- Dobutamine is a synthetic catecholamine with similar actions but that does not increase systemic vascular resistance.
- Milrinone is a widely used inotrope that increases myocardial contractility by inhibiting phosphodiesterase and thereby allowing an influx of calcium into the myocardial cells. Milrinone increases cardiac output by reducing BP through vasodilation. It may not be used in patients with renal disease, HF caused by ischemia, or hypotension.
Heart failure patients who take digitalis preparations experience a reduction in symptoms, expanded exercise tolerance, and improved quality of life. They do not experience decreased mortality rates, however. Digitalis preparations present a significant risk for toxicity, particularly in the presence of electrolyte imbalance. Hyper- or hypokalemia, hypercalcemia, and hypomagnesemia may cause potentially fatal dysrhythmias in patients taking digitalis. The most common medication in this category is digoxin (Lanoxin) (McCustion et al., 2023).
GUIDELINE-DIRECTED MEDICAL THERAPY (GDMT)
In a recent study, GDMT proved to reduce the mortality for patients with HFrEF as well as patients with coronary artery disease in need of surgery. Giving the patients a combination of one or more antiplatelet drugs—a statin to reduce serum cholesterol and triglycerides, a beta blocker to reduce cardiac workload, and an ACE inhibitor or an ARB—reduced mortality rates compared to those with the same diagnoses on other drug regimens (Maddox et al., 2023).
OTHER MEDICATIONS
Three specific beta-adrenergic blocking agents (beta blockers) have been shown to reduce mortality in patients with HF with reduced ejection fraction (HFrEF): metoprolol succinate (Toprol XL), bisoprolol (Zebeta), and carvedilol (Coreg). These may also have a dose-related effect, increasing the EF and therefore increasing cardiac output. Beta blockers are started at a low dose, as they may reduce cardiac contractility (Harding et al., 2022).
The heart failure agent (hyperpolarization-activated cyclic nucleotide gated channel blocker) ivabradine (Corlanor) is a pharmaceutical treatment specifically for heart failure that is intended to prevent patient readmission to the hospital. It may be used in patients with a stable but symptomatic reduced ejection fraction of <35%, sinus rhythm, and an HR >70 bpm. It acts by reducing the heart rate, thus preventing disease progression. Side effects are phosphenes (visual disturbances) and dysrhythmias, the most common of which is bradycardia. Ivabradine is given to HF patients who are already taking the maximum dose or are unable to take beta-blocking agents (Harding et al., 2022).
B-type natriuretic peptide (BNP) treatments given subcutaneously show promise due to the capacity to improve left ventricular function and urine output. The prototype is the recombinant human BNP nesiritide (Natrecor). Initial studies of BNP treatment to prevent recurrence of heart failure symptoms and hospital readmissions showed that the subcutaneous route of administration did not cause hypotension as had previous studies with intravenous medications. Further studies are needed to prove the efficacy of subcutaneous BNP treatments (Pagana et al., 2022).
PHARMACOLOGIC AGENTS HELD OR USED WITH CAUTION IN HEART FAILURE
Antidysrhythmic agents are generally withheld in HF, except amiodarone given for ventricular dysrhythmias. Other antidysrhythmic agents may exacerbate the symptoms of heart failure.
NSAIDs, ACE inhibitors, and diuretics when given in combination may cause renal damage as evidenced by a reduced glomerular filtration rate (GFR). NSAIDs reduce the synthesis of vasodilatory prostaglandins and may inhibit the systemic antihypertensive effect of ACE inhibitors. As ACE inhibitors and diuretics are often used in the treatment of HF, it is recommended that other mid-level–range pain relievers be used instead, such as acetaminophen (Tylenol). Anti-inflammatory medications such as ibuprofen may cause toxicity when used in conjunction with calcium channel blockers, which are commonly used in HF. Anti-inflammatory medications and immunomodular agents such as infliximab often cause fluid retention, which can exacerbate edema in patients with HF.
Thiazolidinediones (glitazones) such as pioglitazone and rosiglitazone are insulin activators that may be used in conjunction with other antidiabetes medications for better control of blood glucose. These drugs increase sodium retention and may aggravate HF symptoms in patients with diabetes with comorbidities for both diseases. These medications may cause fluid retention, edema, and weight gain in class III and IV HF. This may necessitate readmission to the hospital for HF because of the exacerbation of symptoms (McCuistion et al., 2023).
Beta-adrenergic blocking agents in acute HF are used judiciously because of the possibility of reducing cardiac contractility and therefore the strength of the cardiac chambers pumping blood to the rest of the body. When beta blockers are used in HF, they are started in the lowest possible doses to achieve therapeutic effect, and the doses are then titrated up slowly if more drug is needed (Harding et al., 2022). Vital signs are monitored closely, and the patient is observed for signs of reduced cardiac contractility such as blood pressure variations, tachycardia, auscultation of an S3 heart sound, or pulsus alternans (a pulse that alternates strong and weak on palpation).
METFORMIN RESTRICTIONS DROPPED
Previous prohibitions have been dropped against administering metformin to HF patients because of potentially fatal lactic acidosis. Metformin use in chronic diseases such as kidney disease, liver disease, and heart failure are no longer believed to increase mortality. More recent research shows that the use of metformin in HFpEF reduces mortality and hospitalization rates. In a meta-analysis of nine cohort studies including 34,504 patients with both diabetes and HF, metformin was associated with a 20% reduction in mortality (Shen & Greenberg, 2021).
Surgical Interventions
Surgeries can be performed to repair conditions that contribute to severe HF, particularly when nonsurgical treatments alone are ineffective. When the underlying cause of heart failure is corrected, such as by a cardiac valve replacement, the condition itself may improve, depending on the severity of the disease. Hypertrophy of the myocardium, for example, particularly the left ventricle, can only be repaired by a cardiac transplant. The degree of damage to the myocardium, however, may make the patient a poor candidate for transplantation.
VALVE REPLACEMENT / VALVE REPAIR
Cardiac valve malfunction can be caused either by stenosis that does not allow the blood to flow through to the next chamber or by prolapse that prevents the valve from closing completely (atresia), causing regurgitation. Depending on the volume of blood allowed to remain in the cardiac chambers, this regurgitation may cause the affected chamber to have to pump harder to empty, resulting in HF. Of concern is left ventricular dysfunction, as cardiac output is then compromised, causing HF.
The most common valve to be repaired via a surgical procedure is the mitral valve, which prevents regurgitation from the left ventricle into the left atrium. The most common type of valve to be replaced via a surgical procedure is the aortic valve, which prevents regurgitation from the aorta into the left ventricle. The pulmonic and tricuspid valves rarely undergo replacement or repair.
Valve Repair
Valve repair surgery is a closed-heart procedure requiring the patient’s heart to be monitored continuously by a nurse or monitor technician specially trained to recognize dysrhythmias. This patient will be cared for by a cardiologist as well. The placement of clips to prevent the backflow of blood is the most common method of repair of the mitral valve. The average length of stay in the hospital following valve surgery is two to seven days, depending on the degree of invasiveness inherent in the procedure.
Types of surgical valve repair include:
- Patching holes or tears with tissue to provide more support at the base of the valve
- Removing or reshaping tissue so that the valve can close more effectively
- Separating valve flaps that are fused due to a congenital defect
Valve Replacement
The traditional method for valve replacement requires open-heart surgery, necessitating the patient to be transferred to the intensive care unit. Care is provided by critical care nurses as well as a respiratory therapist if the patient requires a ventilator. A thoracic surgeon performs the surgery, and the patient is also cared for in the postoperative phase by a cardiologist.
The surgeon may use a biologic valve from either pig, cow, or human heart tissue, which will last approximately 15 years and require anticoagulation. An artificial valve may also be used; it will last longer but requires that the patient take anticoagulants for life.
Transcatheter aortic valve replacement (TAVR) is an interventional approach. In patients who are not good candidates for traditional valve replacement surgery, a TAVR may be tried. The approach is via an inflatable balloon catheter via the femoral artery or transapically through the ribs. The balloon places the artificial valve within the existing valve. When the balloon is inflated, the artificial valve expands. The new valve then displaces the old, damaged valve and begins to function in its place (NHLBI, 2022b).
IMPLANTATION OF A CARDIOVERTER DEFIBRILLATOR
Ventricular fibrillation and ventricular tachycardia, which are life-threatening dysrhythmias, occur at alarming rates in patients with HF. This is particularly true of those with coronary artery disease as a comorbidity. An internal cardioverter defibrillator (ICD) can sense and correct this dysrhythmia. The device is a small computer implanted in the chest or abdomen and connected to wires inserted into one to three cardiac chambers. When the computer reads a sustained ventricular rhythm, it administers a shock to attempt to restore a more normal heart rhythm (NHLBI, 2023). Its battery is replaced approximately every 20 years.
A wearable cardioverter defibrillator (WCD) vest is another type of defibrillator. It comes equipped with a rechargeable battery and will sense a life-threatening dysrhythmia and discharge a shock to convert to a viable rhythm (NHLBI, 2023). A study of over 5,000 ICD recipients enrolled in five trials assessed the device’s effectiveness and compared mortality due to the shock from the defibrillator with mortality from the dysrhythmia that caused the shock to occur. The results from this study were conflicting (Aktas et al., 2021).
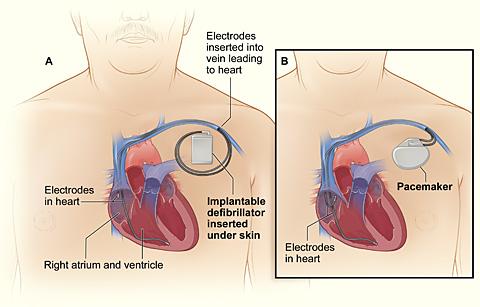
Comparison of an ICD and a pacemaker.
Figure A (left) shows the location and general size of an ICD in the upper chest; the wires with electrodes on the ends are inserted into the heart through a vein in the upper chest.
Figure B (right) shows the location and general size of a pacemaker in the upper chest; the wires with electrodes on the ends are inserted into the heart through a vein in the upper chest.
(Source: NHLBI.)
CORONARY ARTERY BYPASS GRAFT / PERCUTANEOUS CORONARY INTERVENTION
Coronary artery disease is one of the most common comorbidities of HF. The blocked coronary arteries of CAD inhibit the flow of blood—and therefore the oxygen bound to the hemoglobin molecule—to the myocardium, resulting in cardiac ischemia. If this disease progresses, an MI may occur. The infarcted area of the cardiac muscle then becomes akinetic, affecting the ability of the heart muscle to pump with its former strength. This causes the chambers of the heart to diminish in their ability to pump blood and empty with each contraction. If the area of the myocardium affected is the left ventricle, significant changes in the heart’s capacity for pumping blood to the rest of the body may be severely compromised, eventually resulting in HF.
CAD leads to angina pectoris and MI, the two components of acute coronary syndrome (ACS). As stated above, the occurrence of an MI can lead to heart failure by causing left ventricular dysfunction. Heart failure and left ventricular dysfunction are predictors of high mortality risk in patients with ACS. Earlier intervention with revascularization surgery—by grafting the patient’s own veins or arteries to bypass the obstructed coronary arteries—may prevent an MI and the subsequent HF. In a percutaneous coronary intervention (PCI) procedure, the coronary arteries are cleared by a percutaneous transluminal coronary angioplasty (PTCA) and held open by the placement of a stent. This is performed in less severe CAD or for patients who may be poor surgical candidates (Harding et al., 2022).
The patient is cared for in the ICU immediately postoperatively until the patient is extubated from the mechanical ventilator, temporary pacemaker wires have been removed, and the patient is hemodynamically stable. The patient will remain on a cardiac monitor throughout the entire hospital stay and will start on cardiac rehabilitation before being discharged.
HEART TRANSPLANTATION
Cardiac transplantation can be a lifesaving measure and is most commonly performed in the case of end-stage HF that is refractory to medical treatment. In the case of HF caused by akinetic heart muscle or exacerbated by myocardial hypertrophy, nothing else will significantly alleviate symptoms, prevent progression of the disease, or prolong life.
This is a highly specialized surgery performed at only a handful of medical centers. The personnel involved in all aspects of this procedure are rigorously trained and are usually all critical care experts. In the United States, the list of patients awaiting cardiac transplantation is approximately 3,500 in number.
Devices such as the A5000 Circulatory Support System and the BVS 5000 Biventricular Support System are FDA approved as bridge-to-transplant (BTT) systems to support a failing heart until a matching donor heart is available. These are used as lifesaving measures in cases where the patient would otherwise die but has a chance of recovery with a transplant. These patients are critically ill and are monitored in an ICU while awaiting transplantation. (See also “Cardiac Assist Devices” below.)
The one-year posttransplantation survival rate is 90%, and half of the organ recipients are still alive 13 years after the surgery. The most common postoperative complications are rejection (detected by endomyocardial biopsy), infection in conjunction with immunosuppression therapy, sudden cardiac death, malignancy, and cardiac vasculopathy (accelerated CAD) (Harding et al., 2022).
NURSE’S ROLE IN SURGERY
- Ensure diagnostic test results (e.g., laboratory tests, ECG, and X-rays) are on the chart that accompanies the patient to surgery or in the electronic medical record
- Obtain the patient’s signature for informed consent for surgery after the surgeon and anesthesiologist or nurse anesthetist have explained the procedures and possible negative outcomes to the patient (surgeon or anesthesiologist may also obtain the signed consent)
- Fill out a preoperative checklist, including a recent set of vital signs, how long the patient has been taking nothing by mouth (NPO), brief medical and surgical history, allergies, disposition of any belongings, surgical procedure to be performed, any medications taken that day, time of last voiding, and that the consent is signed
- Inform the family or any visitors where to wait until the surgery is completed (surgical waiting room) and let them know that someone will be in touch with them throughout the surgery
- Place an identification band and an allergy band, if necessary, on patient’s wrist
- Remove jewelry and eyeglasses, with eyeglasses to be returned to patient as soon as possible after surgery
- For a patient with heart failure who is on vital cardiac or diabetes medications that should not be held due to the NPO status, administer with one sip of water per physician’s order
- For the operating room circulating nurse, just before the surgical procedure, conduct a “time out” with the surgeon, anesthesiologist, surgical technician, and anyone else involved in the surgery to identify and verify the patient and the procedure to be done
(Harding et al., 2022)
CASE
Alison is a certified respiratory therapist with 27 years of experience. She has worked the past 14 years at City Medical Center as the lead RT on the night shift in the ICU. City Medical Center is an organ transplantation center, and Alison has seen dozens of heart, lung, and a few heart/lung transplants.
Tonight the ICU nurses are recovering a 68-year-old heart transplant patient with end-stage heart failure. He will be transferred out of the ICU when he becomes extubated, has his temporary pacemaker wires withdrawn, and is hemodynamically stable. The postoperative recovery is complicated by the fact that the patient has a comorbidity of moderately severe COPD and is having difficulty being weaned off the mechanical ventilator.
Working as a team, the ICU nurse, the pulmonologist on call, the anesthesiologist still in the hospital after surgery, and Alison work to get the patient extubated. The nurse gradually withdraws pain medication and sedation to allow the patient to become more alert and participate in his care. When he is sufficiently awake to breathe on his own, the patient becomes anxious, causing his heart rate and blood pressure to increase to abnormal levels. The anesthesiologist gives the order by phone for Alison and the ICU nurse to extubate the patient when he is responsive and the tidal volumes measured by Alison are normal. As the ICU nurse prepares to suction the patient, Alison deflates the pilot balloon on the endotracheal tube and slowly withdraws the tube from the patient’s throat. Together they complete a successful extubation.
Cardiac Assist Devices
Cardiac assist devices are used in heart failure patients in whom drug therapy is no longer adequate. They have the effect of improving organ perfusion and decreasing cardiac workload. They are considered temporary measures used to stabilize an HF patient who has sustained an injury or is awaiting heart transplantation.
INTRA-AORTIC BALLOON PUMP (IABP)
This device reduces systolic blood pressure to reduce afterload. This decreases the cardiac workload and enhances the aortic diastolic pressure to improve coronary arterial blood flow. For HF patients, it works primarily as a bridge-to-transplant measure while they are hospitalized and waiting for an appropriate organ to become available.
A balloon is percutaneously inserted through the femoral artery into the thoracic aorta. The pump provides counterpulsation (opposite to the ventricular contractions) that causes the balloon to deflate and inflate, causing a rise in diastolic arterial pressure that improves coronary blood flow to the myocardium.
Contraindications include irreversible brain damage, major coagulopathy such as disseminated intravascular coagulation (DIC), terminal illnesses, abdominal or thoracic aortic aneurysms, moderate to severe aortic insufficiency, and generalized peripheral vascular disease.
Complications include dislodged plaque, aortic dissection, thromboembolism, compromised peripheral circulation, thrombocytopenia, blocked arteries, infection, and improper timing of inflation (Harding et al., 2022).
LEFT VENTRICULAR ASSIST DEVICE (LVAD)
An LVAD provides long- or short-term support for a heart that is failing. It is more compact than an IABP and allows the patient to be more mobile. The device may be inserted internally, such as in the peritoneum, or externally in a blood vessel. LVADs move blood from the left side of the heart to the device and then to the aorta to temporarily support circulation. They may be used in patients with HF for failure to wean from a cardiovascular bypass pump after open-heart surgery, after an MI, or as a bridge-to-transplant measure.
When the patient with heart failure is discharged from the hospital with such a device in place, daily function and the need to protect the device will affect the patient’s ADLs. An occupational therapist will assist the patient to manage the physical manipulation skills required to function with an LVAD. These may include placing the device in a fanny pack for ambulation or a waterproof case for showering, changing the batteries, and using fine-motor control to manipulate the settings. By helping patients and their families learn adaptive skills to accommodate an LVAD, occupational therapists help HF patients maintain independence and return to work, school, and travel more safely (Abramson et al., n.d.).
Contraindications for an LVAD include small body surface area, renal or liver failure not following a cardiac event such as an MI, and comorbidities that limit life expectancy to less than three years. Complications are the same as for an IABP but are less likely to occur.
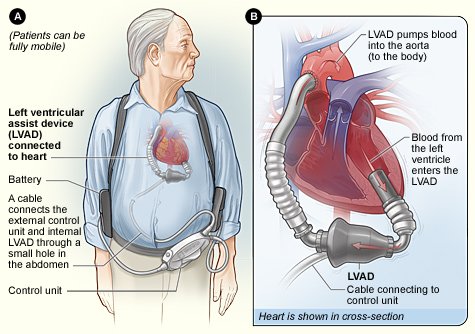
Left ventricular assist device.
(Source: Singhvi & Trachtenberg, 2019.)
CASE
Jesse is an occupational therapist in a regional medical center. Jesse’s patient, Julia McCort, a 74-year-old female, will be discharged with a newly implanted left ventricular assist device (LVAD). Ms. McCort is weak and lacks fine motor control in her fingers. Although the external controller on the device weighs only about five pounds, she complains that the fanny pack that houses the controller when she’s ambulatory “feels like a ton of bricks.”
Jesse works with Ms. McCort on improving her fine motor control by having her practice putting together jigsaw puzzles, tearing paper, sculpting with modeling clay, and threading beads onto a string so that she is better able to adjust the dials on the controller. Jesse also teaches her how to change the battery on the device. Jesse determines that the patient wasn’t wearing the pack correctly and helps her make the appropriate adjustments for it to fit more comfortably.
Supportive Devices for Symptom Management
There are a wide variety of devices and treatments that can be used to treat many of the coexisting symptoms that heart failure patients may experience. These symptoms may be due of the disease itself or related to comorbidities or independently occurring problems.
CPAP / BiPAP / APAP
Continuous positive airway pressure (CPAP), bilevel positive airway pressure (BiPAP), and automatic positive airway pressure (APAP) are commonly used to treat sleep apnea. Sleep apnea is a condition in which a person ceases respirations while asleep for a period of 10 seconds or longer, sometimes gasping for air when respirations resume. The most common cause is obesity. This can result in hypertension, hypoxia, and tachycardia during the period of breathlessness, resulting in a stroke (CVA) or MI occurring during sleep.
A CPAP, BiPAP, or APAP device is connected to a face mask or nasal pillow to deliver positive pressure to force the airway to remain open and allow the passage of air. The differences among these devices include that CPAP delivers a continuous pressure, BiPAP delivers different pressures for inhalation and exhalation, and APAP delivers pressure within a set range only when the person experiences a period of apnea. If the patient continues to be hypoxic during the cessation of apnea, oxygen can be spliced into the PAP tubing to increase the amount of oxygen available to the patient (Harding et al., 2022).
CARDIAC RESYNCHRONIZATION THERAPY (CRT)
CRT is a well-established method for improving cardiac function and coordinating biventricular contractions. CRT improves left ventricular function and therefore hemodynamic efficiency or cardiac output. A small titanium device containing a computer is surgically implanted into the chest wall and connects to wires extending to each ventricle. It acts similarly to a cardiac pacemaker but causes the ventricles to contract in time with each other.
Biventricular pacing improves ventricular function by resynchronizing the heartbeat through pacing both ventricles. This is used for treating HF patients with intraventricular conduction delays, which cause the right and left ventricles to beat out of synch with each other, leading to decreased systolic function, an inefficient pump, and aggravated HF. In patients for whom these therapies are successful, the outcomes are relief of HF symptoms, improved exercise function, and increased life expectancy (Harding et al., 2022).
Human Stem Cell Therapy
Embryonic stem cells that have been altered to become cardiopoietic (cardiac-committed) cells show promise of reversing necrosis in cardiac tissue. These new cardiac cells can then become healthy heart muscle cells. This improves cardiac function and reduces the severity of heart failure by restoring the ability to pump to some previously akinetic (unmoving) areas of the myocardium (Cona, 2023).
Mesenchymal precursor cells (MPCs) from human bone marrow appear to target many of the pathologic factors that contribute to clinical events in patients with HFrEF. In one study, MPCs improved left ventricular ejection fraction by regenerating damaged heart tissue and improved heart function by reducing inflammation (Cona, 2023; Perin et al., 2023).
Stem cells may also be derived from an individual’s own bone marrow or their own healthy cardiac tissue, bringing about an improvement in their cardiac tissue. However, after approximately one week of cardiac muscle damage, the heart stops signaling for help in the form of stem cells, and the damage repair remains largely incomplete. More recent studies have shown that the introduction of highly selected donor stem cells, the individual’s own healthy cardiac tissue given at the time of a myocardial infarction (MI), or the individual’s own healthy cardiac tissue or donor cardiac tissue given late after an MI to be more effective for tissue repair (Cleveland Clinic, 2023).
Cardiac Monitoring
Cardiac monitoring devices, particularly ambulatory devices such as wearable devices, smartphones, and other sensors, have become popular for continual monitoring of cardiac diseases such as HF. Such devices provide early detection of physiologic events and can alert patients and clinicians of the need to seek medical attention. With its significant potential to reduce healthcare costs and improve diagnostics and preventive care, use of remote and ambulatory monitoring devices is expected to increase (Duncker et al., 2021).
Wearable monitoring devices and smartphone-based solutions can provide continuous cardiac monitoring and ambulatory monitoring capabilities for:
- ECG
- Heart rate
- Arrhythmia
- Blood pressure
- Cardiorespiratory fitness
- Stress
- Respiratory rate
- Temperature
- Oxygen saturation
- Ischemia
- Lung fluid
- Apnea
- Step count
- Posture
- Body-weight changes
Implanted devices include the FDA-approved CardioMEMS HF system, which is implanted in the pulmonary artery during a right heart catheterization to measure pulmonary pressures, which can be a predictor of worsening HF as they are increasing. The CardioMEMS HF system is used on HF patients with NYHA Class III heart failure symptoms. HF treatment such as drug dosages are then adjusted based on the pulmonary artery pressure reading. The patient-initiated readings are wirelessly transmitted to a secure website and monitored by the patient’s physicians (Brugts et al., 2021).
Nutritional Therapy
Nutritional therapy is essential in treating an individual with HF. A nutritional consultation with a registered dietitian is usually performed during the initial hospital visit for HF during phase 1 of cardiac rehabilitation (see also “Cardiac Rehabilitation” below). This consultation is usually ordered to provide the patient and close family members with information to control the exacerbation or intensification of HF symptoms. Failure to comply with a low-sodium, fluid-restricted diet is one of the most likely reasons for a patient with HF to be readmitted to the hospital.
WEIGHT REDUCTION
Weight reduction is a key component in treating HF and preventing a worsening of symptoms. Obesity contributes to hypertension, which increases systemic vascular resistance and causes the ventricles to work harder to pump blood. Many HF patients, with or without hypertension, are advised to start a DASH (Dietary Approaches to Stop Hypertension) diet. This diet supports weight loss through consumption of fruits and vegetables, low- or nonfat dairy, lean meats, beans, seeds, nuts, and less sugar (Harding et al., 2022).
FLUID RESTRICTION
Fluid restriction is a treatment modality used to prevent volume overload, which can be caused by the accumulation of fluid in the peripheral tissues (resulting in edema in right-sided HF) or in the lungs (resulting in pulmonary edema in left-sided HF).
Fluid restriction is advised for patients with moderate or severe HF, especially those with renal disease as a comorbidity. Liquids from all sources are usually limited to less than 2,000 mL per day or per healthcare provider orders. HF patients are encouraged to weigh themselves daily at the same time of day and in similar clothing to monitor sudden weight gains indicative of fluid retention.
When the heart is unable to pump fluid effectively, a lower circulating volume reduces the cardiac workload and decreases the possibility of fluid retention and edema. In the hospital, it is the nurse’s responsibility to maintain a strict intake and output (I & O) measurement when a patient has a fluid restriction ordered. The nurse records and reports the I & O fluid balance each shift and every 24 hours to ensure the patient is not retaining fluid. Nursing observations of jugular vein distension, peripheral edema, and lung sounds are also essential (Harding et al., 2022).
SODIUM RESTRICTION
Patients with heart failure are usually counseled to restrict sodium intake (1–2 grams/day) to prevent fluid retention. The affinity that sodium and water have for each other suggests that limiting the intake of sodium will prevent the kidneys from retaining fluid due to the increased serum sodium levels. The retained fluid can then lead to peripheral and dependent edema in earlier stages in HF and hypertension or shortness of breath in later stages.
Studies about the reduction of HF that relate directly to restricting sodium intake are inconclusive. Sodium restriction is usually recommended as an adjunct for HF patients for the possible benefits but is not considered to be effective without the concurrent use of pharmaceutical agents.
This is an area of some controversy, with conflicting study results indicating that the practice of sodium restriction cannot be considered evidence based. A search of the literature does not provide clear proof of a threshold level of acceptable sodium intake for a HF patient. Historical recommendations have been for a moderate restriction of 2,000 mg of sodium per day for a person with HF with mild to moderate fluid retention and 1,000 mg per day for those with severe fluid retention. Recent studies, however, do not support these recommendations (Harding et al., 2022).
AHA HEART FAILURE GUIDELINES
Since 2016, the American Heart Association has provided hospitals with evidence-based guidelines to promote adherence to proven protocols for HF patients designed to shorten hospital stays, reduce hospital readmissions, and maximize reimbursement for hospitals from Medicare, Medicaid, and private insurers. The guidelines upon discharge include:
- Prescription of angiotensin-converting enzyme (ACE) inhibitor, angiotensin reception blocker (ARB), or angiotensin receptor–neprilysin inhibitor (ARNi), if appropriate for that patient
- Prescription of a beta-adrenergic blocking agent (beta blocker), if appropriate
- Prescription of a combination of hydralazine and isosorbide dinitrate for African American patients, if appropriate
- Prescription of an aldosterone agonist, if appropriate
- Assessment of left ventricular function
- Establishment of a postdischarge appointment within seven days of discharge
- Deep vein thrombosis (DVT) prevention measures for nonambulatory patients
- 60 minutes of HF education by a qualified heart failure educator
- Maintenance of BP <140 systolic and <90 diastolic
- A written copy of dietary instructions
- A written copy of discharge instructions
- A follow-up phone call within 48 to 72 hours of discharge
(AHA, 2022)
CARDIAC REHABILITATION
Cardiac rehabilitation is an organized, multidisciplinary therapy proven to advance better outcomes for heart failure patients in the areas of exercise capacity and quality of life. In 2014, the Centers for Medicare and Medicaid Services expanded coverage for cardiac rehabilitation to include patients with stable, chronic HF. This is defined as patients with left ventricular ejection fraction of 40% or less and NYHA functional class II to IV symptoms that occur despite being on optimal medical therapy for six weeks or more (AHA, 2024).
Phases of Cardiac Rehabilitation
There are four distinct phases of cardiac rehabilitation:
- Acute
- Subacute
- Intensive outpatient therapy
- Independent ongoing conditioning
Phase 1 starts while the patient is in the hospital after an initial occurrence of HF or an exacerbation. This phase consists primarily of education of the patient and family and the introduction of an exercise program. During phase 1 the initial goals are:
- Assess exercise tolerance and effects of mobility on exacerbation of symptoms
- Create an individualized plan of care with input from all members of the care team (physicians, nurses, occupational therapists, physical therapists, pharmacist, discharge planners, etc.)
- Begin preliminary safe exercises to improve mobility and cardiac health
- Explain and address any risk factors
- Introduce and train in use of appropriate assistive devices like a cane or a walker and supervise correct use, if needed
Phase 2 starts after discharge and takes place in an outpatient setting such as a physician’s office, clinic, or independent physical therapy practice. This phase lasts three to six weeks. Exercise is performed under observation and often during cardiac monitoring to determine the effect of gradually increasing activity on heart rate, exertion level, and heart regularity during exercise. Aerobic exercises are introduced under controlled conditions to establish tolerance. The patient is taught how to check their own pulse for rate and regularity. (An irregular rhythm is indicative of dysrhythmia that may be potentially life-threatening.) The exercise program started in the hospital in phase 1 is adjusted to patient tolerance and endurance. The primary goal in this phase is to safely return the patient to functional mobility while being closely monitored for physiologic response (Sears, 2022).
Phase 3 includes more independent and group exercises with more self-monitoring. Increased exercises are practiced under the guidance of a physical therapist to monitor for adverse symptomatic response. These exercises may address flexibility, strengthening, and aerobic capacity. The patient is introduced to the concept of rating of perceived exertion (RPE). Exertion parameters include heart rate, respiratory rate, perspiration, and muscle fatigue. The patient may be asked to rate how hard the exercise feels on a subjective scale such as the Borg RPE Scale (see below) (Mayo Clinic, 2024; Sears, 2022).
Phase 4 includes independent and ongoing physical conditioning to preserve optimal health. This is a self-regulated, independent maintenance phase with ongoing monitoring by healthcare personnel to help promote fitness and wellness (Sears, 2022).
CASE
Roberto Pereira is a 61-year-old uninsured landscaper with moderate class II HF, based on his symptoms during periods of exacerbations and slowly, progressively elevating BNP blood levels. He does not have a primary care provider and visits the emergency department (ED) when his symptoms of dyspnea and peripheral fluid retention interfere with his ability to work.
During each ED visit, the nurses encourage Mr. Pereira to instead visit a clinic regularly to take advantage of the continuity of seeing one practitioner. They explain that this would allow him the benefit of having one person to oversee the success of his interventions and assess his lab results. Mr. Pereira states he is afraid of compromising his documentation status and that he is also unable to obtain an appointment when he needs one due to the typical two-month wait caused by the large number of low-income patients using the clinic.
On today’s ED visit, the nurse, who is aware of Mr. Pereira’s lack of insurance, a regular physician, and follow-up care, contacts the hospital’s medical social worker (MSW). The MSW explains to Mr. Pereira and his family what resources are available to them. She offers to make a follow-up appointment at the medical clinic so that Mr. Pereira can get established with an advanced practice nurse who specializes in critical care diseases. The MSW also offers to help him start the paperwork to apply for Medicaid to help pay for today’s ED visit, his medications, and much of his other healthcare expenses. The MSW will also explore whether the city’s public transit system can provide Mr. Pereira with transportation for healthcare appointments.
| Rating of perceived exertion (RPE) | Description | Breathing response |
|---|---|---|
| (Physiopedia, 2024) | ||
| 0 | No exertion | No change, normal |
| 0.5 | Noticeable | |
| 1–2 | Light | Slight increase |
| 3 | Moderate | Lightly puffing, able to hold conversation |
| 4 | Somewhat hard | Noticeable puffing, can only hold a broken conversation |
| 5–6 | Hard | Labored |
| 7–8 | Very hard | Short of breath |
| 9 | Extremely hard | Out of breath |
| 10 | Maximum effort | Gasping |
ADDRESSING PATIENT NEEDS IN ALL AREAS
Cardiac rehabilitation provides information, exercises, and adaptation techniques in activities of daily living to encourage patients with heart failure to perform in an optimal state of function. The various disciplines involved in an individualized cardiac rehabilitation program focus on the following six areas:
- Physiologic (physicians, nurses, physical therapists, exercise physiologists, occupational therapists, respiratory therapists)
- Psychological (mental health workers, social workers, nurses)
- Mental (mental health workers, social workers, nurses, physicians)
- Spiritual (clergy, nurses)
- Economic (social workers, nurses)
- Vocational (occupational therapists)
Exercise-based cardiac rehabilitation is a Class I recommendation from the AHA for any patient with HF. While the patient’s physiologic needs may be the primary focus of most of the healthcare professionals and take the greatest amount of time, the patient’s other needs must also be included in an all-inclusive cardiac rehabilitation program (Harding et al., 2022).
Respiratory Therapy
Pulmonary conditions can exacerbate the difficulty of improving cardiac function via cardiac rehabilitation. Shortness of breath can make cardiac rehabilitation exercises more difficult, if not impossible (Aspen Valley Hospital, 2024).
Therefore, heart failure patients with respiratory comorbidities in cardiac rehabilitation require the same respiratory services as chronic respiratory patients. Use of maintenance supplemental oxygen, chest auscultation, lung capacity measurement, medication administration by inhalation and nebulization, and education are all the responsibility of the respiratory therapist in cardiac rehabilitation.
There is a high prevalence of sleep-disordered breathing (SDB) in patients with heart failure. SDB is known to have a deleterious effect on morbidity and increase mortality in this patient population. Obstructive sleep apnea (OSA) is one form of SDB that requires positive airway pressure to prevent negative outcomes. OSA involves the narrowing of the air passages with relaxation of muscle tone during sleep and/or the tongue and soft palate falling backward and partially or completely obstructing the pharynx.
The treatment of choice for this disorder is the use of a positive airway pressure device while sleeping. The efficacy of treatment with CPAP and BiPAP is monitored by respiratory therapists and pulmonary physicians. Risk factors include obesity with a body mass index (BMI) greater than 30, age over 65 years, neck circumference of 16 inches or greater, male sex, and postmenopausal female sex (Harding et al., 2022).
Physical Therapy
There are numerous documented benefits derived from physical therapy in a cardiac rehabilitation program. Regular exercise, particularly when performed after a prolonged period of sedentary behavior, may produce improvement in oxygen capacity, cardiac output, blood lipid levels, blood pressure, coronary artery blood flow, muscle mass, flexibility, psychological state, and weight loss and control (Sears, 2022).
Physical therapy goals in cardiac rehabilitation are determined by the patient in conjunction with the physical therapist. Including the patient and caregivers in setting these goals is likely to promote better compliance with the agreed-upon exercise regime.
BENEFITS
Direct potential benefits of physical therapy for heart failure include:
- Decrease in the number of hospital admissions
- Decreased mortality in patients with HFpEF
- Improved cardiac capacity and reserve flow, both at rest and with exercise
- Reduced blood pressure in both the short term (i.e., during an exercise session) and long term (i.e., resting blood pressure)
- Improved coronary circulation and myocardial oxygenation
- Improved maximum oxygen uptake (VO2 max)
- Reduction in occurrence and length of depression
- Improved quality of life
(Physiopedia, 2021)
FUNCTIONAL TESTS
Standardized functional mobility tests may be included as part of a comprehensive physical therapy evaluation. Examples of commonly used tests include:
- Timed up and go (TUG): Measures walking ability, dynamic balance, functional mobility, and fall risk
- Six-minute walk distance (6MWD): Measures ADL performance, response to cardiopulmonary exercise, and walking ability
- Berg Balance Scale (BBS): Measures static and dynamic balance, fall risk, and potential needs for assistive devices
- Functional Independence Measure (FIM): Assesses physical, psychological, and social function for patients with ADL-related motor impairments (e.g., feeding, grooming, bathing, dressing, elimination)
- Talk Test (TT): Determines level of intensity of an exercise for the patient by assessing whether they are able to talk easily, sing, or not talk at all after exertion
(Taylor et al., 2021; Sears, 2023)
TREATMENT PLANNING AND INTERVENTIONS
Physical therapists, in collaboration with the other members of the rehabilitation team, establish an individualized plan of treatments and interventions to help cardiac patients optimize their functional mobility and activity tolerance. Therapeutic exercise programs designed to optimize physical functioning and psychological well-being within a patient’s individual activity tolerance are a key part of physical rehabilitation.
The purpose and goals of individualized therapeutic exercise may include optimizing function in multiple areas of patient function, including (but not limited to) aerobic capacity, circulation, endurance, balance and stability, range of motion, functional strength and mobility, ADL performance, injury prevention and/or reduction, and eventual return to highest possible level of desired activities.
In the cardiac rehabilitation setting, physical therapists establish a specific plan of exercise for each patient, including the types and frequency of exercises to be performed. With the goal of helping patients to optimize their cardiopulmonary function, these exercises may include some combination of walking, rowing, cycling, jogging, or other appropriate endurance activities, as tolerated. If indicated, physical therapists may also assist patients with airway clearance techniques, such as specific breathing strategies, body positioning, and manual or mechanical techniques.
Strength and endurance training is also a key component of cardiac rehabilitation, including instructing patients in how to safely and appropriately warm up, stretch, and gradually build their exercise tolerance to a consistent and beneficial level (Mayo Clinic, 2024).
If the patient is able to tolerate them, well-established vigorous exercises will challenge HF patients physically and help them to preserve their ability to function in a beneficial way. Some of these include moderately vigorous–intensity aerobic exercise, high-intensity interval training (HIIT), and sprint interval training (SIT). These exercises increase the HR by alternating bouts of high-intensity exercise with recovery bouts of lower-intensity exercise or no exercise, and intense supramaximal efforts (Taylor et al., 2021).
Functional mobility deficits occur frequently in chronically ill patients, including those with HF. Physical therapists may recommend appropriate assistive devices as needed for patients with mobility issues (such as canes, walkers, crutches, wheelchairs, etc.) and provide training in their safe and correct use. An assistive device may ultimately, though not always, become necessary for some HF patients to maintain at least some degree of mobility independence.
Occupational Therapy
Patients with moderate to severe heart failure require some assistance with ADLs. Poor cognitive function and poor physical fitness may impair patients’ abilities to perform complex tasks, causing them to make mistakes. Disabilities with ADLs are a strong predictor of readmission to a hospital, often within the 30-day period that precludes federal or state agencies paying for the additional admissions.
According to the American Occupational Therapy Association, the following interventions by an occupational therapist serve to reduce the number of hospital readmissions:
- Providing recommendations and training for caregivers
- Determining whether patients can live safely independently or require further rehabilitation or nursing care
- Addressing existing disabilities and fall prevention with assistive devices so patients can safely perform ADLs (e.g., toileting, bathing, dressing, preparing a meal)
- Performing home safety assessments before discharge to suggest modifications to the patient’s home
- Assessing cognition and the ability to physically manipulate objects like medication containers and providing training where necessary
- Assessing the ability to perform social participation and determining strategies to prevent barriers and reduce isolation and depression
- Provide necessary skills knowledge to participate in specific occupations
- Using technology such as videoconferencing and simulation to enhance performance skills and occupational practices
(AOTA, 2024)
Transitional care programs (from facility to home) that include occupational therapy have been documented as reducing readmissions up to 45%. Occupational therapists assist patients with heart failure to address both ADLs and instrumental activities of daily living (IADLs), including dressing, feeding, hygiene, cooking, cleaning, shopping, elimination, and more. Instruction in IADLs requires integration of physical and cognitive factors and skills. OTs may also instruct patients in cardiac rehabilitation in the use of adaptive devices to assist them with the ability to conduct ADLs without concurrent activity intolerance (SCU, 2023).
If the patient will be dependent on mobility-assistive devices in the long term, environmental accommodations may be recommended for the patient’s dwelling. These modifications may require significant structural changes to the patient’s living space and could take a considerable amount of time and expense.
Other recommended assistive devices and adaptive equipment may include environmental modification such as grab bars in the bathtub, in the shower, and by the toilet; shower chairs; and elevated toilet seats. They can also include walkers, canes, and crutches to support issues with ambulation. Patients with difficulty feeding themselves can be instructed in the use of adapted utensils with weighted or built-up handles to promote more independence. Clothing with Velcro closures or elastic waistbands and adaptive devices to aid in reaching shoes and socks can make dressing easier. The occupational therapist can recommend such equipment/devices and educate the patient and family about their use.
Assistive technology includes specialized software for speech recognition or voice-activated typing to simplify tasks. Occupational therapists may train patients to use adapted keyboards, communication boards, or digital communication devices. Therapeutic exercises are taught to help improve strength, flexibility, coordination, or balance (SCU, 2023).
Noncompliance with medications is a common reason that patients with HF may exhibit worsening of their symptoms and be readmitted to the hospital. While nurses, physicians, and pharmacologists educate the patient and their family about the medications, occupational therapists help ensure that patients take medications correctly and improve compliance. They explain the use of medication diaries, calendars, and pill sorter boxes. They also work with patients to improve fine-motor control to manipulate devices such as pill sorters (SCU, 2023).
Severe heart failure (NYHA levels III and IV) may cause cognitive impairment, resulting in a reduced quality of life due to an inability to perform complex cognitive functions and a lack of desire to engage socially in order to cover up the impairment. The occupational therapist works with these patients after a comprehensive activity analysis to encourage them to take on as many cognitive and psychomotor activities as they are capable of doing and to educate the patient and their families about the benefits of remaining socially active to maximize participation in meaningful activities and roles. If the degree of the patient’s cognitive impairment necessitates a caregiver, the occupational therapist conducts a training program for the caregiver in how to accommodate the patient’s impairment (SCU, 2023).
CASE
Bonnie Stevens is a 68-year-old patient in the telemetry unit at Westside, a small community hospital. She is newly diagnosed with left-sided HF with an acute episode and exhibits dyspnea on exertion; orthopnea; a cough productive of pink, frothy sputum; and severe exercise intolerance. She has a history of degenerative joint disease of the right hip and refuses to consider surgery. She also has a history of moderate hypertension, for which she takes lisinopril.
Ms. Stevens dislikes doctors and hospitals and has resisted seeking medical help for her increasingly worsening symptoms to the point of deteriorating health and function. She also prefers to stay at home alone, saying her hip pain makes it too difficult to enjoy the activities she used to do with friends, such as bridge games, book club, and women’s club dinners. Her son and daughter-in-law eventually convinced her to see their family physician, who admitted her to the hospital to improve her breathing, address her mobility issues, and start her on a treatment program.
Ms. Stevens is admitted to the emergency department, where her vital signs are measured at BP 178/98, HR 120, respiratory rate 32, temperature 99.8 °F, and O2 saturation of 89% on room air and 92% on 2 L of oxygen. She complains of a 4-out-of-10 left-sided chest pain with each inspiration. She’s sitting upright on the hospital gurney, bent forward over the over-the-bed table in obvious respiratory distress. She is placed on a cardiac monitor, has an IV started, is placed on an oxygen cannula on 2 L/min. Labs are drawn, a 12-lead ECG performed, and a portable anteroposterior and lateral chest X-ray done. Upon review of the results of the lab and diagnostic tests, the cardiologist on call to the ED admits Ms. Stevens to the hospital.
The significant results are:
- Arterial blood gases on O2 @ 2 L/min:
- pH 7.29 (7.35–7.45 normal)
- pCO2 32 (35–45 mmHg)
- pO2 70 (75–100 mmHg)
- HCO3 20 (22–28 mEq/L)
- Base excess −3 (−2 to +2)
- BNP 1,542 (100–400 pg/mL)
- O2 Sat 90% (94%–100%)
- ECG showing sinus tachycardia with rare PVCs
- Chest X-ray showing left-sided pulmonary edema in the base
The cardiologist writes orders for Ms. Stevens for diuretics and additional antihypertensives and to begin cardiac rehabilitation with consultation by a physical therapist, occupational therapist, a dietitian, and a medical social worker. As part of phase 1 of cardiac rehab, the physician orders occupational therapy and physical therapy consultations for evaluations and recommendations.
Included in the physical therapy evaluation is the TUG exam to help determine Ms. Stevens’s functional mobility and risk of falling. After these tests, Jack, the physical therapist, provides gait training in order to teach the patient the safe and appropriate use of a front-wheeled walker in order to allow her to offload weight from her painful hip while ambulating. Ms. Stevens reports that using the walker significantly reduces her pain while walking, whereupon Jack recommends she continue to use it after returning home. Since her front porch has four stairs, Jack also provides stair safety training using the practice stairs in the physical therapy department. He instructs Ms. Stevens in how to use a “step-to” pattern, leading with the nonpainful leg when ascending and with the painful leg when descending, as well as how to safely manage the walker on the stairs by carrying it folded in one hand while holding the handrail with the other.
Monica, the occupational therapist, discusses with the patient the option of using the powered scooter that is available at most grocery stores to do her shopping. She educates Ms. Stevens on using a pill sorter and the calendar application on her smartphone to remind her when to take the new medications her doctor has prescribed to treat her HF.
Tammy, the registered dietitian, discusses a 2-gram daily sodium diet and a 1,500-mL daily fluid restriction with Ms. Stevens and her family. She teaches them how to read the labels on all grocery products and to set up a beginning diet that will include healthy varieties of as many of the patient’s favorite foods as possible to promote compliance with the diet.
Tran, the social worker, discusses resources in the community such as cardiac rehabilitation exercise classes at the local YWCA and explains the importance of continuing social activities to prevent depression, encourage an active lifestyle, and promote better quality of life.
Follow-up After Discharge/Prevention of Readmission
Checkup calls after discharge are usually performed by the nurse. Whether the HF patient is seen in the emergency department, an acute care hospital setting, or a physician’s office, follow-up calls to inquire about vital signs (BP and heart rate), edema, shortness of breath, increased fatigue, or a sudden weight gain may alert the primary care practitioner to the need for adjustments in treatment. Timely changes in dosages of medications or other treatment may forestall the patient having to be seen in the emergency department or readmitted to the hospital.
NURSE NAVIGATORS
A nurse navigator is a clinician (usually an RN, a nurse practitioner, or a clinical nurse specialist) who integrates long-term healthcare for complex and chronically ill patients. While more common among cancer patients, nurse navigators who specialize in cardiology may serve in this capacity for chronic cardiac diseases such as HF. The purpose is to improve health outcomes and reduce the recurrence of hospital admissions by eliminating short-term, episodic, or fragmented care.
The nurse navigator reviews orders and the clinical pathway to promote effective communication with patients and their families, patient advocacy, holistic patient assessments, improved health literacy, self-management, reduced hospital lengths of stay, and reduction in the number of hospital readmissions.
The ability to improve patient outcomes is enhanced by the nurse navigator’s “soft” skills, including communication, cultural openness, respect for others, compassion, and commitment to patient confidentiality (Kerrigan, 2022).
HOME HEALTH
The use of home health nurses in conjunction with other disciplines, including follow-up physician visits within one week after discharge, has been shown to reduce hospital readmissions for HF patients. Avoidable hospital readmissions may be reduced when home health nurses continue education on acute HF symptoms and risk factors for exacerbations of HF acuity, reinforce medication regimes, and support patient self-management.
This model of nursing care includes detection of disease exacerbation, judging deterioration in patients, ascertaining conditions needing immediate intervention, observation of the patient’s appearance, listening to patients’ concerns and questions, continued management of the patient post discharge, instructing patients and families, and allowing families to participate in the patient’s care (Hashemlu et al., 2023).
HOME TELEHEALTH
Home telehealth can be included in an existing care pathway as an alternate service delivery model for a HF patient following a hospital admission for HF exacerbation. An electronic device monitors daily weight, BP, pulse, and glucose and transmits this data to the patient’s primary care provider’s smartphone. Needed interventions, such as medication dosage adjustment and dietary changes, can then be initiated quickly to prevent further deterioration of physical stability.
Benefits of telehealth include:
- Reduced hospitalizations
- Shorter hospital stays if the patient can be discharged with a remote monitoring device to use at home
- Fewer visits to the emergency room
- Better health outcomes for patients in rural areas
- Better preventive management for chronic conditions
- Reduced risk of illnesses for patients and healthcare workers
(HRSA, 2023)
LABORATORY VALUES
Certain laboratory values must be monitored periodically in HF patients. Early detection and treatment of abnormal laboratory values will promote the patient’s well-being. B-type natriuretic peptide (BNP) is tested initially to determine the presence of HF and then repeated to determine if the HF is worsening. A drop in red blood cells, particularly hemoglobin, could compromise systemic oxygenation. BUN and creatinine values are used to assess renal function. Other common blood tests include electrolytes and metabolic lab work (including magnesium, calcium, and potassium), glucose, albumin, ferritin, and liver tests. Urine sodium levels could be an indication that ACE inhibitors or diuretics are working (Merck & Co, 2024).
BODY WEIGHT
A precipitous increase or decrease in body weight is indicative of fluid shifts. Any gain or loss of more than one to two pounds in a single day suggests retention or release of a large amount of fluid rather than an actual gain or loss of body weight.
SELF-CARE EDUCATION
Readmissions for heart failure are caused by multiple factors, including failed self-care. HF self-care education is therefore standard prior to hospital discharge and is included in a multidisciplinary cardiac rehabilitation program. HF patients who receive a focused education program before discharge are significantly more likely to be compliant with recommendations, have an improved quality of life, and promote their own health.
The quality of patient education in HF directly influences mortality and reduces risk-related behavior and the need for hospital readmissions. Teaching the patient to focus on symptom monitoring, medication management, a low-sodium diet, activity tolerance, and psychosocial strategies promotes self-care that will reduce the frequency of hospital readmissions in HF patients (Jaarsma et al., 2020).
Several evidence-based educational interventions have proven effective in teaching HF patients how to progress in self-care. These interventions include face-to-face teach-back training, home visitation by follow-up phone call, group training, and e-learning.
A cohesive, multidisciplinary plan for education involves all the patient’s caregivers: physicians, nurses (including advanced practice nurses), medical social workers, respiratory therapists, physical therapists, occupational therapists, and discharge planners.
Medication Management Education
Medications are one of the most important treatment parameters in HF. Nonadherence to medication regimes is one of the two most commons reasons that patients with HF are rehospitalized. (The other is noncompliance with dietary restrictions.) Therefore, it is incumbent upon healthcare professionals to ensure patient and family understanding of every aspect of pharmacologic treatment modalities before discharge to home or a long-term care facility. Communication with potential caregivers should be included as well.
Good teaching standards require instructions to be given in a language that is well understood by the person who will be responsible for dispensing the medications, whether that person is the patient, a family member, or a caregiver. Evidence also shows that patients are more likely to be compliant with medication schedules when they understand what they are for and are told how important they are. It is also suggestive of better effectiveness if the patient is told what outcome to expect.
The correct dosage of HF medications is essential to their effectiveness. Unless the importance of giving or taking the correct medication is understood, the patient may not benefit from the intended therapeutic effects. Due to the high cost of pharmaceuticals, the patient or family may occasionally try to reduce the medication dose in order to make the prescription last longer or to share the medications with another. Emphasis must be made about the possible outcomes if the correct dose is not taken as ordered.
Certain medications, such as antihypertensives, cannot be stopped suddenly without producing a rebound effect. This information must be explained to the patient and family.
Teaching Exercise (Activity) Tolerance
Exercise is a crucial component of daily activity for someone with HF. Exercise tolerance is the ability to obtain maximum workload and duration while moving. With the guidance of a physical therapist or exercise physiologist, a person with HF can stay as active as possible for as long as possible and potentially reap the resultant health benefits. The clinician teaches the patient and family specific exercises, within appropriate parameters, to optimize strength and endurance without causing an oxygen deficit secondary to exercise intolerance (Byars et al., 2023). (See also “Cardiac Rehabilitation” above.)
Oxygen consumption is the amount of oxygen used during activity. A certain amount of oxygen is consumed even while at rest. People with HF must pace themselves to ensure that using oxygen stores in the body too quickly does not cause shortness of breath or dyspnea. Medications to regulate heart rate and BP may be given to minimize oxygen consumption. Physical therapists and respiratory therapists work with other care team members to create a physical activity plan to keep the patient with heart failure active while minimizing symptoms of hypoxia (Harding et al., 2022).
Teaching Respiratory Management
Respiratory management is an indispensable aspect of patient and family teaching. Patients with moderate to severe HF may intermittently or permanently require oxygen supplementation or respiratory treatment to remain out of the hospital. Thus, a respiratory therapist is an extremely valuable member of the care team.
OXYGEN SUPPLEMENTATION
Oxygen supplementation may be needed for exacerbations of shortness of breath and dyspnea or may be a permanent feature. Some HF patients may require continuous oxygen even at rest, and others may require oxygen only while active. The supplementation may be in the form of portable oxygen by cylinder, small tank, or an oxygen concentrator. Cylinders and tanks must be refilled periodically, while a concentrator uses the 21% oxygen always available in the surrounding air and concentrates it to deliver a somewhat higher amount, usually measured in liters per minute (L/min).
A nonrebreather mask is a disposable, plastic oxygen mask that covers most of the face and is the only nonventilated oxygen device that can deliver 100% oxygen. But most patients prefer not to wear a mask covering their face and will wear a nasal cannula that can deliver no more than 6 L/min.
Oximetry devices measure the concentration of oxygen in the capillaries to evaluate the need for reduction of oxygen consumption, the need for oxygen supplementation, or the effectiveness of oxygen supplementation to maintain an oxygen saturation of 94% to 100% in the absence of respiratory disease.
(See also “CPAP/BiPAP/APAP” above.)
POSITIONING
Many patients with HF are more comfortable sitting and even sleeping at a high angle up to 90 degrees (orthopnea). In this position, it is more possible to expand the bases of the lungs while inhaling, optimizing the amount of oxygen made available. For this reason, some patients commonly sleep in a chair or have a bed with a movable base in which the head of the bed can be elevated (Harding et al., 2022).
Teaching Weight Management
The closer a person with HF is to their optimal weight, the more active they can be, the less likely they are to need readmission to a hospital with a diagnosis of HF, and the longer their life expectancy. The person or persons in charge of the cooking for the patient may require instruction to promote weight loss and prevent fluid retention while maintaining adequate nutrition and hydration. A registered dietitian or nutritionist may be called in on consultation to help select menus and dishes that will accomplish these goals. Personal preferences, cost, availability, and cultural differences are all typical considerations when meal planning.
Prevention of fluid retention is a fundamental part of patient and family education for patients with HF. Fluid or volume overload can cause the patient to be rehospitalized. In the context of dietary noncompliance, it is the second most common cause for readmission with a diagnosis of HF (noncompliance with a medication regime being the first). Sodium and fluid restrictions are an essential part of self-care education.
(See also “Nutritional Therapy” above.)
CASE
Dorothy is a nurse on a busy telemetry unit in a large medical center in the Southwest, and with 10 years of experience on the telemetry unit, she is now considered expert in teaching HF. She just returned from an education seminar on patient instruction in HF care, where she learned that evidence supports one hour of focused discharge teaching by an expert in HF instruction as successful in prolonging the period between hospital admissions (provided the patient is compliant with teaching).
Dorothy’s patient Nelson Tran is being discharged after his third admission for HF in one year. She arranges for the charge nurse to cover her other patients so that she can devote the time to discharge teaching that her patient needs. Dorothy includes Mr. Tran’s wife in the teaching, since she knows his wife will be helping Mr. Tran take his medications and will continue to do the cooking for the two of them.
As part of her teaching, Dorothy has Mr. Tran explain to her what each discharge medication is and when he will take each one. She discusses sodium and fluid restrictions and has his wife explain how she will be able to include some of Mr. Tran’s favorite foods on the meal plan. She has the physical therapist come into the patient’s room to review his home exercise program with him. She makes his follow-up appointment for him within one week of leaving the hospital and promises to call Mr. Tran within the next two to three days to check on him.
PALLIATIVE / END-OF-LIFE CARE
At the end of life, patients with heart failure tend to exhibit these symptoms:
- Increasing severe pain
- Shortness of breath
- Anxiety
- Depression
- Confusion
Palliative care can provide comfort and symptom management to the end-of-life patient with heart failure, thereby improving the patient’s quality of life (Teoli & Bhardwaj, 2023). But when compared to patients with cancer and other life-threatening conditions, patients with heart failure tend to underutilize palliative care.
The World Health Organization (WHO) stresses the importance of HF patients being treated with prevention and relief of suffering; early identification and assessment of the change in symptoms; and treatment of pain and other physical, psychosocial, and spiritual problems (Årestedt et al., 2021).
Heart failure also frequently results in an unexpected clinical deterioration of the patient or sudden death. For this reason, it is vital that the healthcare team encourage specific planning and goal setting for the end of life. This may include instituting a do-not-resuscitate (DNR) order, internal device deactivation, selection of a care site for the patient’s final days, and bereavement support for the family, usually for up to a year after the patient dies.
Part of the planning and goal setting includes the use of medications to control symptoms and improve quality of life and extend life expectancy.
- Opiates are given for pain and shortness of breath.
- Antiemetics are given to treat and prevent nausea and to promote nutritional intake for well-being and extension of life.
- Oxygen therapy is given for comfort but may also be withheld if thought to extend life unnecessarily.
- Diuretics are given to control pain and symptoms related to severe congestion.
- Renin-angiotensin-aldosterone system blockers, beta blockers, or mineralocorticoid antagonists are given to patients to prevent vasoconstriction and fluid retention and to improve cardiac function.
(Lee & Hwang, 2022)
In order to qualify for federally funded hospice care, a patient’s physician must order that the patient is likely to expire within the next six months. Heart failure frequently qualifies as such a terminal diagnosis.
CONCLUSION
Heart failure is a global epidemic, particularly in countries with a rapidly aging population. In the United States, HF is the most common reason for hospital admission among those over 65 years of age. Half of patients diagnosed with HF die within five years, both nationally and internationally. There are known racial, ethnic, sex, socioeconomic, and age differences that produce varied responses to the disease and to treatments.
There are many different forms of heart failure. Each one represents a distinct set of symptoms leading to the diagnosis. The failure may occur on the right or the left side of the heart, resulting in fluid retention or respiratory symptoms, respectively. Over time, failure of one side of the heart or the other will result in both sides of the heart failing. The patient with HF will exhibit either a reduced (HFrEF) ejection fraction or a preserved (HFpEF) ejection fraction. Systolic HF will exhibit a low EF; diastolic HF will exhibit a normal EF.
Risk factors for heart failure include advanced age, female sex, tobacco use, obesity, excessive drinking, and genetic disposition. Some factors, such as smoking, drinking, and obesity, are modifiable, and lifestyle changes in these areas may prevent HF or slow the progression. Illness caused by the COVID-19 virus has emerged recently as a comorbidity of HF. Similar risk factors and mutual exacerbation of symptoms have also caused HF to be a determining survival factor in COVID-19.
The definitive test to confirm a diagnosis of heart failure is a blood test measuring the level of the neurohormone released in HF, B-type natriuretic peptide (BNP). Normal results are <100 pg/mL. The higher the level of BNP, the more severe the degree of HF. Oher diagnostic tests are performed to determine cardiac and pulmonary function and the presence and degree of any comorbidities such as diabetes, liver or renal failure, COPD, hypertension, or any other heart diseases.
HF may be treated by medications, surgical procedures, or the implantation of devices designed to support the heart that is failing, occasionally as a bridge-to-transplant measure. HF patients are usually on cardiac monitors when they are in an acute care hospital, necessitating time in an intensive care, step-down critical care, or telemetry unit if they are ambulatory.
A great deal of research and clinical trials have been performed to reduce the readmission rate of HF patients to the hospital. Billions of dollars are spent in the United States each year for the treatment of HF, mostly on hospitalization. Patient and family teaching, cardiac rehabilitation, the promotion of self-care, and prevention of the worsening of the HF patient’s condition all serve as methods to prevent the recurrence of HF symptoms that could necessitate readmission to the hospital.
Caring for heart failure patients is a multidisciplinary approach. Physicians, nurses, respiratory therapists, physical therapists, occupational therapists, exercise physiologists, mental health workers, social workers, dietitians, discharge planners, technicians, and families all work together for the benefit of heart failure patients.
HF is included in the list of acceptable primary hospice diagnoses. By itself or in combination with other terminal conditions, a patient with HF may require palliative care at the end of life.
RESOURCES
American Association of Heart Failure Nurses
Heart failure (American Heart Association)
Heart failure (NIH/Medline Plus)
Heart failure (National Heart, Lung, and Blood Institute)
REFERENCES
NOTE: Complete URLs for references retrieved from online sources are provided in the PDF of this course.
Abramson M, Harvey J, Greenfield M, Lauman S, & Metzler D. (n.d.). Partners in therapy: A case study of OT intervention for a recipient of a left ventricular access device. Advance for Occupational Therapy Practitioners, 16–20.
Aktaş MK, Younis A, Zareba W, Kutyifa V, Klein HB, et al. (2021). Survival after implantable cardioverter-defibrillator shocks. Journal of American College of Cardiology, 77(20), 2453–62. https://doi.org/10.1016/j.jacc.2021.03.329
American Heart Association (AHA). (2024). What is cardiac rehabilitation? https://www.heart.org
American Heart Association (AHA). (2022). 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation, 145(18), e895–e1032. https://doi.org/10.1161/CIR.0000000000001063
American Occupational Therapy Association (AOTA). (2024). Domain and process: Intervention. https://www.doi. aota.org/practice/domain-and-process/skills-and-routines
Årestedt K, Brännström M, Evangelista LS, Strömberg A, & Alvariza A. (2021). Palliative key aspects are of importance for symptom relief during the last week of life in patients with heart failure. European Society Cardiology Heart Fail, 8(3), 2202–9. https://doi.org/10.1002/ehf2.13312
Aspen Valley Hospital. (2024). What is cardiopulmonary rehabilitation? https://www.aspenhospital.org
Brugts JJ, Radhoe SP, Aydin D, Theuns DA, & Veenis JF. (2021). Clinical update of the latest evidence for CardioMEMS pulmonary artery pressure monitoring in patients with chronic heart failure: A promising system for remote heart failure care. Sensors (Basel), 21(7), 2335. https://doi.org/10.3390/s21072335
Byars G, Bigler J, & Stephenson S. (2023). Activity tolerance assessment and intervention. https://slcc.pressbooks.pub/otaphysicaldysfunction/chapter/activity-tolerance-assessment-and-intervention/
Centers for Disease Control and Prevention (CDC). (2024). U.S. heart failure death rates by county, 2018–2020. https://www.cdc.gov
Cleveland Clinic. (2023). Stem cell transplant (bone marrow transplant). https://my.clevelandclinic.org
Cona LA. (2023). Stem cell therapy for heart failure. DVC Stem. https://www.dvcstem.com
Diamond J & DeVore AD. (2022). New strategies to prevent rehospitalizations for heart failure. Current Treatment Options Cardiovascular Medicine, 24, 199–212. http://doi.org/10.1007/s11936-022-009
Duncker D, Ding WY, Etheridge S, Noseworthy PA, Veltmann C, Yao X, Bunch TJ, & Gupta D. (2021). Smart wearables for cardiac monitoring—Real-world use beyond atrial fibrillation. Sensors, 7, 2539. https://doi.org/10.3390/s21072539
Elgwairi E, Abdalla A, Elkheshen A, Elharabi Z, & Nugent K. (2024). Pleural effusions in patients with congestive heart failure: Frequency, pathogenesis, diagnosis, and implications. Cardiology Review, 32(2), 91–6. https://doi.org/10.1097/CRD.0000000000000469
Fogoros RN. (2023). How cocaine affects the cardiovascular system. Verywell Health. https://www.verywellhealth.com
Gtif I, Bouzid F, Charfeddine S, Abid L, & Kharrat N. (2021). Heart failure disease: An African perspective. Archives of Cardiovascular Diseases, 114(10), 680–90. https://doi.org/10.1016/j.acvd.2021.07.001
Harding MM, Kwong J, Hagler D, & Reinisch C. (2022). Lewis medical-surgical nursing assessment and clinical management of clinical problems (12th ed.). Elsevier.
Hasbani NR, Ligthart S, Brown MR, Heath AS, Bebo A, et al. (2022). American Heart Association’s life’s simple 7: Lifestyle recommendations, polygenic risk, and lifetime risk of coronary heart disease. Circulation, 145(11), 808–18. https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.121.053730
Hashemlu L, Esmaeili R, Bahramnezhad F, & Rohani C. (2023). A systematic review on clinical guidelines of home health care in heart failure patients. BMC Nursing, 22, 127. http://doi.org/10.1186/s12912-023-01294-w
Huizen J. (2024). Everything to know about end stage heart failure. MedicalNewsToday. https://www.medicalnewstoday.com
Jaarsma T, Hill L, Bayes-Genis A, La Rocca HB, Castiello T, et al. (2020). Self-care of heart failure patients: Practical management recommendations from the Heart Failure Association of the European Society of Cardiology. European Journal of Heart Failure, 23(1), 157–74. http://doi.org/10.1002/ejhf.2008
Kerrigan L. (2022). What is a nurse navigator? https://www.healthecareers.com
Khan E. (2021). Heart failure and COVID-19: Synergism of two inflammatory conditions? British Journal of Community Nursing, 26(1), 18–25.
Lee JH & Hwang KK. (2022). End-of-life care for end-stage heart failure patients. Korean Circulation Journal, 52(9), 659–79. https://doi.org/10.4070/kcj.2022.0211
Lewsey SC & Breathett K. (2021). Racial and ethnic disparities in heart failure: Current state and future directions. Current Opinions in Cardiology, 36(3), 320–8. http://doi.org/10.1097/HCO.0000000000000855
Maddox TM, Januzzi JL, Allen LA, Breathett K, Brouse S, et al. (2024). ACC expert consensus decision pathway for treatment of heart failure with reduced ejection fraction: A report of the American College of Cardiology solution set oversight committee. Journal of American College of Cardiology, 83(15), 1444–88. https://doi.org/10.1016/j.jacc.2023.12.024
Martin SS, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, et al. (2024). 2024 heart disease and stroke statistics: A report of U.S. and global data from the American Heart Association. Circulation, 149(8), e347–e913.
Mayo Clinic. (2022). Stress test. https://www.mayoclinic.org
Mayo Clinic. (2024). Cardiac rehabilitation. https://www.mayoclinic.org
McCuistion LE, Vuljoin DiMaggio K, Winton MB, & Yeager JJ. (2023). Pharmacology: A patient-centered nursing process approach (11th ed.). Elsevier.
Merck & Co. (2024). Classes of drugs for heart failure. Merck Manuals. https://www.merckmanuals.com
National Heart, Lung, & Blood Institute (NHLBI). (2023). What are defibrillators? https://www.nhlbi.nih.gov
National Heart, Lung, & Blood Institute (NHLBI). (2022a). Heart failure symptoms. https://www.nhlbi.nih.gov
National Heart, Lung, & Blood Institute (NHLBI). (2022b). What are heart valve diseases? https://www.nhlbi.nih.gov
National Institutes of Health (NIH). (2023). Cardiac catheterization risks and complications. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
Pagana KD, Pagana TJ, & Pagana TN. (2022). Mosby’s manual of laboratory and diagnostic tests (7th ed.). Elsevier.
Perin E, Borow K, Henry T, et al. (2023). Randomized trial of targeted transendocardial mesenchymal precursor cell therapy in patients with heart failure. Journal of American College Cardiology, 81(9), 849–63. https://doi.org/10.1016/j.jacc.2022.11.061
Physiopedia. (2024). Borg rating of perceived exertion. https://www.physio-pedia.com
Physiopedia. (2021). Physical activity and cardiovascular disease. https://physio-pedia.com
Purwowiyoto SL & Prawara AS. (2021). Metabolic syndrome and heart failure: Mechanism and management. Medicine Pharmacy Reports, 94(1), 15–21. http://doi.org/10.15386/mpr-1884
Rauf D. (2022). How to live longer with congestive heart failure. Everyday Health. https://www.everydayhealth.com
Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, & Coats AJS. (2023). Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovascular Research, 118(17), 3272–87. https://doi.org/10.1093/cvr/cvac013
Schiller R. (2024). How long can you live with heart failure? Verywell Health. https://www.verywellhealth.com
Sears B. (2023). What is a timed up and go (TUG) test? Verywell Health. https://www.verywellhealth.com
Sears B. (2022). A walkthrough of cardiac rehab phases 1–4: Physical therapy after a cardiac event is key to treatment. Verywell Health. https://www.verywellhealth.com
Shen J & Greenberg BH. (2021). Diabetes management in patients with heart failure. Diabetes Metabolism Journal, 45(2), 158–72. http://doi.org/10.4093/dmj.2020.0296
Singhvi A & Trachtenberg B. (2019). Left ventricular assist devices 101: Shared care for general cardiologists and primary care. Journal of Clinical Medicine, 8, 1720. http://doi.org/10.3390/jcm8101720
St. Catherine University (SCU). (2023). 6 common occupational therapy interventions. https://otaonline.stkate.edu
Taylor JL, Bonikowske AR, & Olson TP. (2021). Optimizing outcomes in cardiac rehabilitation: The importance of exercise intensity. Frontiers in Cardiac Medicine, 8, 734278. https://doi.org/10.3389/fcvm.2021.734278
Teoli D & Bhardwaj A. (2023). Hospice appropriate diagnoses. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
U.S. Health Resources and Services Administration (HRSA). (2023). Telehealth and remote patient monitoring. Telehealth.HHS.gov. https://telehealth.hhs.gov
Vecchione A. (2022). Annual heart failure costs in the US could surpass $70B by 2030. Cardiovascular Business. https://cardiovascularbusiness.com





Customer Rating
5.0 / 809 ratings