Pressure Injuries
Risk Assessment and Prevention Measures
Online Continuing Education Course
Course Description
This wound care continuing education course covers risk assessment and prevention measures for pressure injuries. Impacts, risk factors, measuring risk, actions to prevent pressure injuries, and prevention in special populations are all discussed. Applicable CEU for nursing, occupational therapy, and physical therapy.
"Well presented and organized. Complemented my professional education well." - Shannon, OT in Louisiana
"Very, very good and easy to understand." - Juanita, RN in Florida
"Information is very current and educational for my work." - Sarah, RN in Alabama
"Excellent course! Will recommend this to my nurse colleagues." - Linda, PT in California
Pressure Injuries
Risk Assessment and Prevention Measures
Copyright © 2023 Wild Iris Medical Education, Inc. All Rights Reserved.
LEARNING OUTCOME AND OBJECTIVES: Upon completion of this continuing education course, you will have increased your understanding of pressure injury assessment, prevention, and treatment. Specific learning objectives to address potential knowledge gaps include:
- Discuss the impacts of pressure injuries.
- Explain the risk factors for developing pressure injuries.
- Describe the processes for conducting risk assessments and measuring risk associated with pressure injuries.
- Identify actions to help prevent pressure injuries.
- Analyze actions to prevent pressure injuries in special populations.
TABLE OF CONTENTS
INTRODUCTION
The skin is the largest organ in the human body—and essential to life and well-being. Human skin serves many vital functions, the most critical of these being to provide a barrier between the external environment and the internal environment of the body. Similar to any other organ in the body, the human skin is susceptible to disease and injury. Wounds involving the skin are a frequent occurrence in all age groups.
A pressure injury is a wound unlike any other in that its cause is not surgery or trauma but death of the skin and underlying tissues from ischemia due to intense and/or prolonged pressure. A pressure injury can also result from a combination of pressure and shear. There are many factors that contribute to the development of a pressure injury and whether or not it will heal, but the biggest factor in all of these is pressure.
Pressure injury is not new, and the terminology to describe it has varied over time:
- Decubitus (18th century)
- Decubitus ulcer (ca. 1950s)
- Bed sore (ca. 1970s)
- Pressure sore (ca. 1980s)
- Pressure ulcer (ca. 1990s)
- Pressure injury (2016–present)
(PA DOH, 2017)
Defining “Pressure Injury”
Over the years, the definition of a pressure injury has been refined as medical knowledge and understanding of the disease process has advanced, along with improvements in treatment approaches and imaging technology. The National Pressure Injury Advisory Panel (NPIAP, 2016a) definition states:
A pressure injury is localized damage to the skin and underlying soft tissue usually over a bony prominence or related to a medical or other device. The injury can present as intact skin or an open ulcer and may be painful. The injury occurs as a result of intense and/or prolonged pressure or pressure in combination with shear. The tolerance of soft tissue for pressure and shear may also be affected by microclimate, nutrition, perfusion, comorbidities, and condition of the soft tissue.
The localized damage in a pressure injury is the result of compression of soft tissue that interferes with the tissue’s blood supply, leading to vascular insufficiency, tissue anoxia, and cell death. Pressure injuries can develop within 24 hours of the initial pressure but may take as long as a week to present themselves. The first tissues to die are nearest the bone, and as the pressure and anoxia continue, the remaining layers of tissue begin to die. The skin is the last to die. The damage can be compared to an iceberg, with a smaller amount of damage visible at the surface and a large amount of damage below the surface.
Pressure injury usually occurs over bony prominences such as the sacrum, ischium, heel, and trochanter, where there is less tissue to compress. Other factors—such as shearing, skin moisture, heat, poor nutrition, comorbidities, and incontinence—also contribute to the tissue breakdown (WOCN, 2022a).
“PRESSURE INJURY” INSTEAD OF “PRESSURE ULCER”
In April 2016, the National Pressure Injury Advisory Panel (NPIAP) (at that time called the National Pressure Ulcer Advisory Panel, or NPUAP) updated the term pressure ulcer to pressure injury, updated the staging system, replaced the use of Roman numerals with Arabic numerals, and updated the definition of a pressure injury. The two main reasons cited for this change in terminology were:
- Stage 1 pressure injuries and deep tissue injuries were never ulcers.
- An ulcer cannot be present without an injury, but an injury can be present without an ulcer.
The Impact of Pressure Injuries
The impact of pressure injuries is staggering. Over 2.5 million cases of pressure injuries are reported in the United States on an annual basis, making pressure injuries the second most frequent diagnosis in healthcare billing records in this country. According to Centers for Disease Control and Prevention (CDC) data, pressure injury is the eighth most common cause of death in the United States. Veterans Affairs facilities have pressure injury rates double that of the national average (NPIAP, 2021).
Monitoring skin integrity and taking proactive steps to prevent pressure injury development are a primary responsibility for healthcare providers. Failure to do so can have detrimental consequences for patients, providers, and healthcare facilities, such as:
- First and foremost, these wounds are very painful, causing patients a great deal of suffering.
- Quality of life is affected, as the patient must alter activities to help heal the wound, and they may face long-term hospitalization.
- The anatomical location of the injury may result in a loss of dignity.
- The burden of dealing with a chronic wound can result in stress, anxiety, depression, less autonomy and security, and impaired social functioning.
- A nonhealing injury is at high risk for infection, which can be life threatening.
- Caring for a patient with pressure injury negatively impacts the quality of life for family members and caretakers.
(WOCN, 2022a)
Pressure injury is a particular problem for bedbound individuals who are hospitalized, in nursing homes, or have spinal cord injuries. Pressure injuries also increase healthcare professionals’ workloads, as additional time and care must be provided to manage and treat patients’ pressure injuries—more dressing changes, more medications, and more documentation.
Litigation may be brought against a hospital and its staff for neglect, malpractice, and elder abuse if a patient develops a pressure injury while in the hospital. Awards can be in the millions of dollars, and the bad publicity that follows will damage the hospital’s reputation, bottom line, and the trust patients have that they can be cared for safely. Cases involving the occurrence of pressure injuries are the primary malpractice claim in this country (NPIAP, 2021).
Pressure injuries are reportable to state and federal agencies. The information is placed in reports accessible to the public. Regardless of the care setting, all providers must account for the number of pressure injuries that were present on admission and on subsequent reassessments, whether they have closed or worsened (CMS, 2022a).
Governmental agencies may levy fines against a hospital for pressure injuries. The Centers for Medicare and Medicaid Services (CMS) no longer pays a hospital for the additional care needed for a patient who develops a hospital-acquired pressure injury, and the hospital must provide the care nonetheless (CMS, 2022b).
Thus, the assessment, prevention, and treatment of pressure injuries are of major importance to healthcare professionals and to the facilities at which they practice. Most facilities have developed pressure injury prevention programs to put these ideas into practice and prevent negative outcomes for both the patient and the facility.
RISK FACTORS
Risk Groups for Pressure Injuries
Across all settings the three groups most at risk for pressure injuries are:
- Individuals over 65 years of age (with those over age 75 at even greater risk)
- Neonates and children younger than three
- Individuals of any age with spinal cord injury (with spasticity, the extent of the paralysis, a younger age at onset, difficulty with practicing good skin care, and a delay in seeking treatment or implementing preventive measures increasing the risk of skin breakdown)
Other groups of patients also have a high risk for developing pressure injuries. These include patients who:
- Are in critical care
- Have a fractured hip (which indicates increased risk for heel pressure injuries)
- Have diabetes, secondary to complications from peripheral neuropathy
- Are confined to a wheelchair or bed
- Are immobile or for whom moving requires significant or taxing effort (e.g., morbidly obese)
- Experience incontinence
- Have neuromuscular and progressive neurological diseases (e.g., multiple sclerosis, ALS, Myasthenia gravis, stroke)
- Have neurodegenerative disorders (e.g., Parkinson’s disease, dementia)
Risks due to Aging Skin
Changes in both skin structure and function due to aging contribute to the occurrence of skin problems and decrease wound healing:
- A flattening of the epidermal-dermal junction decreases the overall strength of the skin, which increases the risk for skin tears and blistering.
- A decrease in the melanocytes and Langerhans cells increases the risk for allergic reactions and sensitivity to sunlight.
- A decrease in fibroblast function increases the time required to synthesize collagen.
- A decrease in blood flow decreases skin temperature and delays healing.
- A decrease in oil and sweat production contributes to dryness and flaking.
- A decrease in subcutaneous tissue, especially fat, decreases the body’s natural insulation and padding.
- A decline in the reproduction of the outermost layer of the epidermis may lead to the skin’s inability to absorb topical medications.
These changes in skin structure and function (together with changes in cellular DNA that affect cell reproduction and the ability to protect the skin) and the risks that occur with a change in overall health and functional ability put an aged patient at very high risk for the formation of a pressure injury (WOCN, 2022a; Baranoski & Ayello, 2020).
Other Risk Factors
More than 100 additional risk factors associated with the development a pressure injury have been identified. Some of these include:
- General medical conditions such as diabetes, stroke, multiple sclerosis, cognitive impairment, cardiopulmonary disease, cancer, hemodynamic instability (abnormal/unstable blood pressure), peripheral vascular disease, malnutrition, and dehydration
- Smoking
- History of a previous pressure injury (since scar tissue is weaker than the skin it replaces and breaks down more easily than intact skin)
- Increased facility length of stay
- Undergoing surgery longer than four hours
- Significant weight loss
- Prolonged time on a stretcher (since the surface is not conducive to pressure relief)
- Emergency department stays
- Medications, such as sedatives, analgesics, and nonsteroidal anti-inflammatory drugs (NSAIDs)
- Impaired sensation
- Refusal of care, such as when a patient refuses to be turned or moved despite education
- Edema
- Obesity
- Patient not being turned
- An intensive care unit stay, due to the high acuity of illness, presence of multiple comorbid conditions, and:
- Mechanical ventilation
- Vasopressors and hemodynamic instability
- Multiple surgeries
- Increased length of stay
- Inability to report discomfort
(WOCN, 2022a; Baranoski & Ayello, 2020)
RISK ASSESSMENT
The purpose of assessing the risk for developing pressure injuries is to implement early detection and prevention measures. This is of utmost importance, as assessment without intervention is meaningless in the effort to prevent a pressure injury from developing.
Risk Assessment Schedules
The skin is the largest organ in the body, and the clinician must assess it regularly. The assessment of pressure injury risk is performed upon a patient’s entry to a healthcare setting and repeated on a regularly scheduled basis (per facility policy) as well as when there is a significant change in the patient’s condition, such as surgery or a decline in health status.
A schedule for reassessing risk is established based on the acuity of the patient and an awareness of when pressure injuries occur in a particular clinical setting. Recommendations based on the healthcare setting are included below (WOCN, 2022a; Baranoski & Ayello, 2020). A particular facility or setting may have different regulations.
ACUTE CARE
Generally, pressure injuries can develop within the first two weeks of hospitalization, and older adult patients can develop pressure injuries within the first week of hospitalization. The initial assessment is conducted upon admission and repeated:
- At least every 24 to 48 hours
- Whenever the patient is transferred from one unit to another
- Whenever the patient’s condition changes or deteriorates
- Per facility policy
Most medical-surgical units reassess at least daily.
ICU/CRITICAL CARE
Intensive care unit (ICU) patients are at high risk for developing pressure injuries, especially on the heel. ICU patients have been shown to develop pressure injuries within 72 hours of admission. Pressure injuries have been associated with a two- to fourfold increase in the risk of death in older people in the ICU. Pressure injury assessment is to be done at least once every 24 hours (WOCN, 2022a; Baranoski & Ayello, 2020).
PRESSURE INJURIES AMONG CRITICALLY ILL PATIENTS
In a study of factors that placed critically ill patients at the highest risk for developing pressure injury, the presence of diabetes mellitus was found to be the predominant risk factor. In patients with serious healthcare-acquired pressure injuries (HAPIs), almost 30% had diabetes mellitus.
Mechanical ventilation was found to be the most frequent treatment-related risk factor, with the use of vasopressor agents second most frequent. These latter two findings indicate that diminished tissue oxygenation and blood supply are directly associated with pressure injury development. In the United States, respiratory failure necessitating mechanical ventilation is the most widespread cause for admission to intensive care units.
The most frequently occurring type of pressure injury among critically ill patients was found to be deep tissue pressure injury (DTPI).
Two other major findings from the study were that adherence to the implementation of preventive practices decreased the development of HAPI among critically ill patients. Secondly, there is a need to develop a pressure injury risk assessment tool that captures the existence of numerous concurrent conditions in critically ill patients, which are specific risks for pressure injury development (Cox, 2022).
INPATIENT REHABILITATION SETTINGS
The presence of a pressure injury was significantly associated with lower gains in motor function, longer length of stay, and decreased odds of being discharged to the community. Data from one study indicated that 51.1% of patients with spinal cord injury acquired at least one pressure injury during their first rehabilitation stay. Multiple risk factors were identified for the prevalence of pressure injuries in this population, including the severity of the spinal cord injury, development of pneumonia, and longer lengths of stay (Najmanova, 2022). Assessment is on admission and per facility protocol.
LONG-TERM CARE
Nursing home patients are at high risk for the development of pressure injuries. In long-term care settings, most pressure injuries develop within the first four weeks.
- In skilled nursing facilities, initial assessment is conducted upon admission and repeated weekly thereafter.
- In nursing homes with long-term patients, assessment is conducted upon admission, repeated weekly for the first month, and repeated monthly thereafter, or whenever the patient’s condition changes.
Among nursing home residents, it has been found that there is a substantial connection between the occurrence of pressure injuries and the presence of dementia, cerebrovascular disease, and female sex (Elli, 2022).
HOME HEALTH
In home healthcare settings, most pressure injuries develop within the first four weeks. The initial assessment is conducted upon admission and repeated:
- At resumption of care
- Recertification (assessment and approval of the need for continuation of patient care)
- Transfer or discharge
- Whenever the patient’s condition changes
Some agencies reassess with each nursing visit.
An important factor in preventing pressure injury development in the home care setting is the education of family and caretakers about interventions needed to maintain skin integrity. This must be an ongoing process and frequently evaluated by the clinician (Wound Source, 2019).
COMMON QUESTIONS ABOUT PRESSURE INJURIES
Q: Can a pressure injury be treated at home?
A: Yes, pressure injuries can be treated at home after they have been diagnosed by a healthcare provider and a plan of care is put in place. This may include help from a home care clinician who is skilled in wound care and who will educate the patient, family, and caregivers in dressing changes, patient repositioning, and other strategies to assist with wound healing.
HOSPICE AND PALLIATIVE CARE
One study showed that of eight pressure injuries that developed during the study, five occurred within two weeks prior to death. Assessment occurs at admission and as patient condition changes. The development of pressure injuries during the end-of-life phase can significantly increase pain and suffering (Seton, 2022). However, preventing pressure injury development at the end of life can be challenging. Skin care must focus on hygiene, promoting comfort, pain control, and limiting distressing symptoms such as wound drainage, bleeding, or odor (Vickery, 2020).
Elements of an Assessment
Prevention of pressure injuries must begin with frequent and routine assessment of the patient’s skin and of the risk factors that, if left unmanaged, will contribute to the development of an injury. Risk assessment without interventions to modify the risk is meaningless.
A head-to-toe, front and back inspection of the skin must be done on admission and at least daily (or per facility regulation). Five parameters for skin assessment are recommended by the Centers for Medicare and Medicaid Services:
- Skin color
- Skin temperature
- Skin texture/turgor
- Skin integrity
- Moisture status
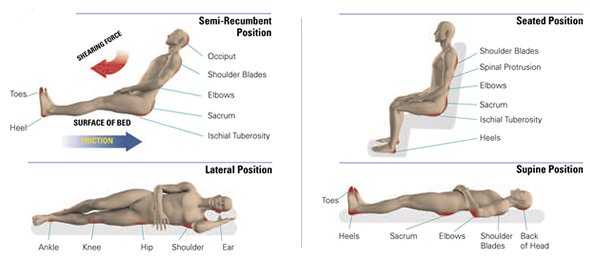
The assessment focuses on high-risk areas such as bony prominences, areas of redness, and under medical devices. The specific areas to assess are shown in the table and diagram below. The assessment must not be limited to a visual inspect of the skin surfaces but also include tissue palpation to determine skin temperature, presence of edema, and overall tissue uniformity (WOCN, 2022a).
| If the patient’s position is: | Then focus on these areas: |
|---|---|
| Lateral | Ear, shoulder, trochanter, knee, ankle |
| Supine | Occiput, shoulder blades, elbows, sacrum, heels, toes |
| Semi-recumbent | Occiput, shoulder blades, elbows, sacrum, ischial tuberosities, heels |
| Seated | Shoulder blades, spinal protrusions, elbows, sacrum, ischial tuberosities, heels |
Bony prominences are high-risk areas for pressure injuries. (Source: © Invacare Corporation. Used with permission.)
Blanchable erythema is a reddened area that temporarily turns white or pale when pressure is applied with a fingertip. This is an early indication to redistribute pressure. Nonblanchable erythema is redness that persists when fingertip pressure is applied. It means that tissue damage has already occurred. (See image below.)
It can be difficult to identify skin problems in patients with dark skin. Redness may not be easy to see. The clinician needs to compare the at-risk area (such as the coccyx or hip) with skin next to it and look for color differences or changes in temperature or pain (WOCN, 2022a).
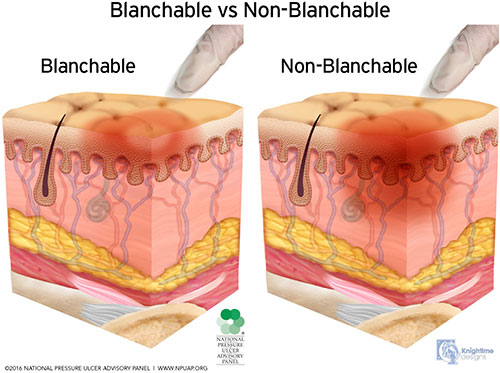
Blanchable vs. nonblanchable erythema. (Source: © NPIAP, used with permission.)
ASSESSMENT AND MEDICAL DEVICES
Medical devices such as shoes, heel and elbow protectors, splints, oxygen tubing, face masks, endotracheal tube holders, compression stockings and TED hose, and others must be removed and the skin inspected daily. For example, oxygen tubing can cause pressure injuries on the ears, and compression stockings and TED hose have been known to cause heel injuries.
If the device cannot be removed—such as a nasogastric (NG) tube, urinary catheter, tracheostomy holder, neck brace, or cast—then the skin around the device must be carefully inspected: the nares for an NG tube, the neck for a tracheostomy, the mucosa for a urinary catheter, etc. If the patient complains of pain under an unremovable device, the physician is notified (WOCN, 2022a).
Consider all adults with medical devices to be at risk for pressure injuries.
- The Joint Commission Quick Safety defines a medical device–related pressure injury (MDRPI) as an event that results from the use of devices applied to patients for diagnostic or therapeutic reasons (TJC, 2022). Studies indicate that as many as 50% of HAPIs are associated with medical device use (Sermersheim, 2021).
- The most frequently used medical devices that cause pressure injuries in adults include those related to respiratory care, tubes and drains, braces, compression wraps, and splints (Pittman, 2020).
- The most commons sites for MDRPIs are the ears, nose, face, chin, and lips (Brophy, 2021).
- Among pediatric patients, the prevalence of MDRPIs has been reported to be as high as 70%. This population is at high risk for serious injury related to medical devices due to factors such as immature skin development (especially in the neonate population), the lack of availability of correctly fitting medical devices, and the use of attachment and immobilization products (Stellar, 2020).
Injuries caused by medical devices are reportable to state and federal agencies, just as are those caused by pressure on bony prominences (EPUAP/NPIAP/PPPIA, 2019).
ASSESSMENT AND MOBILITY
Immobility is the most significant risk factor for pressure injury development. More frequent monitoring to prevent pressure injuries is conducted for patients who have some degree of immobility, including those who are:
- Nonambulatory
- Confined to bed, chairs, wheelchairs, recliners, or couches for long periods of time
- Paralyzed and/or have contractures
- Wearing orthopedic devices that limit function and range of motion
- Dependent on assistance to ambulate or reposition themselves
(WOCN, 2022a)
COMMON QUESTIONS ABOUT PRESSURE INJURIES
Q: Can patients who are conscious and alert still develop pressure injury?
A: Yes. The primary cause for the development of pressure injury is immobility. Patients who are alert and conscious but are fully or partially immobile are at risk for pressure injury development.
ASSESSMENT FOR FRICTION VS. SHEARING
Friction is the rubbing of one surface against another. Patients who cannot lift themselves during repositioning and transferring are at high risk for friction injuries. Friction may contribute to the development or worsening of a pressure injury due to the shear it creates. There are two types of friction:
- Static friction is the force that resists motion when there is no sliding; for example, static friction prevents an individual from sliding down in bed when the head of the bed is elevated.
- Dynamic friction is the force between two surfaces when there is sliding, for example, when a person is sliding down in bed. Skin trauma can result.
Shear is the mechanical force that is parallel to the skin; it can damage deep tissues such as muscle. Shear can result when friction stretches the top layers of skin as it slides against a surface or deeper layers when tissues attached to the bone are pulled in one direction while the surface tissues remain stationary.
Shearing most commonly occurs when the head of the bed is elevated above 30 degrees and the patient slides downward. Friction is most common when patients are turned or pulled up in bed (WOCN, 2022a; EPUAP/NPIAP/PPPIA, 2019).
ASSESSMENT FOR INCONTINENCE
Moisture from incontinence can contribute to pressure injury development by macerating the skin and increasing friction injuries. Fecal incontinence is an even greater risk for pressure injury development than urinary incontinence because the stool contains bacteria and enzymes that are caustic to the skin. When both urinary and fecal incontinence occur, the fecal enzymes convert the urea in the urine to ammonia, which raises the skin’s pH. When the skin pH is elevated (alkaline), the skin is more susceptible to damage. Pressure injuries are four times more likely to develop in patients who are incontinent than in those who are continent (WOCN, 2022a).
COMMON QUESTIONS ABOUT PRESSURE INJURIES
Q: Are in-dwelling urinary catheters used to prevent pressure injury?
A: Indwelling urinary catheters are not recommended as a means of preventing pressure injury due to the risks associated with their long-term use, such as infection. Short-term use of an indwelling catheter may be recommended when healing an existing pressure injury, but this is done on an individual basis and after thorough wound assessment and consideration of other options.
ASSESSMENT FOR NUTRITIONAL STATUS
Although individual nutrients and their specific roles in preventing pressure injury have not been determined, malnutrition is associated with overall morbidity and mortality. A nutritional assessment is conducted upon admission or when there is a change in the patient’s condition that would increase the risk of malnutrition, such as:
- Patient’s refusal to eat or eating less than usual
- Prolonged NPO status
- Development of a wound or other conditions that increase metabolic demand
- When a pressure injury is not progressing toward healing
The clinician must also keep in mind that patients who are overweight or with obesity can be malnourished and should undergo a nutritional assessment.
Parameters to assess include:
- Current and usual weight
- History of unintentional weight loss or gain
- Food intake
- Dental health
- Ability to swallow and/or feed oneself
- Medical interventions (such as surgeries of the gastrointestinal tract that may affect absorption of nutrients such as vitamin B12)
- Psychosocial factors (such as the ability to obtain and pay for food)
- Cultural influences on food selection
Serum albumin and prealbumin are no longer considered reliable indicators of nutritional status, as there are multiple factors that will decrease albumin levels even with adequate protein intake. These include inflammation, stress, surgery, hydration, insulin, and renal function. Therefore, laboratory evaluation is only one part of a nutrition assessment (WOCN, 2022a; Baranoski & Ayello, 2020).
ASSESSMENT FOR PREVIOUSLY HEALED PRESSURE INJURIES
During patient assessment, the clinician notes the presence of scar tissue or evidence of wound healing over bony prominences, such as the sacral area, and if possible, determines if this is a healed pressure injury. The existence of a previously healed pressure injury places a patient at greater risk for pressure injury recurrence. As a pressure injury heals, it decreases in depth, but the body does not replace the lost bone, muscle, subcutaneous fat, or dermis. Instead, the full-thickness injury is filled with granulation or scar tissue and then covered with new epithelium (EPUAP/NPIAP/PPPIA, 2019).
Various studies have indicated that patient populations most at risk for pressure injury reoccurrence include those with spinal cord injury and patients with surgical reconstruction, such as flap-closure. Diabetes mellitus and scoliosis were also found to be risk factors for pressure injury reoccurrence (Tsai, 2023).
CASE
Mr. Frank is a 90-year-old man who has been admitted to the hospital with pneumonia. He fell at home three months ago and was also hospitalized at that time. His equally elderly wife denies that she is having any difficulty caring for him and says that he eats well and takes all his medications.
The admitting nurse finds Mr. Frank to be very thin and that he weighs 10 pounds less than when he was hospitalized after his fall. His incontinence brief is saturated with urine, and his perineal skin is raw. He does not move himself in the bed. The nurse recognizes that Mr. Frank is at high risk for developing a pressure injury due to his poor nutrition, his immobility, and his incontinence. The nurse discusses with the physician the patient’s need for a dietitian referral, a pressure reduction mattress, and a barrier product to protect his skin. She alerts the discharge planner that Mr. Frank may also require home health, with personal care services daily if that is available with his insurance coverage.
The physician also requests physical therapy (PT) and occupational therapy (OT) evaluations and recommendations to improve the patient’s mobility and address self-care needs in order to reduce Mr. Frank’s risk for developing a pressure injury. Prior to discharge, a physical therapist and occupational therapist evaluate and work directly with Mr. Frank on various aspects of mobility and activities of daily living (ADL) performance. They also provide pertinent education and hands-on training for Mrs. Frank in order to optimize her ability to safely care for her husband at home.
For instance, the physical therapist begins to teach Mrs. Frank how to safely assist her husband with bed mobility, transfers to/from a bedside chair and/or commode, and ambulating household distances with an appropriate walker. The occupational therapist teaches Mrs. Frank how to safely assist her husband with ADLs (such as dressing, bathing, and toileting). Both therapists recommend continued PT and OT services in the home setting in order to progress the patient’s functional mobility and independence with ADLs.
If Mr. Frank and his wife continue to have difficulty with his care at home, additional home-based help or an alternate level of care placement may eventually need to be considered.
Risk Assessment Tools and Scales
Several risk assessment tools or scales are available to help predict the risk of a pressure injury, based primarily on those assessments mentioned above. These tools consist of several categories, with scores that when added together determine a total risk score.
The Braden Scale for Predicting Pressure Sore Risk is the most validated and widely used pressure injury risk assessment tool in the United States. It was first published in 1987 and has thus been in use for decades across a variety of settings. Two other common scales are the Norton Scale and the Cubbin-Jackson Scale, which are described below (Baranoski & Ayello, 2020; WOCN, 2022a).
The clinician uses these tools to help determine risk so that interventions can be started promptly. These tools are only used for assessing adults, although the Braden-Q Scale has subcategories that relate to assessing children.
It is important that when the clinician uses a scale, the scale must not be altered in any way, meaning there cannot be shortcuts or changes to the definitions. Any changes would alter the accuracy and usefulness of the scale in predicting the risk of developing pressure injuries. The same scale should be used consistently throughout the facility, and if this is not a standard practice, it is one that clinicians should advocate for.
Assessment tools notwithstanding, if a patient has other major risk factors present (such as age, fever, poor perfusion, etc.), the patient may be at higher risk than a risk score would indicate. Clinicians must work to assure that, regardless of the specific risk assessment tool being used, the professionals using it are proficient in its use and knowledgeable regarding potential risk factors within their patient population that are not accounted for in the assessment tool they are using (WOCN, 2022a).
BRADEN SCALE
The Braden Scale consists of six categories:
- Sensory perception: Can the patient respond to pressure-related discomfort?
- Moisture: What is the patient’s degree of exposure to incontinence, sweat, and drainage?
- Activity: What is the patient’s degree of physical activity?
- Mobility: Is the patient able to change and control body position?
- Nutrition: How much does the patient eat?
- Friction/shear: How much sliding/dragging does the patient undergo?
There are four subcategories in each of the first five categories and three subcategories in the last category. The scores in each of the subcategories are added together to calculate a total score, which ranges from 6 to 23. The higher the patient’s score, the lower their risk.
- Less than mild risk: ≥19
- Mild risk: 15–18
- Moderate risk: 13–14
- High risk: 10–12
- Very high risk: ≤9
It is recommended that if other risk factors are present (such as age, fever, poor protein intake, hemodynamic instability), the risk level be advanced to the next level. Each deficit that is found when using the tool should be individually addressed, even if the total score is above 18. The best care occurs when the scale is used in conjunction with nursing judgment. Some patients will have high scores and still have risk factors that must be addressed, whereas others with low scores may be reasonably expected to recover so rapidly that those factors need not be addressed (WOCN, 2022a).
(See also “Resources” at the end of this course.)
NORTON SCALE
The very first pressure injury risk evaluation scale, called the Norton Scale, was created in 1962 and is still in use today in some facilities. It consists of five categories:
- Physical condition
- Mental condition
- Activity
- Mobility
- Incontinence
Each category is rated from 1 to 4, with a possible total score ranging from 5 to 20. A score of less than 14 indicates a high risk of pressure injury development.
CUBBIN-JACKSON SCALE
The Cubbin-Jackson Scale was created specifically to determine pressure injury risk in critically ill patients. In one study, it was found to have comparable prognostic validity to the Braden Scale, and in a second study, its prognostic validity was found to surpass the Braden Scale when used in a group of critically ill trauma patients (Higgins, 2020).
The Cubbin-Jackson Scale consists of ten items:
- Patient age
- Patient weight
- Skin condition of the entire body
- Mental status
- Mobility
- Nutrition
- Respiratory status
- Incontinence
- Hygiene
- Hemodynamic status.
It utilizes a four-point scale, with scores ranging from 10 to 40. The lower the score, the higher the risk for pressure injury (Choi, 2013).
THERMAL IMAGING
Research has shown that changes in temperature frequently occur before there are changes in skin color. Thermal imaging has therefore become an established tool in the assessment of deep tissue pressure injury (DTPI). Human skin produces infrared radiation, which allows long-wave infrared thermography (thermal imaging) to detect changes in skin temperature. It has been found that alterations in skin color related to unrelieved pressure more often develop into a pressure injury when the temperature of the area at baseline is below that of the adjacent skin.
In a study of 114 patients in an ICU, thermal imaging was used along with clinical assessment to evaluate anatomical sites at high risk for DTPIs, namely, the coccyx, sacrum, and bilateral heels. Thermal assessments were performed using the Scout Device, which is FDA approved for this procedure. Detecting early changes in skin temperature before there were any visible signs of DTPI allowed for proactive interventions, and data from the study demonstrated about a 60% reduction in the number of DTPIs compared to the unit’s usual rate.
Study authors pointed out that thermal imaging can result in significant cost savings, reduced expenditure in treating pressure injury, and reduced settlements related to legal liability for hospital-acquired pressure injury. Clinicians require training in the correct use of thermal injury equipment (Koerner, 2019).
Another study demonstrated that infrared thermography has the ability to identify tissue damage one day before visual discovery of a pressure injury. It was found that the infrared thermography model used in the study could perform an objective evaluation of subcutaneous tissue abnormalities. Early-stage detection allows for rapid implementation of preventive measures (Jiang, 2022).
PRESSURE INJURY PREVENTION
It is more cost efficient to prevent a pressure injury than to cure one. Interventions that help the clinician prevent pressure injuries include:
- Minimizing pressure through regular repositioning
- Using a support surface for body weight distribution
- Managing incontinence to prevent skin damage from moisture
- Managing nutrition and hydration to support the body in preventing damage and healing any damage that has occurred
(Baranoski & Ayello, 2020; EPUAP/NPIAP/PPPIA, 2019; WOCN, 2022a)
COMMON QUESTIONS ABOUT PRESSURE INJURIES
Q: Is it always possible to prevent or avoid a pressure injury?
A: In the past, clinicians have argued that pressure injuries are not avoidable when the patient is too sick to be turned; when there are more vital organs to worry about than the skin; or when it is too difficult, expensive, or there is not enough staff to implement all preventive measures. Yet in 2008, the Centers for Medicare and Medicaid Services (CMS) determined that hospital-acquired conditions could be reasonably prevented with evidence-based guidelines and stopped reimbursing hospitals for the treatment and care of pressure injuries that were not present on admission.
To the contrary, in their position statement on avoidable versus unavoidable pressure injuries the Wound, Ostomy and Continence Nurses Society has stated: “Given the clinical complexities and constellation of comorbidities commonly encountered in today’s healthcare environment, it is reasonable to state that not all pressure ulcers/injuries are avoidable or preventable” (Schmitt, 2017).
Regular Repositioning and Early Mobilization
While the underlying cause and formation of pressure injuries is multifaceted, by definition a pressure injury cannot form without pressure on the tissue. Thus, immobility is the most significant risk for the development of pressure injuries. High pressures over bony prominences for a short time and low pressures over bony prominences for a long time are equally damaging (Baranoski & Ayello, 2020). In order to decrease the risk, it is important to reduce the time and amount of pressure the patient is exposed to.
All patients must have their positions changed on a regular schedule. How often this is done is determined by each patient’s activity/mobility level, tissue tolerance, skin condition, overall medical condition, treatment goals, type of pressure redistribution support surface used, and the comfort of the patient (Baranoski & Ayello, 2020; WOCN, 2022a).
Physical therapists may be involved in all aspects of pressure injury prevention, including the initial patient assessment, evaluation of patient mobility, positioning-related recommendations, and recommendation and implementation of treatment/interventions. An occupational therapist can also provide interventions for transfers, skill training for mobility, and independence skills for hygiene and toileting. Skills learned from these therapists can reduce incontinence and immobility, which can reduce the risk of pressure injury development (Baranoski & Ayello, 2020).
INTERDISCIPLINARY APPROACH
Improving the mobility of patients, or mitigating the effects of immobility, requires the assistance of many in the healthcare team:
- Primary care nursing staff: Assess for all risk factors; see that needed interventions are provided; reassess outcomes frequently
- Physical therapists: Evaluate functional mobility and/or provide wound evaluation/treatment for patients with potential or actual pressure injury; provide interventions including direct wound care, optimization/maintenance of joint range of motion, strength training, seating and positioning recommendations, evaluation of support surfaces, functional mobility and/or balance training, gait training, and/or assistive device recommendations/training
- Occupational therapists: Address lifestyle factors that can lead to increased incidence of pressure injuries, including assessing the patient’s cognitive and functional capacity and recommending adaptive equipment and interventions to meet individual patient needs
- Medical equipment department: Determine what equipment is available for the patient
- Social workers: Uncover what resources are available to the patient
(Baranoski & Ayello, 2020)
BED-BOUND PATIENTS
For bed-bound patients, the standard “turn every two hours” may be more than adequate for some but not at all adequate for others. Evidence suggests that turning/repositioning every four hours, when combined with a pressure-redistributing mattress, is as effective for prevention of pressure injury as repositioning or turning every two hours (WOCN, 2022a).
It is important to keep in mind that when lateral rotation mattresses are used for pulmonary and cardiovascular care, such rotation does not off-load the skin; the patient must still be repositioned off the bed surface and the skin checked frequently. Lateral rotation is a characteristic of specialized mattresses that rotate a patient around a longitudinal axis in a recurring sequence. Lateral rotation places the patient in positions that maintain one lung higher than the other. The goal of lateral rotation is to prevent pneumonia, not to treat or prevent pressure injury (Baranoski & Ayello, 2020).
If the medical condition is so severe that repositioning the patient regularly is not possible, then a support surface designed to decrease pressure must be used and the patient repositioned with frequent small shifts (e.g., mini or low-angle turns, elevating heels off the bed, repositioning the head and extremities every hour, and passive range of motion). (See also “Using Support Surfaces” below.)
ALTERNATING PRESSURE OVERLAY
Patients who undergo long, complex surgical procedures are at particularly high risk for hospital-acquired pressure injury occurrence due to prolonged immobility on a relatively hard surface. Recent studies have examined the use of a dynamic alternating pressure (AP) overlay in both the operating room setting and in intensive care units. The overlay facilitated low-profile recurrent tissue off-loading. The low-profile creation of the overlay accomplished micro pressure relief and diminished shear forces while minimizing significant shifts in the surgical site. In the ICU setting, the AP overlay was positioned on the low–air loss beds that were normally used.
Results of the studies show that when used in conjunction with standard pressure injury prevention protocol, the AP overlap decreased pressure injury occurrence, decreased cost, and validated staff satisfaction (Pitman, 2021).
When turning the patient, clinicians often think that the patient must be completely over on a side. This can be difficult for the clinician/caregiver to do, is uncomfortable for the patient, can result in cardiopulmonary compromise, and can increase pressure on the side of the body.
Instead, frequent small position changes, rather than completely turning the patient, is faster, easier, and safer for all. Any change in position is beneficial. The patient need only be tilted to the side, no more than 30 degrees, with pillows or wedges to help support and reduce the pressure over bony prominences. A small pillow behind the shoulder or the hip alters position without having to move the entire body. Bending the knee alters the pressure on the sacrum and hip. A pillow between the knees prevents pressure when one bony prominence is lying directly on top of another. A small pillow behind the heel will elevate the heel off the surface and prevent pressure.
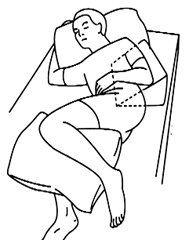
A small turn using a bolster can be as effective as a full turn in repositioning the patient. (Source: C. Melter.)
NPIAP provides the following general recommendations for repositioning patients in bed:
- Reposition the patient in such a way that pressure is relieved or redistributed.
- Avoid positioning the patient on bony prominences with existing nonblanchable erythema.
- Avoid subjecting the skin to pressure and shear forces, and use manual handling aids to reduce friction and shear. Lift—do not drag—the patient while repositioning. Dragging the patient will cause skin damage due to friction. In most situations, simple devices like lift sheets can be used.
- Use a split-leg sling mechanical lift device when available to transfer a patient into a wheelchair or bedside chair when the patient needs total assistance to transfer. A split-leg sling incorporates wide straps that circle the legs. This device allows for comfortable positioning of the patient and has the added advantage of the patient’s knees not being pushed together, which is the typical position in a hammock sling (Preferred Health Choice, 2019).
- Do not leave moving and handling equipment under the patient after use unless the equipment is specifically designed for that purpose.
- Avoid positioning directly onto medical devices such as tubes, drainage systems, or other foreign objects.
- Do not leave the patient on a bedpan longer than necessary.
- Use principles of safe patient handling to prevent injury to both the patient and the staff.
Additional recommendations for repositioning in bed include:
- Use the 30-degree tilted side-lying position, alternating between right side, back, left side, or prone position if patient can tolerate this and the medical condition allows.
- Encourage individuals who can reposition themselves to sleep in a 30- to 40-degree side-lying position or flat in bed if not contraindicated.
- Avoid lying postures that increase pressure, such as a 90-degree side-lying position or the semirecumbent position.
- Limit head-of-bed elevation to 30 degrees for an individual on bedrest unless contraindicated by medical condition or feeding considerations. If not contraindicated, lower the head of the bed one hour after eating or intermittent bolus tube feedings (WOCN, 2022a).
- If sitting in bed is necessary, avoid head-of-bed elevation or a slouched position that places pressure and shear on the sacrum and coccyx.
For a patient with an existing pressure injury:
- Do not position the patient directly on the injury or on areas of nonblanchable redness or deep tissue injury; pressure reduces perfusion to the injured tissues and will delay healing and may cause deterioration of the wound.
- Continue to turn and reposition the patient regardless of the support surface in use.
- Inspect the skin for additional damage each time the patient is turned or repositioned.
Preventing Heel Pressure Injuries
The reduction of pressure and shear at the heels is very important in clinical practice. Studies indicate the heels are the most common place for the occurrence of deep tissue pressure injury (DTPI), and they are the second most common sites for the occurrence of all pressure injury (WOCN, 2022a). The posterior heel sustains intense pressure even when a pressure reduction surface is used. Because the heel has so little tissue, the pressure is transmitted directly to the bone. Patients diagnosed with diabetes mellitus and those with diminished circulation have a significant risk for the development of heel pressure injuries (Greenwood, 2021).
Ideally, heels should be free of all pressure, sometimes called “floating” the heels. Pressure can be relieved by elevating the lower leg and calf from the mattress by placing a pillow under the lower leg or using a suspension device that floats the heel. The pressure will then be spread to the lower leg, relieving the heel. The recommended position for the pillow is lengthwise under the calf, without hyperextension of the patient’s knee, and with the heel suspended off the pillow. Consideration should be given to the size of the pillow used. If it is too thin, offloading of the heel may not be achieved (Greenwood, 2021). The patient must still be turned at regular intervals to promote pulmonary, renal, and vascular function along with protecting skin integrity.
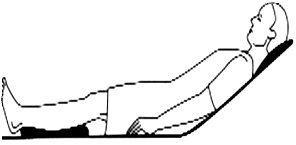
Heels are properly floated. (Source: C. Melter.)
Padding devices such as synthetic sheep skin, fleece-lined “bunny boots,” and rigid splints protect the heels and remove friction and shear but do not remove the pressure, and regular skin checks are still required. Common devices such as intravenous bags, rolled towels or sheets, cut-out rings, and water-filled gloves are not designed to redistribute pressure and can actually increase pressure.
All appropriate heel pressure–relieving devices are used in conjunction with good nursing care, including frequent, individualized heel inspections. Patients are reminded to promptly report any heel discomfort, and this is followed by an immediate assessment by the clinician (Baranoski & Ayello, 2020; EPUAP/NPIAP/PPPIA, 2019).
PROPHYLACTIC DRESSINGS
A polyurethane foam dressing can be applied prophylactically to bony prominences (e.g., heels, sacrum) to reduce friction, shearing, and moisture damage and thereby help prevent pressure injuries in anatomical areas frequently subjected to friction and shear.
Trials have indicated that the application of specialty foam dressings to the sacrum is effective in reducing the rate of pressure injury in ICU patients and in nursing home residents who are at high risk for pressure injury development. Research also indicates that the application of multilayer foam dressings to the sacrum of patients before undergoing cardiac surgery greatly reduces the incidence of pressure injury in the immediate postoperative period (first five days in the ICU and progressive care units) (Strauss et al., 2019; EPUAP/NPIAP/PPPIA, 2019).
Two recent systemic reviews and meta-analysis of published trials support the use of foam dressings in reducing HAPI in patients in acute settings, in particular sacral and heel pressure injuries. Using prophylactic foam dressings is believed to diminish shear and friction and decrease the temperature and moisture in localized skin microclimate. To date, no study has determined if a specific brand of foam dressing is more successful in pressure injury prevention compared to its counterparts. The varying composition and structure of foam dressings may effect mechanisms such as shear, friction, and microclimate (Sillmon, 2021; Sugrue, 2023).
Considerations when selecting a prophylactic dressing include:
- Ability of the dressing to manage microclimate
- Ease of application and removal
- Ability to regularly assess the skin
- Anatomical location where the dressing will be applied
- The correct dressing size based on area to be protected
Foam dressings have a greater ability to absorb moisture than film or hydrocolloids and often have easy-to-lift borders. They are also permeable to water vapor and gases. Some dressings adhere well but can damage fragile skin on removal. All other preventive measures must be continued along with the dressings; the skin must still be inspected daily and the dressings replaced as needed (Ayello & Baranoski, 2020; EPUAP/NPIAP/PPPIA, 2019).
CHAIR-BOUND PATIENTS
A chair-bound patient must be repositioned as well. When a patient is seated, the weight of the body causes the greatest amount of pressure to occur over the ischial tuberosities. Since this area of the body is relatively small, the ischia bear intense pressure when a person is seated; without pressure relief, a pressure injury will occur quickly. If the patient cannot sit upright but slouches in the chair, then the sacral area is at risk as well. Pressure remains unrelieved in a paralyzed person because the small, involuntary movements that restore blood flow to the tissues are absent.
Specialized wheelchairs that offer a tilt and/or recline option may be indicated for positioning patients at risk of developing pressure injuries. Tilt and recline, though often confused, actually serve distinct and complementary positioning roles. Reclining a chair changes the hip angle and provides some pressure relief, but shearing forces may remain on the back. A tilt-in-space chair both tilts the head back and raises the feet up concurrently, thereby providing more pressure relief and less shearing forces.
Research has found that wheelchairs with tilt-in-space and recline mechanisms positively decrease sitting interface pressure and increase ischial blood flow. The bulk of the patient is moved from the seat of the wheelchair and is supported by the backrest. The degree to which this happens is proportionate to the tilt-and-recline angle of the wheelchair. Data suggest that even a small amount of tilt-and-recline angle can result in pressure relief to the tissues. However, meaningful increases in ischial bold flow were found only at greater angles of tilt-and-recline. At this time, there is no clear agreement on the period of time that tilt-and-recline should be done in order to effectively prevent pressure injury (Zemp et al., 2019).
General recommendations for the chair-bound patient include:
- Stand the patient and reseat them in the chair frequently, if possible.
- Provide adequate seat tilt to prevent sliding forward in the chair and adjust footrests and armrests to maintain proper posture and pressure redistribution.
- Elevate the legs or place the feet on a stool if the feet do not reach the floor in such a way as to slightly tilt the pelvis forward by positioning the thighs slightly lower than horizontally. This will prevent sliding forward out of the chair and reduce pressure on the sacrum.
- Elevate the feet and recline the chair by 30 degrees to reduce pressure.
- If the patient can change their own position, encourage pressure relief every 15 minutes. This includes chair pushups, leaning forward, leaning side to side, or tilting backwards. Leaning forward is the most effective and might be easier than chair push-ups.
- Acutely ill patients at risk for pressure injuries should not sit for longer than two hours at a time and not return to sitting for at least an hour.
- Patients who are incapable of changing their position while sitting should be repositioned at least every hour by a caregiver.
(EPUAP/NPIAP/PPPIA, 2019)
For a patient with an existing pressure injury:
- Minimize sitting time and consult a seating specialist if the injury worsens on the seating surface selected.
- Consider periods of bed rest to promote ischial and sacral pressure injury healing.
- Avoid sitting a patient with an ischial pressure injury in a fully erect posture.
- Patients with existing pressure injuries on the ischial areas should limit time sitting up in the chair to three times a day for 60 minutes or less, and they must use a cushion (gel or air cushions are best) that redistributes pressure.
(WOCN, 2022a)
A Cochrane review of literature found no studies that examine the effectiveness of pressure-redistributing static chairs in the prevention and treatment of pressure injuries. Pressure-redistributing static chairs encompass a range of products, from hospital chairs to those with integrated pressure-redistributing surfaces and tilt functions. The authors of the study noted this as a “priority area” for research (Stephens, 2022).
PHYSICAL AND OCCUPATIONAL THERAPY AND WHEELCHAIR POSITIONING
Physical and occupational therapists are of great importance in assessing and managing the immobile patient’s activities and instructing staff, patients, and families in proper techniques to prevent pressure injuries. This may include assessing the seating and positioning needs of individuals who are wheelchair-dependent. Proper wheelchair positioning with an individualized seating system can promote good posture, enhance breathing and digestion, prevent complications such as pressure injuries and skin irritation, slow further loss of mobility, minimize pain, and maximize functioning.
Components of a wheelchair seating system include appropriate size and width as well as specialized supportive cushions, backrests, headrests, and trunk, arm, and leg supports when indicated. The depth of the wheelchair, measured from back to front, must be adequate to provide support from the buttocks to the back of the knees. The floor-to-seat height, measured from floor to seating surface, must be sufficient to keep the patient’s thighs in a position parallel to the floor, with hip flexion as close to 90 degrees as possible (WOCN, 2022a).
PATIENTS WHO ARE UNABLE TO MAINTAIN REPOSITIONING
Some patients are unable to maintain position changes, such as patients with dementia or delirium. Since delirium diminishes a patient’s ability to recognize pressure and discomfort, it is recognized as a potential risk factor for pressure injury development. Delirium has been found to affect up to 50% of hospitalized older adults, depending on the healthcare setting and pre-existing conditions (WOCN, 2022a).
Since delirium is reversible, every effort must be made to find the underlying cause, the most prevalent being:
- Pre-existing dementia
- Postoperative delirium
- Medications
- Dehydration and electrolyte disturbances
- Infection (e.g., urinary tract infection)
- Reduced sensory input
(WOCN, 2022a; Health in Aging, 2020)
Around 5%–8% of all persons over the age of 65 years suffer from some type of dementia. Beyond this age, the number of those with dementia doubles every five years. It is projected that close to half of those 85 years and older have some form of dementia, which places them at a significantly high risk for pressure injury development (Cleveland Clinic, 2023).
Each patient will have different risk factors for pressure injury, and each plan of care must be individualized. Pressure injury prevention for confused patients requires a holistic approach. Interventions include:
- Assessing whether the patient’s position looks comfortable:
- Is the patient’s shoulder jammed against the bed?
- Is the side-lying position more than 30 degrees?
- Is the sheet under the patient wrinkle-free and free from debris such as crumbs?
- Placing the patient in a quiet environment
- Minimizing bright lights
- Controlling the temperature
- Promoting rest and comfort (e.g., is the patient hungry, thirsty, in pain, or in need of toileting?)
(WOCN, 2022a; Vasquez, 2022)
PATIENTS WHO REFUSE CARE
Some patients may refuse care, refuse to be repositioned, refuse to use a pressure-relieving cushion in their chair, or refuse to participate in PT or OT. The first and most important action by the clinician is communication: Why is the patient refusing care?
- Is it related to their diagnosis, for example, a traumatic injury such a spinal cord injury, a chronic condition such as rheumatoid arthritis, or a condition with an uncertain progression, such as multiple sclerosis?
- Is the patient exhibiting signs and symptoms of depression, such as irritability, fatigue, or hopelessness?
- Is a referral to a mental healthcare professional needed?
Open, honest communication with the patient and, if appropriate, their caretakers should address the importance of pressure injury prevention and the consequences of not implementing recommended interventions. When discussing potential complications of refusing care, clinicians must be candid and accurate in their descriptions of the complications so that the patient is left in no doubt about the seriousness of the decision they are making in refusing care and to protect the facility and clinicians against liability and future legal action.
Full, accurate, and objective documentation in the patient’s record is essential. The medical record must show:
- Education provided to the patient
- Frequency of patient education
- Consistent approach among clinicians
- Other interventions, such as involvement of the facility’s ethics committee
(Pirotte, 2022)
It is the patient’s right to refuse care, but clinicians must ensure that facility policies and procedures are followed and that the patient is afforded every option to receive care.
Strategies clinicians can employ when treating patients who refuse care include:
- Empathize with the patient. Allow them to verbalize their feelings about their injury or condition. Remember that a loss of control in one’s life is frightening to everyone. Be a good listener.
- Avoid dictating and assuming control. Rather than telling the patient they are going to be repositioned, ask them if they would be more comfortable receiving help to change position themself.
- Look for the root cause of why the patient is refusing care. Remember that in the majority of cases there is a reason why the patient is not compliant with care.
- Ask the patient if there is anyone they would like to talk to, such as a close friend, a spiritual advisor, or someone who has had a similar traumatic injury.
- As a clinician, do not take the patient’s refusal of care personally. Do not allow personal emotions to interfere with patient interactions.
- Aim for small steps. If the patient will agree to a pressure-relieving cushion in a chair, it is a step in the right direction.
- Continue to educate the patient about why pressure relief is important.
- Remember that the patient is the primary member in the care team and continue to elicit their input on care.
- Remain friendly and caring in word and action; it is a known fact that patients cooperate more readily with clinicians they like.
(James, 2023; Bennett, 2020)
CASE
Patricia is a 61-year-old female with multiple sclerosis, leaving her bedridden and unable to move her legs. Despite being on a pressure reduction surface, she has developed a stage 3 pressure injury at her sacrum because of refusing to be turned due to the severe pain she experiences from muscle spasms that are triggered each time her right leg is moved. This has made it very difficult for the staff to provide wound care and keep Patricia clean. Furthermore, pain medication has not been effective for Patricia’s very intense but brief episodes of pain.
The nurse asks the physical therapist for recommendations to make moving Patricia less painful for her and less stressful for the staff. After evaluating Patricia, the therapist recommends daily PT treatment, including localized heat treatments to her right leg and gentle active-assisted range of motion to her trunk and extremities prior to attempting bed mobility. PT sessions also focus on improving Patricia’s independence with functional bed mobility (as tolerated).
After several treatment sessions, Patricia is able to tolerate and actively assist with turning toward her right side and staying in position for the time needed to care for her wound and assist her with personal hygiene. The therapist also instructs the staff about less painful ways to assist Patricia with bed mobility and positioning to allow wound care, in order to minimize discomfort to her lower extremities. As a result, Patricia is able to tolerate and even actively assist in turning from side to side, and her leg pain when repositioned is reduced to a more tolerable level.
Using Support Surfaces
Factors in the development of pressure injuries include prolonged pressure, friction/shear, and moist, warm skin. Each of these factors can be at least partially controlled by an appropriate surface for the bed and/or chair. A support surface is a specialized mattress or mattress overlay, chair cushion, or stretcher/operating room pad designed for the management of pressure loads and microclimate. (Microclimate is the term used to describe the local tissue temperature and moisture at the body/support interface, and microclimate control is a function of some support surfaces.) (See also “Emerging Therapies” later in this course.)
Pressure redistribution is the most important feature of a support surface. The body’s tissues can withstand higher loads of pressure for short periods of time and lower loads for longer periods of time. A surface that effectively redistributes pressure across the entire body (contact) surface effectively reduces the amount of pressure and extends the time a patient can safely remain in one position (WOCN, 2016b; EPUAP/NPIAP/PPPIA, 2019).
It is critical to remember, however, that there is no mattress, cushion, or bed available today, at any price, that will eliminate pressure and relieve the clinician or caregiver from having to reposition the patient. Patients must still be repositioned no matter what surface is used. Likewise, pressure is not the only contributing factor to skin breakdown and does not replace attention to perfusion, nutritional support, and management of comorbidities (WOCN, 2022a).
COMMON QUESTIONS ABOUT PRESSURE INJURIES
Q: Can a support surface be used at home to provide pressure relief?
A: Yes, a support surface can be used at home to provide pressure relief. If possible, seek the assistance of a wound care nurse, physical therapist, or occupational therapist when choosing a pressure relief surface to use at home. Depending on which pressure-relief surface is chosen to best meet the patient’s needs, Medicare, Medicaid, or private insurance may pay for all or part of the cost.
Q: Which is the best pressure relieving mattress for home use to prevent pressure injury development?
A: The first step is to discuss concerns with the healthcare provider. When choosing a pressure relieving surface, it is also beneficial to have a wound care/pressure injury prevention specialist complete a home assessment and assist with recommendations. The best pressure-relieving surface for home use will depend on several factors such as the type of bed that the pressure relieving mattress will be placed on and the patient’s mobility status. If a patient requires a pressure relieving surface while in bed, they will probably also need a pressure relieving surface/cushion when out of bed.
IMMERSION, ENVELOPMENT, AND BOTTOMING OUT
In order to redistribute pressure, a support surface must conform to the contours of the body through immersion and envelopment. Immersion is the depth to which the body “sinks into” the surface. As the body does this, the pressure is spread out along the body surface. Immersion is dependent on the stiffness and thickness of the support surface and the flexibility of its cover.
Envelopment is the ability of the support surface to conform to irregularities such as clothing, bedding, and bony prominences without causing substantial increase in pressure. This maximizes pressure redistribution.
In contrast to the functions of immersion and envelopment, the term bottoming out is used to indicate excessive penetration of the surface, meaning the body sinks so deeply into the surface that its bony prominences are actually resting on the underlying bed frame. Factors that contribute to bottoming out include:
- Patient weight that exceeds the support surface’s limits
- A disproportion between weight and size, such as in a patient with bilateral leg amputations, which results in more of the body weight being concentrated in the trunk
- Consistently keeping the head of the bed over 30 degrees
- Inadequate support settings such as under- or overinflation
For some support surfaces, bottoming out can be evaluated by placing one’s hand palm-up beneath the support surface in the area underlying the patient’s bony prominence. If the patient’s bony prominence can be felt by the hand, then the support surface is not supporting the patient properly. However, many support surfaces cannot be assessed this way; the clinician must contact the equipment department of the facility or the supplier or manufacturer of the support surface for information on how to assess the functioning of the support surface.
COMPONENTS OF A SUPPORT SURFACE
The most important component of a support surface is the medium used to provide the pressure redistribution. This can be air, fluid, or solid, alone or in combination.
Foam is a solid material and is available in all configurations. Foam surfaces are generally low-cost and lightweight and require minimal maintenance. Disadvantages include the fact that foam does not last long since it compresses over time; it absorbs moisture (which can be a potential for infection); and it is hard to dispose of. Closed-cell foam does not allow air through, which can increase skin temperature, while open-cell foam does allow air to enter and exit, making it more conformable to the body. One type of open-cell foam is memory foam. If a foam pad is used on top of a mattress (known as an overlay) to redistribute pressure, it needs to be at least three inches thick.
Gel pads contain a mix of substances that allow them to respond like memory foam. They are good at preventing shear, but they can result in increased skin moisture.
Fluid support surfaces include a viscous substance that is thick but free-flowing, which allows it to redistribute weight. Water-filled surfaces reduce pressure better than a standard mattress but are undesirable for use in a hospital due to multiple concerns, such as temperature control, leakage, difficulty with transfers, performance of CPR, and the time and labor involved in draining the mattress and moving the bed.
Air is frequently used in support surfaces; however air-filled surfaces have the potential to leak if damaged and require either periodic manual reinflation (if nonpowered) or an electric pump to remain inflated. Studies show that low–air loss surfaces prevent the accumulation of moisture and resultant skin maceration (Baranoski & Ayello, 2020; EPUAP/NPIAP/PPPIA, 2019).
Some support surfaces have low-friction covers (e.g., Gore-Tex) to reduce friction so that the skin slides more easily over the surface without putting strain on the skin that could cause damage. However, even these support surfaces cannot provide total prevention against the shearing that occurs when the patient slides down in bed when the head is raised; other interventions are needed to prevent that (WOCN, 2022a; McNichol 2020).
COMMON QUESTIONS ABOUT PRESSURE INJURIES
Q: When selecting a foam overlay for pressure relief, does the thickness matter?
A: Yes, to provide pressure relief, the foam overlay should be at least three inches thick. A thinner overlay may provide comfort but will not properly distribute pressure.
CATEGORIES OF SUPPORT SURFACES
Support surfaces are commonly used in a variety of applications:
- Mattresses and mattress overlays
- Operating room bed surfaces
- Examination and procedure table surfaces
- Pads for emergency and transport stretchers or gurneys
General categories of support surfaces include mattresses, overlays, and integrated bed systems. Specific features include cushions and pads. They may be powered or nonpowered, or active or reactive. Added features may include low–air loss, air-fluidization, lateral rotation, and alternating pressure (see below).
Rings, foam cutouts, or donuts under the patient should not be used as support surfaces, as these concentrate pressure on surrounding tissue, causing swelling and decreasing circulation. The fact that they can be found in medical supply stores does not mean they are safe to use.
With the use of any support surface, the number of linens and other items used under the patient must be kept at a minimum or the pressure-reducing ability of the surface will be altered significantly. Staff, patients, and family members must be instructed to use no more than two items between the patient and the surface (e.g., one pull sheet and one incontinence pad or product).
General Categories
Mattresses can be composed of any medium or a combination, and may require a specialized bed frame. They create much less risk of bottoming out and can provide other therapeutic functions such as reduced friction and shear and improved microclimate management between the patient’s skin and the surface.
Overlays can be composed of any medium and are placed on top of an existing mattress. They are thinner than mattresses, putting the patient at risk for bottoming out. They may also include other drawbacks, such as elevating the height of the sleep surface, complicating patient transfers, altering the fit of linens, and increasing the risk of falls or entrapments.
Integrated bed systems are composed of the support surface and bed frame combined into a single unit. Their advantage is that the frame may include many features to make the bed easier and safer to use, such as alarms, scales, and the ability to support more weight.
Specific Categories
Procedure, transport, ER, and OR mattresses are used for patients who require a support surface in bed, since this means they also need one while on gurneys or tables. Many companies provide pressure redistribution pads for surfaces other than beds.
Chair cushions are utilized for patients who sit for a long time in order to reduce the risk of ischial pressure injuries. These cushions must be matched to the patient based on size, posture, mobility, and lifestyle needs. Some cushions include covers that can dissipate heat, while some specialized wheelchair cushions also address incontinence.
COMMON QUESTIONS ABOUT PRESSURE INJURIES
Q: Do regular household cushions provide pressure relief?
A: A regular cushion can provide comfort; however, they are not designed for pressure relief. For patients who sit for long periods of time and have limited ability to change position, a pressure-relief cushion should be used.
Active surfaces can be either a powered mattress or an overlay that changes its load distribution whether or not someone is on the surface (called alternating pressure). The air cells in such surfaces cyclically inflate and deflate, which changes the areas of the body under pressure. These are recommended for patients at high risk and for whom frequent manual repositioning is not possible.
Reactive surfaces move only in response to the patient’s body. These can be powered or nonpowered (nonpowered also being referred to as static air surfaces). These are low-tech, compact, and light-weight. They are available as chair cushions, overlays, mattresses, and procedure pads. All reactive support surfaces are appropriate for pressure injury prevention in patients who are frequently repositioned. Some are appropriate for patients with existing pressure injuries.
A review of Cochrane data undertaken in 2019 indicated that using reactive air surfaces may lessen the possibility of pressure injury occurrence when compared to the use of foam surfaces. When looking at time-to-event outcomes, the review also found that in nursing home environments, the use of reactive air surfaces may diminish the risk of pressure injury development within a 14-day timeframe compared with active air surfaces. However, current evidence on the effectiveness of reactive air surfaces compared to other forms of pressure relief surfaces remains uncertain (Shi, 2021).
Low–air loss surfaces have a pump that provides a slow, continuous airflow into the mattress for even pressure distribution and continuous airflow across the skin for microclimate management. The amount of pressure in the mattress can be adjusted for the height and weight of the patient, and the mattress further adjusts when a patient sits up in bed to prevent bottoming out. Controls allow instant deflation for CPR. These surfaces cannot be used on patients with unstable spines.
Air-fluidized surfaces contain silicone-coated beads that provide both air and fluid support. When air is pumped through the beads, the beads behave like a liquid and the patient floats, with two thirds of the body immersed in the warm, dry beads. When the bed is turned off, it becomes hard enough for repositioning or CPR. Some beds now combine air-fluidized therapy in the lower half of the bed and low air loss in the upper half, allowing the bed to be adjustable. This is a very expensive therapy and should only be used for patients who require very high-level care such as those with multiple wounds or flap procedures (a surgical procedure to close a pressure injury). The beds are also extremely heavy and may not be safe in a home.
Lateral rotation is used to prevent and treat certain cardiopulmonary conditions. With this feature, the patient is continually rotated from side to side. While some low–air loss beds may incorporate lateral rotation, this does not eliminate the need for routine manual repositioning. This is because when the bed turns the patient, the patient’s skin and tissues never leave the surface of the bed, and thus pressure is not relieved (WOCN, 2022a; Baranoski & Ayello, 2020).
CHOOSING A SURFACE
A support surface must be selected to meet the patient’s needs for pressure redistribution based on the following factors:
- Level of immobility and inactivity
- Need for microclimate control (see below) and shear reduction
- Size and weight of the patient
- Risk for development of new pressure injuries
- Number, severity, and location of existing pressure injuries
(WOCN, 2022a; EPUAP/NPIAP/PPPIA, 2019; Baranoski & Ayello, 2020)
Other factors to consider include:
- Fall and entrapment risk (overlays and mattresses can increase the height of the sleep surface)
- In a patient who is a candidate for progressive mobility, a surface that makes it easier to get out of bed
- Compatibility of the surface with the care setting
- Availability of the product
- Previous support surface usage and patient preference
- Achievement of specific outcomes (i.e., prevention of pressure injury)
(WOCN, 2022a; EPUAP/NPIAP/PPPIA, 2019; Baranoski & Ayello, 2020)
Determining the appropriate support surface is based first on the patient’s condition and the healthcare setting. Overall, if the patient is able to be turned and has at least two intact turning surfaces, meaning the skin is intact on two sides of the body (e.g., the right and left trochanters), then a mattress overlay or an alternating pressure pad can be used over a regular mattress. If the patient has skin breakdown on more than one side of the body, then a mattress replacement should be used. Depending on the healthcare setting, patients who already have stage 3 or 4 pressure injuries on their trunks qualify for a mattress replacement.
Cost and product availability are secondary considerations in choosing a surface. The healthcare setting will also determine the product used. For instance, in the home setting, the weight of the bed, the structure of the home, the width of the doors, and the availability of uninterrupted electrical power will have a major impact on the support surface available for use. A patient’s health insurance may be a significant determining factor as to which surface will be available to the patient.
In general, a standard hospital mattress should not be used with at-risk patients. It is important to contact the medical equipment department to determine what is available for pressure reduction (WOCN, 2022a).
Microclimate Control
Microclimate is the term used to describe the local tissue temperature and moisture at the body/support interface, and microclimate control is a function of some support surfaces. This feature can be of benefit to patients who are diaphoretic (sweat heavily) or with elevated temperatures and perspiration, since they are at increased risk for developing pressure injuries. For instance, older adult patients have a reduced ability to dissipate excess heat, resulting in skin warming. High levels of moisture from perspiration, incontinence, or drainage result in maceration, which reduces skin strength and increases damage caused by friction (WOCN, 2022a; EPUAP/NPIAP/PPPIA, 2019; Baranoski & Ayello 2020).
The need for moisture and temperature control is considered when selecting a support surface. A support surface with microclimate control can help maintain normal skin hydration and temperature through the use of porous covers that promote air transfer between the skin and surface, which results in the decrease of moisture and body heat. Other surfaces pump air through microperforations in the support cover to decrease moisture and heat (WOCN, 2022a).
Fabrics
Silk-like fabrics rather than cotton or cotton-blend fabrics can be used to reduce friction and shear. Research has shown that the use of silk-like fabric undergarments, booties, and linen (alone or in combination) significantly reduces the incidence of pressure injury development and reduces the deterioration of existing pressure injuries (EPUAP/NPIAP/PPPIA, 2019).
A study conducted with 112 residents over 65 years of age in a care facility demonstrated the benefits of using a “breathable,” silk-like, three-layer ventilating mattress sheet. The residents in the sample were all deemed at high risk for pressure injury occurrence, were on pressure-reducing surfaces covered with a cotton sheet, and were unable to change position independent of staff. At the end of the 12-week study, almost 70% of the subjects were able to complete independent position changes in bed. There was also a significant reduction in pressure injury occurrence compared to residents who were placed on cotton sheets.
There is a high friction coefficient with cotton sheets, which makes repositioning difficult for patients and requires staff assistance. An advantage of the three-layer ventilating mattress sheet is its reduced friction coefficient compared to its cotton counterpart, resulting in less effort needed to make position changes. Combining the lower friction coefficient with therapy to increase muscle strength and improve coordination increases patient ability to perform self-repositioning. This in turn decreases the risk for pressure injury development and decreases staff workload (Van Leen, 2022).
Patient Size and Weight
The support surface chosen must be approved for the patient’s body size and weight. Most conventional support surfaces are for patients who weigh 300 pounds or less. If the patient weighs more than this, then a bariatric mattress and frame must be used.
Even in patients who weigh less than the weight limit, where their weight is concentrated may make a difference in the mattress needed. The mattress may not be able to support the patient when the body weight is not evenly distributed. For instance, a paralyzed patient or an amputee may weigh under the limit, but most of the weight will be concentrated in the trunk. If it looks as though the patient is lying or sitting in a “well,” the surface may not be able to support the patient’s weight.
In the event a surface does not appear to be supporting a patient’s weight—and provided the support surface is the correct one—it is important to check for any disconnected hoses or improper machine settings. It may also be necessary to contact the appropriate hospital department, supplier, or manufacturer to have the support surface inspected as needed. It is also important to ensure that all staff caring for the patient are aware of the features of the support system (EPUAP/NPIAP/PPPIA, 2019; WOCN, 2022a; Baranoski & Ayello, 2020).
Managing Moisture
Moisture can lead to skin damage of various types. Incontinence-associated dermatitis (IAD) is the most common form of moisture-associated skin damage and is frequently misidentified as a pressure injury. While they are not the same thing, preventing IAD can also help to prevent the formation of pressure injuries.
TYPES OF SKIN DAMAGE DUE TO MOISTURE
Moisture-associated skin damage (MASD) is defined as inflammation and erosion of skin caused by prolonged exposure to various sources of moisture, including urine or stool, perspiration, wound exudate, mucus, or saliva. The four forms of MASD are:
- Incontinence-associated dermatitis (IAD)
- Intertriginous dermatitis (ITD)
- Periwound moisture-associated skin damage
- Peristomal moisture-associated skin damage
(WOCN, 2022a; EPUAP/NPIAP/PPPIA, 2019)
Incontinence-associated dermatitis due to urinary incontinence may involve the perineum and the area between the vulva and scrotum or anus. IAD due to fecal incontinence may involve the anus, buttocks, coccyx, perigenital areas, groin folds, and posterior thigh regions. Its characteristics are moist, bright-red skin, inflammation, denudement (skin stripped raw), erosion, and blisters. The damage is usually superficial but can progress to full-thickness lesions. The patient will complain of burning, pain, and itching.
Moisture alone causes maceration, but acute inflammation and skin loss are due to a combination of maceration and some other source of injury, such as a chemical irritant, friction, or pathogenic invasion. Wet skin loses strength and is more prone to damage from friction and shearing. It allows irritants to penetrate the epidermal layers of skin, which then allows common pathogens such as Candida and Staphylococcus to enter (WOCN, 2022a).
Skin injury by friction appears as redness and progresses to abrasion. It occurs when skin is rubbed vigorously during cleaning or when skin rubs against incontinence garments or bed or chair surfaces. Areas of skin rubbing against each other cause “kissing” lesions such as are seen between the buttocks cheeks.
Mechanical damage (friction) abrades and disrupts the skin from the “top down,” whereas pressure and shear cause blood vessel compression and ischemic damage from the “bottom up.” Determining the cause of the lesion—and distinguishing between IAD and a pressure injury—includes an assessment of the location, characteristics, and most importantly, the patient’s history.
| Friction (top down) |
IAD (top down) |
Pressure/Shear (bottom up) |
|
|---|---|---|---|
| (WOCN, 2022a) | |||
| Location | Fleshy surfaces exposed to repetitive rubbing | Perineal area, inner thighs, buttocks | Bony prominences, under medical devices |
| Depth | Superficial | Superficial | Full thickness |
| Characteristics | Pink/red wounds without necrosis | Edges indistinct, fungal rashes common, pink/red wounds without necrosis | Well-defined lesions, tunneling and undermining, tissue necrosis |
| History | Restlessness, frequent sliding, malnourished, diaphoretic, on steroids | Prolonged or recurrent exposure to urine and/or stool | Prolonged immobility, sliding, use of medical device at site of damage |
MANAGING INCONTINENCE
It is important to cleanse skin gently with a pH-balanced cleanser at each incidence of soiling. Perineal skin cleansers are more effective for prevention and treatment of IAD than traditional soap and water. This is because bar soap, which is alkaline and very drying, disrupts the skin’s protective abilities. Vigorous cleaning, as well as the use of rough washcloths, can also lead to skin erosion. Soft, disposable cloths are easier on the skin. Fragrance or alcohol are irritants, and cleaning products containing these are to be avoided. Some facilities use no-rinse foams, which are another good option.
An incontinence skin barrier product should be used to protect the skin after cleansing. Products such as creams, ointments, pastes, or those that form a film on the skin are all useful. Protective products with dimethicone, petroleum, or zinc oxide are recommended for patients with fecal incontinence or both urinary and fecal incontinence to protect against IAD. Several manufacturers offer products that both clean and protect, saving time for the caregiver and increasing the likelihood that perineal care will be performed.
Absorbent underpads or incontinence briefs are chosen to wick moisture away from the skin instead of trapping the moisture against the skin. However, all briefs increase moisture at the perineal region because they are occlusive and do not “breathe.” This creates warmth near the skin that, when combined with moisture, ammonia, and enzymes, increases skin breakdown. There is an increased risk with the use of briefs because they may not be changed as often as they should be due to the difficulty in seeing when a patient has voided. Briefs are not recommended for fecal incontinence because they can trap stool against the skin.
Many hospitals have moved away from using briefs except when a patient is ambulating or going off the unit. Instead, they use underpads that are especially designed to keep the skin dry and breathable and do not allow heat or moisture to be trapped against the skin.
A toileting program can also decrease incontinence and thus IAD. In situations where the severity of urinary incontinence has contributed to or may contaminate an existing pressure injury, placing a urinary catheter may be indicated (WOCN, 2022b).
Much attention has been paid to infections related to the use of indwelling catheters, particularly since 2008, when the Center for Medicare and Medicaid Services changed reimbursement regulations, calling catheter-associated urinary tract infections (CAUTI) “preventable harm” and withholding payment for additional costs related to CAUTI treatment (WOCN, 2022b). Significant bacterial colonization occurs within a few days of catheter insertion, which can lead to infection. The prevention of CAUTI begins with the decision to not insert a catheter.
The CDC has developed guidelines for the appropriate use of indwelling catheters, as follows:
- To manage urinary retention or bladder outlet obstruction
- To provide accurate urine output measurements
- To manage bladder short term following select surgical procedures
- To assist in the healing of perineal wounds at risk of contamination by urine
- To improve comfort at end of life
(WOCN, 2022b)
Therefore, incontinence is not a valid reason for insertion of an indwelling catheter to replace alternative means of care for the incontinent patient. It can be used in the treatment of a wound but not in prevention, since the risk of CAUTI is too high (WOCN, 2022b).
| Type | Description | Causes/Associated Factors |
|---|---|---|
| (WOCN, 2022b) | ||
| Urge incontinence | Involuntary loss of urine with an abrupt strong need to void; occurs with the involuntary contractions of the detrusor muscle or uncontrolled urethral relaxation | Associated neurological disorders include stroke, paraplegia, multiple sclerosis, Parkinsonism, or dementia. |
| Stress incontinence | Involuntary leakage of small amounts of urine with a rise in intra-abdominal pressure that occurs during coughing, sneezing, laughing, and physical activity | Often seen in women; causes include estrogen deficiency, weakness in pelvic floor musculature, urethral sphincter weakness, childbirth, and obesity. |
| Functional incontinence | Having a functional urinary tract but unable or unwilling to get to the toilet to urinate | Often occurs in older adults; contributing factors include use of physical restraints, musculoskeletal dysfunction, unavailability of a urinal, visual impairment, impaired mobility, cognitive deficits, unfamiliarity of environment, and psychosocial difficulties. |
| Overflow incontinence | Involuntary loss of urine secondary to overdistension of the bladder; results in the leakage of small amounts of urine due to an outflow obstruction or a hypotonic bladder | Common causes include medications, neurological conditions such as diabetic neuropathy or spinal cord injury, prostate enlargement, detrusor weakness, or urethra stricture. |
| Mixed incontinence | Combination of other types of urinary incontinence, typically urge and stress incontinence | Common in older adults. |
OCCUPATIONAL AND PHYSICAL THERAPY AND INCONTINENCE
Occupational and physical therapists are both specialists in assessing, identifying, and treating the underlying impairments associated with urinary incontinence. Common interventions that can be implemented by these professionals may include:
- Pelvic floor muscle training (PFMT): A program of repeated pelvic floor muscle contractions, which involves specific and progressive learned exercises to consciously contract the pelvic floor muscles, found to be useful in preventing urinary incontinence, overactive bladder, and postvoid dribbling
- Scheduled toileting (i.e., timed voiding): Monitoring and then matching of the individual’s typical toileting schedule
- Habit retraining: Identifying the individual’s natural voiding pattern and developing an individualized toileting schedule
- Prompted voiding: Establishing a routine in which a caregiver suggests voiding and provides assistance as needed
- Biofeedback: Using an external sensor placed in close proximity to the anal orifice to detect contraction of the pelvic floor muscles in order to help the patient to identify and strengthen those muscles
- Electrical stimulation: Using electrodes placed in either the vagina or rectum to stimulate and strengthen pelvic floor muscles, thereby aiding pelvic floor muscle contractions
Research indicates that the use of a toileting routine in combination with medication and education results in decreasing urinary incontinence.
(APTA, 2022; Vasavada, 2019; WOCN, 2022b)
TREATING FECAL INCONTINENCE
Fecal incontinence occurs when structures controlling defecation do not function correctly, usually because of diarrhea, trauma to the structures, or nerve damage.
- Causes of diarrhea include anything that increases gut motility or decreases water absorption, including medications, certain foods, and diseases that cause intestinal inflammation.
- Trauma to the structures includes scarring from chronic inflammation (as in irritable bowel syndrome), radiation, surgery, and childbirth.
- Nerve damage can also be the result of childbirth or of spinal cord injury, multiple sclerosis, Parkinson’s disease, stroke, and diabetic neuropathy.
Those with cognitive difficulties may not perceive the need to defecate. Patients with fecal incontinence are referred to a specialist for accurate diagnosis and appropriate treatment.
When the underlying cause cannot be treated, regulation of bowel function by promoting ideal stool consistency can lead to few or no periods of incontinence. Modifying the diet can help, as certain foods can exacerbate fecal incontinence, such as alcohol, caffeine, fruit, gas-producing foods, sugar-free products that contain sorbitol, and dairy products for those who are lactose-intolerant. Dietary fiber adds bulk and can improve passage of stool.
Adequate fluid intake is essential because dietary fiber pulls water into the feces, and without adequate water, the patient will have very dry stools that are difficult to pass.
Emptying the bowel at regular intervals through timed toileting, digital stimulation, or use of suppositories prevents incontinence because an empty rectum will not leak.
Incontinence containment products are useful in community settings, but a plan for skin protection must be in place. In hospital settings, fecal management systems can be used. A colostomy is also an option if everything else has failed and quality of life is severely restricted due to fecal incontinence (WOCN, 2022b).
CASE
Ruth is an older adult living in a residential treatment facility. She ambulates with assistance, and requires hands-on assistance with toileting and self-care. She has a stage 3 pressure injury on her sacrum that the nurse is treating per physician’s orders.
The nurse is aware of the importance of keeping Ruth’s perineal skin clean, dry, and protected, and that exposure to urine can delay or prevent wound healing. Remembering that frequent dressing changes can delay healing, the nurse advises the physician of Ruth’s incontinence and wound status. The nurse also asks the physician for referrals to physical therapy and occupational therapy in order to further evaluate Ruth.
After completing an initial evaluation, the physical therapist believes that Ruth would benefit from gait training with a front-wheeled walker in order to improve her ambulation safety. The occupational therapist also evaluates the patient and recommends a timed voiding program along with verbal voiding prompts, a raised toilet seat, and grab bars in the bathroom; he believes that Ruth’s mild cognitive decline would not make her a good candidate for biofeedback.
At the end of four weeks of following the timed voiding schedule, the nurse compares the wound’s condition and measurements and finds that there is now granulation tissue in the wound bed and that the wound size has decreased by 75%. The nurse and occupational therapist recommend long-term continuation of the individualized toileting regime to maintain continence and prevent further pressure injury.
Managing Nutrition
Malnutrition is associated with overall morbidity and mortality. Assessing the patient’s nutritional status must be part of the total assessment for pressure injury risk. A nutrition assessment should be performed upon admission and whenever there is a change in the patient’s condition that puts them at risk for malnutrition.
Malnutrition places patients at greater risk for developing pressure injury. Malnutrition is defined as the presence of two or more of the following characteristics:
- Unintended weight loss
- Loss of muscle mass
- Loss of subcutaneous tissue
- Localized or generalized fluid accumulation
- Decreased functional status
A low body mass index (BMI) is a factor related to pressure injury development. With a low BMI due to poor nutritional intake, there is a diminished quantity of subcutaneous tissue and decreased protection of the skin. Studies show that the probability of pressure injury development is higher among underweight patients. A study found that almost 1 in 5 patients with a BMI less than 18.5 kg/m2 developed a pressure injury. For patients with obesity, research shows that those with a higher BMI (60 kg/m2 and above) have the greatest risk for pressure injury (VanGilder, 2021).
NUTRITION ASSESSMENT PARAMETERS
- Current weight and usual weight
- History of unintentional weight loss or gain (>5% change in 30 days or >10% change in 180 days)
- Body mass index (BMI)
- Food intake
- Dental health
- Ability to chew, swallow, and feed oneself
- Medical and/or surgical history that influences intake or absorption of nutrients
- Drug-food interactions
- Psychosocial factors that can affect food intake
- Ability to obtain and pay for food
- Facilities for cooking and eating
- Food preferences
- Cultural and lifestyle influences on food selection
- Over 65 years of age
The patient is monitored for signs of dehydration, such as decreased skin turgor and/or urine output or elevated serum sodium. Serum protein tests, such as for albumin and pre-albumin, may be affected by inflammation, renal function, and hydration and may therefore not correspond with overall nutritional status. Thus, laboratory tests are considered as only one part of the nutritional assessment.
Recent weight loss in older adults is a key factor in mortality risk. The “anorexia of aging”—which includes appetite decline, weight loss, and decreased metabolic rate—places the older adult at risk for malnutrition. While unintended weight loss is a risk factor for malnutrition, bariatric adults may also be poorly nourished (Cox, 2020).
The following are dietary recommendations for those who are at risk for pressure injury or have an existing pressure injury and who are at risk for malnutrition:
- Calorie intake of 30–35 kcal per kg of body weight
- Protein intake of 1.25–1.5 g protein per kg of body weight
- High-calorie, high-protein oral nutrition supplements (ONSs) containing arginine (an amino acid) and vitamins and minerals when nutritional needs cannot be met with dietary intake
- Adequate daily fluid intake
Renal function is also assessed to ensure that high levels of protein are appropriate for the patient (EPUAP/NPIAP/PPPIA, 2019; WOCN, 2022a; Baranoski & Ayello, 2020).
Any patient with nutritional and pressure injury risks, suspected or identified nutritional deficiencies, or a need for nutritional supplementation to prevent undernutrition should be referred to a registered dietitian. Any patient with a pressure injury should be referred to the dietitian as well (WOCN, 2022a).
PREVENTING PRESSURE INJURY IN SPECIAL POPULATIONS
Patients with Medical Devices
With the recognition that the use of medical devices can contribute to pressure injury formation, NPIAP has also developed “Best Practices for Prevention of Medical Device-Related Pressure Injuries (MDRPI)” (EPUAP/NPIAP/PPPIA, 2019; WOCN, 2022a). These include:
- Choose the correct size of medical devices to fit the individual.
- Cushion and protect the skin with dressings in high-risk areas (e.g., nasal bridge).
- Remove or move the device daily to assess skin.
- Avoid placement of devices over sites of prior or existing pressure injury.
- Educate staff and caregivers on correct use of devices and prevention of pressure injury.
- Be aware of edema under devices and the potential for pressure injury.
- Confirm that devices are not placed directly under an individual who is bedridden or immobile.
- Ensure that all members of the patient care team, particularly respiratory therapy, are involved in the plan for reduction of MDRPI.
(WOCN, 2022a; Baranoski & Ayello, 2020)
COMMON QUESTIONS ABOUT PRESSURE INJURIES
Q: Can a patient who sleeps with a breathing machine (BiPAP or CPAP) develop pressure injuries?
A: To work effectively, a breathing machine must fit snugly around the patient’s face. It is usually worn nightly and, depending on the patient’s condition, any time the patient naps. Since breathing machines are normally prescribed for long-term use, there is a risk of pressure injury developing, especially if the straps are worn too tightly. If there are changes in the contours of the patient’s face, resizing the mask may be necessary. The patient or family should discuss these issues with the healthcare provider.
Bariatric Patients
Pressure injury prevention and treatment for bariatric patients is similar to that for nonbariatric patients. However, it is also more challenging for a number of reasons:
- The bariatric patient has increased difficulty moving, either independently or with assistance.
- Increased body weight makes it difficult to view bony prominences and to redistribute pressure.
- Shear and friction are often increased, as the patient is inclined to drag the heels and sacrum when getting out of bed.
- The increased pressure on the bowel and bladder from abdominal weight increases the risk of stress incontinence and diaphoresis, which increases the risk of skin maceration.
- Obesity can compromise respiration due to impaired diaphragmatic movement and lead to subsequent impaired tissue perfusion.
- Pressure injuries develop over bony prominences but may also result from tissue pressure across the buttocks and other areas of high adipose tissue concentration. They may develop in unique locations, such as underneath folds of skin and in locations where devices may have been compressed between skin folds.
- The weight of the pannus (the skin “apron”) can cause pressure injuries to develop in areas such as the hip, thighs, trunk, and torso.
- Skin must be checked for maceration, which is common due to increased diaphoresis.
- Additional positioning devices may be needed to offload the pannus or other large skin folds.
- Infection and delayed healing are more common.
- Deeper tissue layers can impede assessment of cavity wounds and increase the risk of retained wound dressings.
- Equipment must be provided that is the appropriate size, and great care taken that neither the patient nor the staff are injured during the provision of care.
- Bariatric patients appear to be at higher risk for medical device–related pressure injuries.
- Bariatric patients may be more susceptible to deep tissue injury.
(EPUAP/NPIAP/PPPIA, 2019; Baranoski & Ayello, 2020; WOCN, 2022a)
COMMON QUESTIONS ABOUT PRESSURE INJURIES
Q: What can a person with obesity who has an abdominal pannus do to a prevent pressure injury from developing?
A: It is important to gently wash and dry under the pannus daily using a pH-balanced soap or cleanser. Checking for redness or excoriation under the pannus should also be done at least once a day; a hand-held mirror can help with this. Placing a piece of dry cotton material under the pannus can help with moisture control. Wearing tight-fitting clothing or placing synthetic fabrics under the pannus should be avoided. If excoriation develops, a healthcare professional should be consulted in order to determine the best treatment option. It is recommended not to apply lotions or creams under the pannus, as this can cause further excoriation.
Pediatric Patients
There is growing awareness that pressure injuries can be the basis of illness, suffering, and increased cost of care in the pediatric population. A study assessing neonatal ICU patients found that almost 80% of pressure injury present was device-related and that over 90% of MDRPI developed in premature infants. Another study found that 35% of pressure injuries resulted from tracheostomy fixation devices. Pressure injury can occur directly under the tracheostomy tube or at different location under the ties used to secure the tube in place (Delmore et al., 2019; Odom et al., 2020).
Examples of pediatric pressure injury risk assessment tools include the Braden Q and the Pediatric Pressure Ulcer Prediction and Evaluation Tool (PPUPET). The Braden QD scale incorporates the five subsets of the Braden Q scale along with the inclusion of two new subsets: the number of medical devices in use and patient repositionability/skin protection (Curley et al., 2018).
Several factors are associated with pediatric pressure injury development:
- Low birth weight
- Skin texture (e.g., neonatal skin is very thin)
- Incubator temperature and humidity
- Support surface used
- Limited position changes
- Endotracheal intubation
- Incontinence
- Poor tissue perfusion
- Fever
- Larger head proportion to the body, putting the occiput at high risk for pressure injury development
Consider children with medical devices to be at greater risk for pressure injuries. At particularly high risk are those with mechanical ventilation, including tracheostomies, CPAP or BiPAP, and ECMO. Increased temperatures, moisture, and humidity under medical devices and their securement can greatly increase the risk for pressure injury in pediatric patients. Proactive, preventive care should be the primary goal for clinicians, and the use of absorptive dressings under medical devices should be used whenever feasible (Boyar, 2019b).
Risk for MDRPI is also associated with the frequent use of noninvasive pressure ventilation (NIPPV) with pediatric patients in the management of respiratory distress, preservation of lung volume, and enhanced gas exchange. When the securement straps are too loose, there is a high risk for friction and shear injuries caused by the mask rubbing against the patient’s skin. Several studies indicate that when the patient mask leakage amount is between 25 and 55 liters per minute, this provides the optimum effectiveness of NIPPV treatment while preserving patient skin integrity and reducing MDRPI. Further large-scale studies are needed to validate this strategy (Lauderbaugh, 2022).
Many pediatric patients with MDRPI are younger, with premature infants being particularly vulnerable. One of the major reasons for this vulnerability is immature skin, with neonates and infants under 2 years of age at the highest risk level. Neonates have a much thinner stratum corneum, which is the outermost layer of the epidermis, compared to full-term infants. For neonates less than 24 weeks of gestational age, the stratum corneum may be almost completely absent. Another factor that predisposes the premature skin of pediatric patients to pressure injury is the reduced interconnection between the epidermal and dermal layers of the skin. This places the premature skin of neonates at greater risk for damage associated with friction (Delmore et al., 2019).
There is limited information available on healing times for pressure injuries in the pediatric population. One small study that included pediatric patients with pressure injuries at stages 2 to 4 demonstrated an average healing time of 13 days. However, more detailed research needs to be done in this area, taking into consideration the gestational age of the infants and comorbidities. A retrospective study done at Arkansas Children’s Hospital found that the use of a foam dressing and wound-filler silver product during the first 14 days of treatment assisted with healing of tracheostomy-related pressure injuries (Odom et al., 2020).
Immobility-related pressure injury is another major area of concern in the pediatric population. The occipital region is the area most often affected by immobility-related injuries especially in infants from birth to 3 years of age. In infants, the head encompasses a higher percentage of body weight and surface area, and when the pediatric patient is lying supine, the occipital area is the main pressure point and at substantial risk for pressure injury (Bryant & Ayello, 2020).
Currently there are no guidelines proposing the use of a specific type of pressure redistribution surface for patients in neo-intensive care units. A recent study found that most patients in neonatal units are placed on standard mattresses with rolled blankets and soft repositioning devices used to provide pressure off-loading (Razmus, 2021).
As in adults, assessment and monitoring, involvement of the family, nutritional management, support surfaces, and repositioning are important. Wound care strategies and dressing selections must be taken with great care, as baby skin is more permeable and more fragile than adult skin, with the result that products commonly used on adults may not be appropriate for children, such as skin preps, barrier products, antimicrobials, and adhesives. Wound care product choices should be discussed with pediatric specialists (EPUAP/NPIAP/PPPIA, 2019).
COMMON QUESTIONS ABOUT PRESSURE INJURIES
Q: Are children at risk for developing pressure injuries?
A: Yes, children with health conditions that include mobility restrictions or require medical devices are at risk for developing pressure injuries. Frequent skin checks must be performed, with special attention to the skin surfaces under any devices. Parents and guardians of children who are living at home with limited mobility or the use of assistive devices (e.g., splints) should receive education and guidance on skin care from a healthcare professional.
Spinal Cord Injury Patients
The development of pressure injury is of special concern to individuals with spinal cord injury. Pressure injury is the second most frequently occurring medical complication among those with spinal cord injury. Data demonstrate that >95% of adults with spinal cord injury will develop one or more pressure injuries during their lives. Lack of mobility and paralysis are key factors in pressure injury occurrence; the greater the level of paralysis, the greater the risk for the development of pressure injury.
In a study of community-dwelling individuals with spinal cord injury, it was found that those with spinal cord injury at the cervical level had a higher risk of developing a pressure injury compared to those with a spinal cord injury at the lumbar level (Cowan et al., 2019).
In another study of individuals with spinal cord injury living in long-term care facilities, researchers found a higher incidence of pressure injury in patients who were paraplegic than those who were tetraplegic. This may be because patients who are tetraplegic require total care from the facility staff, whereas patients who are paraplegic have more responsibility for making position shifts aimed at relieving pressure, especially when sitting. Another finding was a greater risk for malnutrition in patients with paraplegia compared to those with quadriplegia, perhaps due to a greater calorie use in paraplegic patients due to upper body mobility and potential unaddressed difficulties with independent feeding in this group.
Recommendations include the implementation of greater preventive measures among long-term care patients who are paraplegic to prevent pressure injury. Specific suggestions include closer and more consistent monitoring of this high-risk population, better utilization of assistive technology, and increased patient motivation and education regarding pressure injury prevention (Cowan et al., 2019; Baranoski & Ayello, 2020; WOCN, 2022a).
COMMON QUESTIONS ABOUT PRESSURE INJURIES
Q: What can a person with a spinal cord injury can do to prevent a pressure injury from developing?
A: There are many interventions for a person with a spinal cord injury to minimize their risk for pressure injury, such as good nutrition and pressure relief. The single most important intervention is frequent skin checks. These should be performed at least once or twice a day and more frequently if an area of redness develops.
Pressure Injuries at End of Life
Skin changes or unusual wounds can occur at the end of life and may include deep tissue injury, pressure injuries, or ischemic/mottled wounds. Due to the underlying etiologies, these wounds are generally thought to be unavoidable. For patients at the end of life, it is important to determine the goals of the patient and caregiver(s). Some may wish to achieve healing of the pressure injury, whereas others may desire only palliative care, including reducing pain, odor, drainage, bleeding, and infection, and simplifying dressing changes for comfort. The patient and family must be educated as to realistic expectations for wound healing.
One example of skin changes at the end of life is the Kennedy terminal ulcer. This type of ulcer was first described in 1989. It is located on the sacrococcygeal area, and it appears as a purple, red, blue, or black discoloration of the skin with a butterfly or pear shape that has irregular borders. It has a sudden onset, develops rapidly into a full-thickness wound despite appropriate care, and may precede death in days to weeks (WOCN, 2022a).
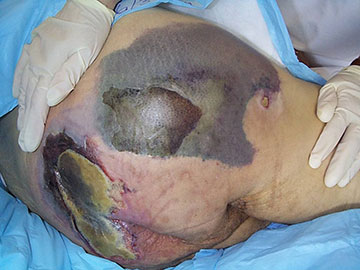
Terminal ulcer. (Source: C Melter.)
CONCLUSION
Pressure injuries are a life-threatening problem among vulnerable individuals, including those who are bed- or chair-bound and those who are critically ill. Nearly all pressure injuries are considered preventable, and this requires a full commitment by the healthcare facility and individual clinicians so that a pressure injury will not occur.
There are many factors that contribute to the formation of a pressure injury, including comorbidities, incontinence, poor nutrition, and advanced age, but the most significant risk factor is immobility. Patients who are dependent on others for repositioning are at greatest risk of developing a pressure injury, for nonhealing of a pressure injury should it occur, and for the recurrence of a pressure injury.
Pressure injuries can be prevented through both an “outside” approach (which includes minimizing pressure through regular repositioning, using a support surface, and managing incontinence) and an “inside” approach (which includes managing nutrition and hydration to support health and healing).
Finally, healthcare professionals, patients, and caregivers must be vigilant about monitoring for pressure injury recurrence. Failing to protect the development of pressure injuries or to care for existing pressure injuries puts all patients and the healthcare system in jeopardy for what is often a costly but avoidable complication.
RESOURCES
Braden-Q Scale (for pediatric population)
Pressure Injury Prevention in Hospitals Training Program (AHRQ)
REFERENCES
NOTE: Complete URLs for references retrieved from online sources are provided in the PDF of this course.
American Physical Therapy Association (APTA). (2022). Physical therapists’ guide to incontinence. https://aptapelvichealth.org
Baranoski S & Ayello EA. (2020). Wound care essentials practice principals (4th ed.). Wolters Kluwer.
Bennett WL, Pitts S, Aboumatar H, et al. (2020). Strategies for patient, family, and caretaker engagement. National Library of Medicine. https://www.ncbi.nlm.nih.gov
Boyar V. (2019a). Pressure injuries in the pediatric population: Many questions, few answers. Wound Management & Prevention, 65(4), 12–6.
Brophy S, Moore Z, Patton D, O’Connor T, & Avsar P. (2021). What is the incidence of medical device-related pressure injuries in adults within the acute hospital setting? A systemic review. Journal of Tissue Viability, 30(4), 489–98.
Centers for Medicare and Medicaid Services (CMS). (2022a). Hospital-acquired conditions (present on admission indicator). https://www.cms.gov
Centers for Medicare and Medicaid Services (CMS). (2022b). Hospital-acquired condition (HAC) reduction program. https://www.cms.gov
Choi M. (2013). Operational definitions of Cubbin and Jackson scale. ResearchGate. https://www.researchgate.net
Cleveland Clinic. (2023). Dementia. https://my.clevelandclinic.org
Cowan LJ, et al. (2019). Pressure ulcer prevalence by level of paralysis in patients with spinal cord injury in long-term care. Advances in Skin & Wound Care, 32(3), 122–30.
Cox NJ, Morrison L, Ibrahim K, Robinson SM, Sayer AA, & Roberts HC. (2020). New horizons in appetite and the anorexia of aging. Age and Ageing, 49(4), 526–34. https://doi.org/10.1093/ageing/afaa014
Cox J, Edsberg LE, Koloms K, & VarGilder CA. (2022). Pressure injuries in critical care patients in US hospitals. Journal of Wound, Ostomy and Continence Nursing, 49(1), 21–8.
Curley MA, Hasbani NR, et al. (2018). Predicting pressure injury risk in pediatric patients: The Braden QD scale. Journal of Pediatrics, 192, 196–202.
Delmore B, et al. (2019). Pressure injuries in the pediatric population: A National Pressure Ulcer Advisory Panel whitepaper. Advances in Skin & Wound Care, 32(9), 394–407.
Elli C, Novella A, Nobili A, Janes A, & Pasina L. (2022). Factors associated with a high-risk profile for developing pressure injuries in long-term residents of nursing homes. Med Princ Pract, 31(5), 433–8. https://doi.org/10.1159/000527063
European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel, and Pan Pacific Pressure Injury Alliance (EPUAP/NPIAP/PPPIA). (2019). Prevention and treatment of pressure ulcer/injuries: Clinical practice guideline (3rd ed.). Author.
Greenwood C. (2021). Heel pressure ulcers: Understanding why they develop and how to prevent them. Nurs Stand, 37(2), 60–6. https://pubmed.ncbi.nlm.nih.gov
HealthinAging.org. (2020). Delirium: Causes. https://www.healthinaging.org
Higgins J, Casey S, Taylor E, Wilson R, & Halcomb P. (2020). Comparing the Braden Scale and Jackson/Cubbin pressure injury risk scales in trauma-surgery ICU patients. Critical Care Nurse, 40(6), 52–61.
International Society for Pediatric Wound Care (ISPeW). (2019). 7th annual meeting in Houston. https://ispew2019.dryfta.com
Jaing X, Wang Y, Wang Y, Zhou M, Huang P, Yang Y, Fang P, et al. (2022). Application of an infrared thermography-based model to detect pressure injuries: A perspective cohort study. British Journal of Dermatology, 187(4), 571–9. https://onlinelibrary.wiley.com
James TA. (2023). Building empathy into the structure of health care. Harvard Medical School. https://postgraduateeducation.hms.harvard.edu
Koerner S. (2019). Use of thermal imaging to identify deep-tissue pressure injury on admission reduces clinical and financial burdens of hospital-acquired pressure injuries. Advances in Skin & Wound Care, 32(7), 312–20.
Lauderbaugh DL, Popien TL, Lesser DJ, Bhattacharjee R, & Pfrommer Puleo C. (2022). Reducing mask-related pressure injuries in pediatric patients during noninvasive ventilation by targeting patient mask leak. Journal of Wound, Ostomy and Continence Nursing, 49(6), 522–7.
McNichol L, Mackey D, Watts C, & Zuecca N. (2020). Choosing a support surface for pressure injury and prevention. National Library of Medicine. https://www.ncbi.nlm.nih.gov
Najmanova K, Neuhauser C, Krebs J, Baumberger M, Schaefer DJ, O’Sailer C, Wettstein R, et al. (2022). Risk factors for hospital acquired pressure injuries in patients with spinal cord during first rehabilitation: prospective cohort study. Spinal Cord, 60(1), 45–52. https://doi.org/10.1038/s41393-021-00681-x
National Pressure Injury Advisory Panel (NPIAP). (2021). 2021 fact sheet: About pressure injuries in healthcare. https://cdn.ymaws.com
Odom BH, Yates C, et al. (2020). Pediatric tracheostomy wound healing: A retrospective cohort study. Advances in Skin & Wound Care, 33(1), 36–42.
Pirotte BD & Benson S. (2022). Refusal of care. National Library of Medicine. StatPearls. https://www.ncbi.nlm.nih.gov
Pittman J & Gillespie C. (2020). Medical device-related pressure injuries. Critical Care Nursing Clinics of America, 32(4), 533–42.
Pittman J, Horvath D, Beeson T, Bailey K, Mills A, Kaiser L, Hall DK, & Sweeney J. (2021). Pressure injury prevention for complex cardiovascular patients in the operating room and intensive care unit. Journal of Wound, Ostomy and Continence Nursing, 46(6), 510–5.
Razmus S & Keep SM. (2021). Neonatal intensive care nursing pressure injury prevention practices. Journal of Wound, Ostomy and Continence Nursing, 48(5), 394–402.
Schmitt S, Andries MK, Ashmore PM, Brunette G, Judge K, & Bonham PA. (2017). WOCN society position paper: Avoidable versus unavoidable pressure ulcers/injuries. Journal of Wound, Ostomy, and Continence Nursing, 44(5), 458–68. https://doi.org/10.1097/WON.0000000000000361
Sermersheim ER, Hall L, Boudreau L, Ambutas S, & Gulczynski B. (2021). Reducing nares acquired pressure injuries: “Protecting the nares because I care” project in adult patients. Journal of Wound, Ostomy and Continence Nursing, 48(5), 389–93.
Seton JM, Hovan HM, Bogie KM, Murray MM, Wasil B, Banks PG, Burant CJ, et al. (2022). Interactive evidenced-based pressure injury education program for hospice nursing. Journal of Wound, Ostomy and Continence Nursing, 45(5), 428–35.
Shi C, Dumville JC, Cullum N, Rhodes S, Leung V, & McInns E. (2021). Reactive air surfaces for preventing pressure ulcers. National Library of Medicine. https://pubmed.ncbi.nlm.nih.gov
Sillman K, Carter M, Shook L, Lawson C, & Burfield AH. (2021). The use of prophylactic foam dressings for prevention of hospital-acquired pressure injuries. Journal of Wound, Ostomy and Continence Nursing, 48(3), 211–8.
Steller JJ, Hasbani NR, Kulik LA, Shelley SS, Quigley S, Wypj D, & Curley MAQ. (2020). Medical device-related pressure injuries in infants and children. Journal of Wound, Ostomy and Continence Nursing, 47(5), 459–69.
Stephens M, Bartley C, & Dunville JC. (2022). Do pressure redistributing static chairs help to prevent pressure ulcers? Cochrane. https://www.cochrane.org
Strauss R, et al. (2019). Silicone foam dressing for prevention of sacral deep tissue injuries among cardiac surgery patients. Advances in Skin and Wound Care, 32(3), 139–42.
Sugrue C, Avsar P, Moore Z, Patton D, O’Connor T, Nugent L, & Budri A. (2023). The effect of prophylactic silicone dressings on the incidence of pressure injuries in the acute care setting. Journal of Wound, Ostomy and Continence Nursing, 50(2), 115–23.
The Joint Commission (TJC). (2022). Quick safety 25: Preventing pressure injuries. https://www.jointcommission.org
Tsai YJ, Lin CH, Yen YH, Wu CC, Carvajal C, Molte NF, Lin PY, & Hsieh CH. (2023). Risk factors for pressure ulcer recurrence following surgical reconstruction: A cross-sectional retrospective analysis. Front Surg, 10. https://doi.org/10.3389/fsurg.2023.970681
Van Gilder CA, Cox J, Edsberg LE, & Koloms K. (2021). Pressure injury prevalence in acute hospitals with unit-specific analysis. Journal of Wound, Ostomy and Continence Nursing, 48(6), 492–503.
Van Leen MWF, Van Ratingen WU, & Schols JMGA. (2022). Effects of a breathable silk-like, 3-layer ventilating mattress sheet on self-repositioning, repositioning support and pressure ulcer incidence: A pragmatic observational study. Wounds International, 13(1), 38–45.
Vasavada SP. (2019). Urinary incontinence treatment & management. Medscape. https://emedicine.medscape.com
Vasquez MS. (2022). Prevention and treatment of pressure injuries for frail elderly with serious illness. University of Texas at El Paso. https://www.alz.org
Vickery J, Compton L, Allard J, Beeson T, Howard J, & Pittman J. (2020). Pressure injury prevention and wound management for the patient who is actively dying: Evidence-based recommendations to guide care. Journal of Wound, Ostomy and Continence Nursing, 47(6), 569–75.
Wound Care Advisor. (2019). Nutritional considerations in patients with pressure ulcers. https://woundcareadvisor.com
Wound, Ostomy, and Continence Nurses Society (WOCN). (2022a). In D Doughty & K Morre (Eds.), Core curriculum: Wound management. Wolters Kluwer.
Wound, Ostomy, and Continence Nurses Society (WOCN). (2022b). In D Doughty & K Morre (Eds.), Core curriculum: Continence management. Wolters Kluwer.
Wound Source. (2019). Review: Pressure injury progression in the home palliative care setting https://www.woundsource.com
Zemp R, Rhiner J, Plüss S, Togni R, Plock JA, Taylor WR. (2019). Wheelchair tilt-in-space and recline functions: Influence on sitting interface pressure and ischial blood flow in an elderly population, BioMed Research International, 2019, article ID 4027976, 10 pages. https://doi.org/10.1155/2019/4027976





Customer Rating
5.0 / 709 ratings