Wound Care
Online Continuing Education Course

Course Description
Wound Care CEU. 11-contact-hour continuing education course for nurses and other healthcare practitioners on wound assessment, treatment, and management for patients with acute and chronic wounds in various clinical settings. Bestseller course!
Course Price: $49.00
Contact Hours: 11
Pharmacotherapeutic Hours: 1
Course updated on
October 31, 2025
"Actually very interesting. No one is thrilled to take CE, but this was presented in an interesting and informative manner. Well done!" - Leisa, RN in Arizona
"Excellent. Great review. Really enjoyed it." - Sylvia, RN in Texas
"I searched for a wound care course to fulfill my CEU requirements, and this was the most thorough and in-depth course I found. It will help me provide a higher level of care for my patients." - Jeannie, LPN in Tennessee
"Was instructional enough to be a good learning tool for a nurse just entering wound care, and it had several points that were new to me even after five years in the specialty." - Julie, OT in North Dakota
Wound Care
Copyright © 2025 Wild Iris Medical Education, Inc. All Rights Reserved.
LEARNING OUTCOME AND OBJECTIVES: Upon completion of this continuing education course, you will demonstrate an understanding of wound assessment and management for patients with acute and chronic wounds in various clinical settings. Specific learning objectives to address potential knowledge gaps include:
- Describe the role of the skin.
- Discuss the types of wounds and the wound-healing process.
- List the steps in immediate treatment of acute wounds.
- Explain what is included in a detailed wound assessment.
- Summarize the various wound cleansing techniques and dressing options.
- Recognize the signs and symptoms of wound infection.
- Identify impediments to wound healing.
- Discuss wound care for specific types of wounds and special populations.
- Describe advanced wound care treatment modalities.
- Review patient and caregiver wound care education.
TABLE OF CONTENTS
- Introduction
- Skin--The External Barrier
- Definitions of Wounds
- Wound Healing
- Wound Care Team
- Immediate Treatment for Acute Wounds
- Comprehensive Wound Assessment
- Wound-Cleansing Techniques
- Wound Dressings
- Wound Infection
- Impediments to Wound Healing
- Treating Specific Types of Chronic Wounds
- Other Types of Wound Care
- Advanced Wound Care Modalities
- Patient Teaching
- Conclusion
- Resources
- References
INTRODUCTION
The skin is the largest organ in the human body, comprising approximately 15% of total adult body weight. The skin maintains our internal environment while protecting us from the external environment. It allows us to experience a wide range of stimuli, from pleasure to pain.
A break in the continuity of the skin surface is the first step in the formation of a wound and provides a potential portal of entry for infection. A wound can be as simple as a surface abrasion, or it can be an extensive, life-threatening destruction of tissue that reaches down to and includes the internal organs of the body.
While the healing process is basically the same for all wounds, there are many extenuating factors that will either expedite or impede healing.
Wound care is not exclusive to any one healthcare profession. Successful outcomes are achieved when an interdisciplinary team approach is used, calling on the expertise of many different clinicians and employing many different treatment modalities—from simple dressings to advanced treatments such as negative-pressure wound therapy and hyperbaric oxygen therapy.
Clinicians encounter wounds in every healthcare setting, from the penetrating gunshot wound that is rushed to the emergency department to acute and chronic wounds that need to be treated in the hospital setting, outpatient clinics, skilled nursing facilities, hospice care, and in-home care.
Chronic wounds are described as a “silent epidemic” in this country (Sen, 2025). Overall, chronic wound care is estimated to cost Medicare around $22.5 billion annually, and healthcare clinicians are called upon to provide cost-effective, state-of-the-art care of increasingly complex wounds.
- 1 in 6 Medicare recipients are affected by nonhealing chronic wounds (Sen, 2025).
- 6.7 million people have nonhealing chronic wounds of a lower extremity (UT Health East Texas, 2022).
- In the United States, the prevalence rate for the occurrence of a venous ulcer in those older than 65 years of age is 1.7%, with associated healthcare costs averaging over $10 billion annually (Dunlop et al., 2025).
- Chronic venous disease is the seventh most frequently occurring chronic condition and the leading cause of leg ulcers, and it is the determinative cause in 95% of lower extremity ulcers (Baranoski & Ayello, 2020; Attaran & Carr, 2023).
ANCIENT WOUND CARE
Wound care can be traced back to the earliest civilizations. The ancient Greeks were among the first to highlight the importance of wound cleansing. They washed wounds with clean water, which was frequently boiled first; they used vinegar and wine as cleansing agents.
The Egyptians are thought to have been the first people who applied honey to wounds. Their wound dressings, which they referred to as plasters, were a composite of honey, grease, and lint. In the first known records of wound care in the Ebers Papyrus, dated 1534 BCE, bleeding blood vessels were described as being “burnt with fire” (i.e., cauterized).
One of the earliest descriptions of the “four cardinal signs of inflammation”—rubor, tumor, calor, et dolor (redness, swelling, heat, and pain)—came from the Romans (Shah et al., 2018).
SKIN—THE EXTERNAL BARRIER
To properly understand the occurrence, continuation, and healing of wounds, it is necessary to first look at the skin.
The skin is comprised of two layers: the epidermis, made up of four to five thin layers stacked on top of each other, and the dermis (often referred to as the “true skin”), a layer of connective tissue directly below the epidermis. Beneath the dermis is the subcutaneous tissue, which separates the skin from the underlying muscles, tendons, joints, and bones. Skin varies in thickness from less than 1 millimeter in the eyelids to greater than 4 millimeters on the soles of the feet.
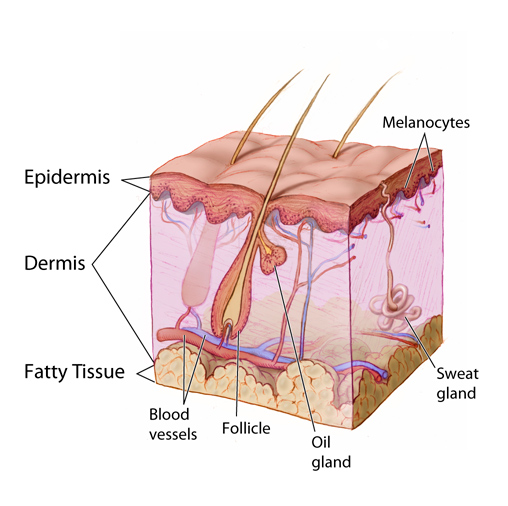
The layers of skin and associated glands and vessels (epidermis, dermis, fatty tissue, blood vessels, follicle, oil gland, sweat gland, and melanocytes). (Source: Don Bliss/National Cancer Institute.)
Epidermis
The deepest layer of the epidermis is known as the stratum basale or stratum germinativum. It is a layer of dividing, reproducing cells that migrate upward. As they travel, they differentiate and become filled with keratin, a tough, fibrous protein. The mature cells are pushed to the surface, where they die; thus, the outermost layer of the epidermis is made of flat, dead keratinocytes. The stratum basale also contains melanocytes, the cells responsible for producing melanin, the pigment that adds color to the skin and also protects against the damaging effects of ultraviolet light.
Shallow wounds to the epidermis usually heal rapidly and without complications. Usual skin thickness is reestablished, and there is no scar formation.
Dermis
The layer of skin directly beneath the epidermis is the dermis. A basement membrane separates these two layers. The dermis is mainly connective tissue and is therefore much stronger than the epidermis. The dermis varies in thickness across the surface of the body, but everywhere it is significantly thicker than the overlying epidermis.
The dermis is itself made up of two layers: the papillary layer is directly beneath the epidermis, and the reticular layer is below that. The primary function of the papillary dermis is to supply nutrients to the epidermis. The reticular dermis contains fibroblasts, which are cells that synthesize the connective tissue proteins, collagen, and elastin. These cells are responsible for the strength and elasticity of the skin. The reticular layer of the dermis also contains macrophages, which are essential for wound healing, and mast cells, a key component of the immune system.
The tissue of the dermis also contains small blood vessels, lymph vessels, nerves with their endings, and the smooth muscle fibers of hair follicles. Hair, nails, sweat glands, and sebaceous glands are sunken epidermal appendages that lie in deep valleys in the dermis surrounded by a row of germinative epidermal cells.
Subcutaneous Tissue
Beneath the dermis is a layer of subcutaneous tissue (also known as the hypodermis) containing fat. The thickness of the subcutaneous layer varies throughout the body. It is thickest along the anterior thigh and thinnest on the back of the hands.
Besides fat cells, subcutaneous tissue contains blood vessels, lymph vessels, and nerves. The subcutaneous layer is held together by a continuous sheet of fibrous membrane that runs parallel to the surface of the skin. This membrane is called the superficial fascia. The subcutaneous tissue provides insulation to the body, and it is also important in pressure redistribution (Baranoski & Ayello, 2020).
Sex differences are noted in the thickness and distribution of subcutaneous tissue. Males are inclined to have more subcutaneous tissue in the abdominal area and shoulders, while females have more subcutaneous tissue around their buttocks, hips, and thighs (Jones, 2024).
Individuals with a thin layer of subcutaneous tissue and limited mobility are at high risk for the development of pressure ulcers/injuries. Those with excess subcutaneous tissue can have a harder time with wound healing since subcutaneous tissue is not well perfused, and this in turn decreases the blood supply to the dermis and epidermis (WOCN, 2022).
The subcutaneous tissue is a loosely organized compartment. When skin wounds extend deeper than the dermis, dirt is easily pushed into and spread within the subcutaneous tissue. This increases the risk of infection and requires that deep wounds be cleaned thoroughly.
Beneath the subcutaneous tissue layer, structures such as muscles and organs are enclosed in their own separate connective tissue sheaths. The generic name for these sheaths is deep fasciae. Deep fasciae generally look off-white in fresh wounds. When treating a wound, tears in the deep fasciae are repaired whenever possible.
DEFINITIONS OF WOUNDS
A wound occurs when there is an interruption of the normal makeup and function of the skin. Wounds may be described as either acute or chronic (Baranoski & Ayello, 2022). Wounds interfere with the structure and function of tissues beneath the skin surface and can extend into body cavities and deeper organs. Wounds can occur in otherwise healthy human beings at any stage in their lifespan. They can be as simple as a scrape or as a complex as a deep, life-threatening wound affecting internal organs. Wounds affect millions of individuals, making it imperative to develop standard terminologies to define and classify wounds (WOCN, 2022).
By Depth
One way of classifying wounds is according to the amount of tissue destruction involved (WOCN, 2022). The terms partial thickness and full thickness are used to describe wounds of varying depths. A wound that is limited to the epidermis and the dermis is a partial-thickness wound. A wound that extends beyond the dermal layers is considered a full-thickness wound.
By Time
Another way to classify wounds is according to the length of time they have existed. Acute wounds are newly formed wounds that move through the process of healing in an orderly and efficient manner. The length of time it takes an acute wound to heal varies depending on the size, depth, and manner of wound closure (WOCN, 2022; Shah et al., 2018). Chronic wounds, on the other hand, do not adhere to the normal healing trajectory and can be present for months or years (Baranoski & Ayello, 2020).
ACUTE WOUNDS
Acute wounds happen suddenly and are normally related to trauma or injury. Such a traumatic wound is also often a mixture of types. Types of acute wounds are described below.
- Lacerations are tears. When made by a knifelike object, a laceration is a narrow, deep wound with sharp edges. When made by a blunt object, a laceration is a rip with jagged edges.
- Crushes or contusions are compression wounds. A crush wound bruises and damages the skin and the underlying tissue, although the skin can remain closed in some crush wounds.
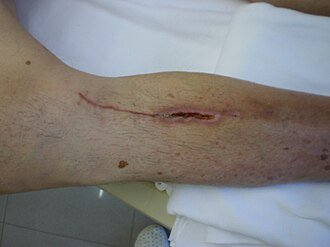
- Punctures are narrow, deep wounds. Typically, punctures have small openings with sharp edges. Puncture wounds have a relatively high risk of infection (see photo below).
- Avulsions are wounds in which tissue has been torn out. Sometimes, the avulsed tissue remains partly connected to its normal surroundings.
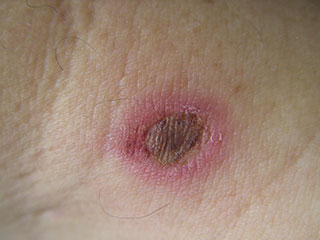
- Burns are wounds made by external destructive energy (e.g., heat) or by external chemicals (e.g., acid). First-degree burns are superficial and red. Second-degree wounds include damage to the dermis and produce blisters. Third-degree burns go deeper than the dermis and produce dry, dead tissue. (See photo below.)
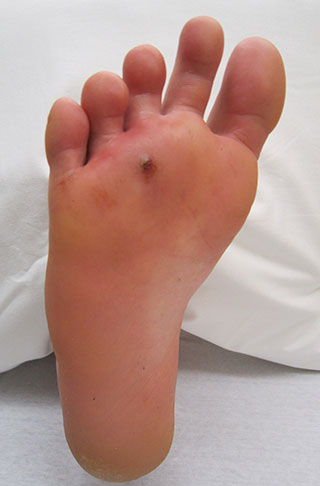
Infected puncture wound. (Source: James Heilman, MD, CC BY-SA 3.0.)
Second-degree burn, with some evidence of infection around central sloughing area. (Source: Peter Ellis, CC BY-SA 3.0.)
CHRONIC WOUNDS
There are several definitions for chronic wounds. They can be defined as wounds that do not heal within a realistic time frame, usually three months (Wound Source, 2022). Chronic wounds can also be defined as acute wounds that are physiologically compromised due to an interruption in the wound-healing phases, which can be caused by several factors, including diminished cellular migration (Evans & Orapallo, 2025). Other terms used to describe chronic wounds include delayed healing, recalcitrant, stalled, and hard to heal (Baranoski & Ayello, 2020).
Chronic wounds are also classified by the underlying causative factor or disease process and include pressure ulcers/injuries, diabetic ulcers, arterial and venous ulcers, and nonhealing palliative wounds.
It is important to remember that all chronic wounds start out as acute wounds. A surgically dehisced wound is an example of an acute wound that develops into a chronic wound.
WOUND HEALING
Wounds heal in one of two possible ways: regeneration or scar formation. In regeneration, the tissue that has been destroyed or damaged is replaced by tissue of the same type. This is the preferable way for wounds to heal because it preserves proper functioning of the injured site and its normal appearance. In scar formation healing, the lost tissue is replaced by fibrous scar tissue, which does not have the same properties as the original tissue and is unable to carry out the same functions (WOCN, 2022).
One of the main factors controlling how a wound heals is the depth of the wound. Shallow wounds that encompass only the epidermis and a portion of the dermis are capable of healing by regeneration because the damaged tissue can reproduce itself. However, with deeper wounds in which multiple tissue layers are involved, including subcutaneous tissue down to muscle, tendons, and bone structure, regeneration is not possible. These structures are unable to reproduce themselves, and the only option for healing is scar tissue formation.
Phases in Wound Healing
The body has a sequential mechanism to heal acute wounds through regeneration. A noncomplicated surgical incision, for example, will go through the following healing process:
- Hemostasis
- A short inflammatory phase
- Proliferation
- Maturation
HEMOSTASIS
Hemostasis begins immediately after a wound occurs, and it is initiated by blood coming in contact with the collagen in the tissues. This triggers what is called the clotting cascade. The purpose of hemostasis is to stop bleeding. Activated platelets in the wound bed release platelet-derived growth factors, which control and hasten the healing process. Activated platelets are also involved in creating the fibrin structure that leads to the formation of a fibrin clot, which stops the bleeding. The fibrin clot also serves as an initial matrix within the wound area into which cells can transfer. It also provides a temporary barrier to bacterial intrusion from the outside environment.
This process of clot formation happens rapidly if there are no bleeding abnormalities. Individuals with an impaired clotting mechanism will experience an impaired healing process (Baranoski & Ayello, 2020; Shah et al., 2018, Bryant & Nix, 2024).
When larger blood vessels are severed in an acute wound, further measures to stop bleeding are usually needed, such as the application of manual pressure, cauterization, or suturing.
INFLAMMATION
Once a clot is formed and bleeding ceases, the inflammatory phase begins. The inflammatory phase is a normal and essential part of wound healing that establishes a clean wound bed. The platelet-derived growth factors attract white blood cells to the wound. The first white blood cells on the scene—polymorphonuclear cells, also called neutrophils—are the initial line of defense; their function is to remove bacteria from the wound through enzymatic activity.
The various biologically active molecules being released into the wound also hypersensitize the endings of local pain nerves, causing them to react to smaller amounts of chemical and mechanical irritation, thus making the wound site tender. Together, these processes produce local inflammation.
The number and activity of the neutrophils decline as the inflammatory process continues, and by the third day of wound healing, macrophages are the predominant white blood cells in the wound. Macrophages are scavengers that continue to debride (or cleanse) the wound biologically by removing dead and dying bits of tissue, dirt, and bacteria. Macrophages, which are derived from tissue monocytes, are an essential component of the initial phases of wound healing.
A decreased level of macrophage activity in the wound is associated with prolonged and delayed wound healing. Individuals with uncontrolled diabetes and diabetic wounds are noted to have low macrophage counts and difficulty with wound healing. Macrophages also release growth factors, chemicals that stimulate the growth of fibroblasts, endothelial cells, and epithelial cells, all of which quickly transition the wound into the proliferative phase of healing.
It is important for the clinician to recognize that induration, warmth, redness, and swelling are normal findings during the inflammatory phase of wound healing and are not, at this stage of the process, an indication of wound infection. It is also good practice to share this information with the patient (Baranoski & Ayello, 2020; Bryant & Nix, 2024).
PROLIFERATIVE PHASE
The next set of events in wound healing constitute the regenerative, or proliferative, phase. This phase begins when fibroblasts (the cells responsible for the synthesis of the new connective tissue) are attracted to the wound by growth factors and white blood cells. Fibroblasts are the only cells capable of synthesizing connective tissue, and it is important to note that they can be damaged by certain antiseptics.
In acute wounds, collagen fiber production normally begins around the fifth day after injury. Collagen is a structural tissue protein found in various forms throughout the body. Collagen fibers are composed of the protein collagen, which is the most frequently occurring protein in the human body. Collagen provides strength and support to connective tissue, and adequate collagen production is an essential part of tissue repair and wound healing. At the same time, new blood vessels are growing into the wound.
Presently, 28 separate collagens have been isolated in vertebrate tissue. Collagen makes up 30% of the complete protein content in the human body. Collagen is crucial to the process of tissue scaffolding and adds significant tensile strength to the extracellular matrix (ECM). Type I collagen is the most common type and is the principal collagen found in the human dermis (Amirrah et al., 2022).
Together, the newly forming cells, blood vessels, and loose extracellular matrix are called granulation tissue. Granulation tissue fills the base of an open wound. Healthy granulation tissue contains newly growing blood vessels and should be beefy red with a bumpy, uneven surface resembling velvet.
MATURATION
Maturation, also called remodeling, is the last stage of wound healing. This phase can last up to one year after the wound occurrence and is characterized by strengthening, defining, and debulking of the final scar tissue.
A wound that heals without complications will achieve 80% of its normal tensile strength. Tensile strength refers to the skin’s ability to resist breakdown under tension, and it is a very important factor in maintaining normal skin integrity. It will never regain 100% tensile strength, something for clinicians to keep in mind with caring for patients with a healed wound, especially with healed pressure ulcers/injuries. This lack of regular tensile strength makes these areas more prone to further wound development.
The above phases of wound healing are usually discussed as separate entities, but in reality, wound healing is an intricate process with overlapping phases (Bryant & Nix, 2024).
THE HEALING PROCESS AND CHRONIC WOUNDS
A chronic wound will not move through the healing process described above. Research into why this occurs includes the study of the molecular and cellular changes that happen in the conversion of an acute wound into a chronic one. These research findings are the basis for new and innovative therapies that seek to selectively correct the abnormalities that impede wound healing.
Whereas acute wounds are found to have high levels of growth factors, these are markedly decreased in chronic wounds. One important discovery is that acute wounds that are subjected to frequent episodes of injury can evolve into chronic wounds.
What has been discovered is that chronic wounds stall in the inflammatory phase of wound healing because of the presence of free-flowing (planktonic) bacteria in the wound and the formation of biofilm. Biofilm is defined as groups of microorganisms (bacteria and fungi) that attach themselves to a surface and become entrenched in a hydrated matrix composed of an extracellular polymeric substance (Baranoski & Ayello, 2020; Bryant & Nix, 2024). The symbiotic relationship between the bacteria in the biofilm allows their collective strength to create a formidable barrier to attack, and their densely packed matrix does not readily allow penetration by white cells and antibodies.
Research has also found that the bacteria found in the center of the biofilm become dormant and produce no metabolic activity; this greatly increases their resistance to antibiotic therapy, since antibiotics attack actively dividing bacteria. This provides the biofilm with a high level of immunity to standard treatment that readily kills planktonic bacteria, and it presents one of the major challenges in chronic wound care—the eradication of biofilm and preventing it from regrouping. (See also “Biofilm and Infection” later in this course.)
Scar Tissue Formation
Scar tissue formation is one of the two ways in which wounds heal, and all full-thickness wounds will form scars. There are noticeable differences between scar tissue and normal skin. There is less elasticity in scar tissue; it has fewer blood vessels, resulting in a decreased blood supply; and it appears lighter in tone than the surrounding skin.
Scars are the natural patches produced in a healing wound. They are the end product of the wound-healing process and have diminished strength compared to normal tissue. Even years after a wound heals, it has been found that scar tissue never fully recovers the strength of normal tissue (Baranoski & Ayello, 2020; Bryant & Nix, 2024).
In the first few days after an injury, closed skin wounds are being knit weakly together by the forming scar tissue. By about day five, the basic architecture of the wound patch has been established, and from then on, the healing process consists largely of strengthening and remodeling the scar.
Scars can take six to nine months to mature. New scars tend to be red and thick for a month or two before gradually becoming less vascular (i.e., paler), less bulky, and flat. It can take as long as five years for a scar to reach its final color.
MINIMIZING SCARS
The width of a scar can be minimized by:
- Thorough debridement
- Careful suturing (avoiding inversion of the skin edges)
- Removing excess granulation tissue
- Good secondary wound care (especially preventing the wound from becoming infected)
- Removing sutures promptly
PROBLEM SCARS
Scars are a natural result of healthy healing, although scars are imperfect replacements for damaged tissue. Normal scars can lead to problems. Even under the best healing conditions, some normal scars may end up interfering with the movement of the skin and the underlying tissue. In addition, some normal scars are unsightly.
When the healing situation is not ideal, scars are more likely to become problems. After poor healing, some scars become unnecessarily large or unnecessarily weak. For example:
- Infections, tissue necrosis, sebaceous skin, and wounds perpendicular to natural lines of minimal skin tension all lead to scars that are larger than normal.
- If a wound reopens before it is effectively sealed (called dehiscence), the scar will be wider and usually weaker.
- If too few capillaries grow into the forming scar tissue, leading to ischemia, the scar will be very weak and may develop into an ulcer.
The wound patching process may also become excessive and generate too many new cells or, more commonly, too much collagen in the scar. Such scars will enlarge and bulge from the wound. Scars built of too many cells (mainly fibroblasts) are called desmoids or aggressive fibromatoses. Scars built from too much collagen are either hypertrophic scars or keloids. When excessive scars form tight ridges along the skin and permanently interfere with normal movement, they are called contractures.
Keloids
Keloids are benign tumors that grow beyond the bounds of a wound and do not regress. Keloids are caused by the excess deposition of collagen in a healing wound. The tendency to form keloids is genetic, and there are, at present, no preventive measures. Patients with darkly pigmented skin are particularly susceptible.
Unlike hypertrophic scars (discussed below), keloids develop late in the healing process; they can appear months or even years after the injury. Keloids bulge out beyond the edges of the wound, and some keloids can become sizeable. The typical presentation of a keloid is as a raised, hyperpigmented nodule that is firm to the touch. Keloids that do not regress spontaneously are usually found on the upper half of the body.
Successful treatment of keloids is challenging. Treatments that have shown some success include corticosteroid injections, chemotherapy, radiation, lasers, and surgical excision. However, surgical removal of the keloid is not normally performed unless the tissue becomes pendulous (WOCN, 2022).
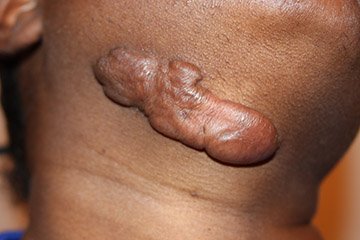
Keloid scar on the neck. The tendency to develop keloids is a genetic trait. (Source: Htirgan, CC BY-SA 3.0.)
Hypertrophic Scars
Hypertrophic scars are caused by excess deposition of collagen fibers in a healing wound. This happens in burns, infected wounds, and wounds healing under tension. In hypertrophic scars, the excessive formation of collagen usually stops within a few weeks. The result is a scar that is thicker than normal and raised above the plane of the skin, but unlike a keloid, a hypertrophic scar does not expand out beyond the actual wound. Hypertrophic scars, which usually get smaller spontaneously, can occur anywhere on the body (Bryant & Nix, 2024).
Hypertrophic scars are also produced in wounds that have a long reaction (inflammatory) healing phase and in which reepithelialization has been delayed, such as in many burn wounds. For burn patients, continuous pressure (constant pressure lasting 6 to 12 months) can help to reshape and flatten hypertrophic scars. Specialized secondary pressure dressings are available for hypertrophic-susceptible and burned areas such as the face and hands. Laser therapy can also be used in the treatment of existing hypertrophic scars and as a mechanism to avoid scar formation in the early postoperative phase (Mony et al., 2023).
Hypertrophic scar, four months after incident. (Source: Cgomez447, CC BY-SA 3.0.)
Contractures
All scars go through a process of shrinking or contracting. Enlarged scars, however, sometimes contract excessively, with extreme tightening and constriction of the skin surface, leading to physical defects and functional disability (Johns Hopkins Medicine, 2025). When contractures form over joints, the scars can make bending difficult or impossible. Disabling contractures most commonly form across finger joints, along the neck, across the axilla, and across the antecubital fossa.
Contractures after amputation surgery of a lower extremity can occur in up to 5% of cases and can begin to develop within a few days after the surgery. Contractures usually occur in the joint nearest to the amputation site, such as the tibiofemoral (knee) joint or the acetabulofemoral (hip) joint.
A contracture is a permanent fixture of the skin, and it cannot be repaired by stretching, massaging, or applying ointments, lotions, or creams. The most successful treatment for a contracture is to have it excised surgically. Early consultation with physical and/or occupational therapy can be an important step in the prevention of contractures during the wound-healing process (Friedlander et al., 2023).
Types of Wound Closure
Wound closure is described as:
- Primary closure (primary intention)
- Secondary closure (secondary intention)
- Tertiary closure (tertiary intention)
In primary closure the layers of involved tissue are brought together, and the wound edges are approximated and then surgically closed with either sutures or skin staples. Wounds closed by primary intention require only a limited amount of collagen to repair the tissue damage.
Secondary wound closure occurs when wounds are left open after surgery. An example of this is an abdominal wound repaired to the level of the fascia, with the remaining layers above this left open. These wounds fill in with new granulation tissue over a period of time, followed by wound contraction and reepithelialization. How long this takes depends on the overall condition of the patient and is affected by the presence of concomitant conditions such as cardiac disease and diabetes. Secondary wound closures tend to leave a larger scar. Other examples of wounds that heal by secondary intention are dehisced surgical wounds and pressure ulcers/injuries.
Tertiary intention, or closure, combines primary- and secondary-intention wound repair. The wound is allowed to fill in with granulation tissue and is then surgically closed. Delayed primary closure is used for highly contaminated wounds that may need repeated debridement or may need to be treated with antibiotics before being closed.
The main factors determining whether a wound will be closed immediately by primary intention or left open to heal by secondary or tertiary intention is whether there is a high risk of infection and whether the degree of tissue loss is such that the wound edges cannot be easily approximated without putting undue tension on the incision line. The immediate primary closure of a well-cleansed wound protects it from new contamination and allows the most control over the size and appearance of the final scar.
Wounds closed with sutures add new foci for infection (i.e., the suture holes), and sutures should not be left in place longer than is necessary. It is recommended that sutures be removed within one to two weeks after their placement. However, sutures should not be removed prematurely, since this may increase the risk of dehiscence and an increase in scar tissue. The following table lists optimal suture removal times for specific anatomic locations.
| Location | Time (days) |
|---|---|
| (Ratner, 2020) | |
| Face | 5–7 |
| Neck | 7 |
| Scalp | 10 |
| Arms and trunk | 10–14 |
| Lower extremities | 14–21 |
DEHISCED WOUNDS
Wound healing by primary intention, showing partial dehiscence. (Source: Intermedichbo, CC BY-SA 3.0.)
When a wound that has already been closed spontaneously reopens, this is referred to as a dehisced wound. Incomplete dehiscence occurs when the skin edges separate but the deeper layers of tissue remain together. In complete dehiscence all layers of the wound separate, and this can extend down to and beyond the fascia. Evisceration happens when the intestine protrudes into the wound; it is a medical emergency (WOCN, 2022).
WOUND CARE TEAM
Successful wound care is team based and holistic in its approach. The collaboration of the various team members enhances and expands the perspectives of the team, provides broad knowledge, and ensures that the patient receives the best possible care.
A wound care team draws from the expertise of several disciplines and at a minimum will include a physician with specialized training in wound care, nursing, physical therapy, occupational therapy, a dietitian, and a case management/discharge planner. Depending on the type, severity, and location of the wound, the team may also include a general surgeon, plastic surgeon, podiatrist, foot and ankle specialist, CWOCN (certified wound ostomy continence nurse), and certified diabetes educator.
Certification
Specialized certification in wound care is available for clinicians in different practice areas. The Wound Ostomy and Continence Nursing Certification Board (WOCNCB) offers the CWOCN credential to RNs who have a bachelor’s degree in nursing, have graduated from an accredited wound care program, and have passed the required certification exams. If the clinician’s focus is entirely on wound care, there are programs that offer only wound care or wound and ostomy care. There is also an advanced practice certification for nurses who have completed an MSN or other graduate nursing degree (WOCNCB, n.d.).
The National Alliance of Wound Care and Ostomy (NAWCO) also provides wound certification to a wide variety of healthcare professionals, including physicians, nurses, physical therapists, and occupational therapists (NAWCO, 2020).
The American Professional Wound Care Association (APWCA) provides several certifications, including Certified Hyperbaric and Wound Specialist (CHWS) and Certified Skin and Wound Specialist (CSWS). The requirements for CHWS certification include at least two years of experience as a hyperbaric technician with cross-training as a wound care assistant or comparable clinical position. This certification is open to several professions, including physicians, respiratory therapists, nurses at different practice levels, nurse practitioners, RNs, and LPNs. The CSWS certification requires at least 500 hours of clinical wound training and active practice experience in wound care yearly during the previous two years. This certification is also open to the professionals listed above (APWCA, n.d.).
The American Physical Therapy Association (APTA) offers a wound management specialist certification for physical therapists (APTA, n.d.).
While certification is not required to work in wound care, it can be regarded as part of continuous professional development and a commitment to a lifelong learning process. Certification demonstrates a level of expertise that enhances the professional standing of the clinician. Employers recognize and value the extra qualification, and it boosts the level of confidence patients have in the care they are receiving.
Team Member Functions
Each member of the wound care team contributes unique skills and therapeutic interventions that complement each other and provide for a fully comprehensive approach to wound treatment and healing. Among team members, there are both overlapping and unique functions, as described below (Bryant & Nix, 2024; Mackenzie, 2020).
PHYSICIAN
- Takes on the role of team leader
- Oversees the treatment plan
- Consults with the other team members
- Utilizes the services of other professionals as needed, such as general surgery, podiatrists, and orthotists
- Performs sharp surgical debridement when clinically indicated
- Evaluates the appropriate use of new treatments or procedures
- Applies bioengineered skin products to wounds as needed
PHYSICAL THERAPIST
- Completes wound assessments, sets goals, makes recommendations for treatment, and provides hands-on wound care
- Performs specialized wound treatments such as electrical stimulation to the wound bed, pulsed lavage, and sharp debridement (where permitted under their state practice act)
- Applies compression if necessary
- Administers negative pressure wound therapy
- Utilizes laser therapy in the prevention and treatment of scar formation
- Employs ultrasound therapy to aid in decreasing wound scale
- Assesses strength, sensation, bed mobility, and transfer ability
- Recommends assistive technology, such as wheelchairs, wheelchair cushions, ambulatory devices, etc.
- Maintains range of motion in joints affected by a wound or in close proximity to a wound; maintains and improves overall strength, positioning, and orthotic use
- Performs pressure mapping for patients at high risk for ulcer formation
- Teaches crutch walking to patients with contact casting
- Assists patients to titrate activities to decrease the risk of wound recurrence
(APTA, 2020; Guarino et al., 2023)
As with other licensed professions, individual state regulations define and limit any specialized training and the therapeutic interventions physical therapists are allowed to perform.
OCCUPATIONAL THERAPIST
- Evaluates the patient’s ability to maintain self-care
- Recommends a program of therapy to increase the patient’s capacity to perform activities of daily living
- Provides hands-on wound care and monitors the wound status
- Conducts conservative sharp debridement of devitalized wound tissue
- Administers negative-pressure wound therapy
- Utilizes treatment interventions such as electrical stimulation, ultrasound, and whirlpool
- Constructs special splints and orthotic devices to protect areas that are healing and to prevent deformity
- Applies wound closure strips
- Removes sutures and wound closure strips
- Provides individualized therapeutic interventions
- Teaches the appropriate use of adaptive devices for self-care activities such as bathing, dressing, and meal preparation
(Amini, 2018)
As with other licensed professions, state regulations define and limit any specialized training and the therapeutic interventions occupational therapists are allowed to perform.
NURSE
- Performs a comprehensive assessment of the patient using the nursing process
- Completes a thorough wound assessment and documentation of findings
- Carries out local wound treatments
- Monitors the status of the wound
- Performs conservative sharp debridement of devitalized wound tissue
- Administers negative-pressure wound therapy
- Applies compression if needed
- Removes sutures
- Collaborates with other team members in the development of the patient treatment plan
- Helps to ensure that the plan of care is followed, reviewed, and adjusted as needed at regular intervals
- Advocates for the patient in voicing concerns about their care and having their questions addressed
- Helps to formulate the discharge plan and follow-up care for the patient
As with other licensed professions, state regulations define and limit any specialized training and the therapeutic interventions nurses are allowed to perform.
IMMEDIATE TREATMENT FOR ACUTE WOUNDS
Depending on the level of injury involved, acute wounds are often medical emergencies. The most frequent causes of acute wounds are trauma related to:
- Motor vehicle accidents. Injuries to the driver of the vehicle, passengers, and pedestrians may result in wounds that reach from skin level down to the internal organs.
- Gunshots. These wounds often have extensive tissue damage that is not always evident at the time of injury. It may take several days for the full extent of the injury to become apparent.
- Agricultural and industrial accidents. Many of these injuries cause amputation or partial amputation of a limb and can result in highly contaminated wounds.
- Natural disasters. Severe weather conditions such as earthquakes, hurricanes, tornadoes, and freezing conditions can all lead to wounds from flying debris, crush injuries, or frostbite.
- Animal bites. These range from a nip from the neighbor’s dog to a penetrating bite from a wild rodent. In all of these cases, infection from saliva is a serious concern.
Stabilization
For individuals with acute wounds, the immediate concern is stabilization of the patient due to injuries that may be life-threatening. Maintaining a patent airway, ventilation, and adequate circulation are the first considerations, along with controlling blood loss. Wound evaluation and cleaning will be done in conjunction with several other simultaneous interventions, such as placement of intravenous lines and cardiac monitoring.
Wound History
Once the patient is stable, obtaining a wound history is important (see also “Wound History for Chronic Wounds” below). The most pertinent information is:
- How did the injury and wound occur?
- How much time has passed between the injury and treatment? This establishes how long the wound was exposed to possible contaminants; bacterial contamination and infection is a major concern in the treatment of acute traumatic wounds.
- What is the patient’s immunization status? When did the patient last have a tetanus booster vaccination? (Clostridium tetani is an anaerobic organism capable of causing serious infection in wounds that have been exposed to soil, feces, or saliva or wounds that result from crush injuries or frostbite.)
- Was there exposure to rabies? Was the wound caused by a bite by a wild animal or a domestic pet that has not been vaccinated against rabies?
Cleaning
Trauma wounds must be thoroughly cleaned of all debris, and potentially infectious material must be removed. Depending on the size and depth of the wound, debridement and wound irrigation may need to be done in the operating room under anesthesia. Whether it is performed in the emergency department or in surgery, the aim of debridement is to establish a clean, healthy wound free of nonviable tissue and foreign particles.
Closing
The risk for infection often determines whether an acute trauma wound is 1) closed by primary intention, 2) left open to granulate (grow new healthy tissue in the wound bed) in secondary-intention healing, or 3) surgically closed at a later stage (tertiary intention). Primary closure is not the preferred route for trauma wounds; experts recommend leaving the wound open to heal by secondary intention. Primary closure is the best choice, however, for facial wounds and wounds where there is scant tissue loss.
COMPREHENSIVE WOUND ASSESSMENT
A complete and accurate wound assessment is the foundation on which successful wound care is built. The information obtained from this assessment provides the criteria for identifying the wound etiology and subsequent classification of the wound. The clinician must invest the time required to gather the objective data that is an essential requirement of a consistent and reliable wound assessment.
Patient History
The process begins with an overall patient history, which serves the dual purpose of clinical evaluation and establishing a therapeutic relationship with the patient. The optimum environment for a patient history is a quiet, comfortable, well-lit room. The overall history will include the components described below.
HEALTH HISTORY
- Patient’s perception of their current state of wellness
- Diagnosed medical conditions and treatments
- Past health status
- Surgeries
- Injuries, accidents
- Disabilities and their impact on the patient’s life
- Current medications (including over-the-counter medications, supplements, and herbal remedies)
- Allergies (since several wound treatments contain substances that can cause an allergic reaction)
- Alcohol, tobacco, caffeine, and substance use, both past and present
- Family health history, including any possible genetic conditions, such as diabetes mellitus, heart disease, or circulatory conditions
(WOCN, 2022l Baranoski & Ayello, 2020)
SOCIAL HISTORY
- Employment, full time or part time. What amount of sitting, standing, and walking does the patient normally do during work activities?
- Recreational pursuits.
- Family composition. Who comprises the patient’s main support system? Is there anyone the patient wants to be involved in providing assistance? Is anyone willing to learn wound care and help with dressing changes at home?
- Home environment. Does the patient live in a family home, rented accommodation, or trailer home, or do they lack permanent accommodation?
- Health insurance. Does the patient have private insurance? The team social worker or case manager will need to verify what wound care benefits the patient’s insurance policy will cover and if there are any provisions for home care. All out-of-pocket expenses need to be discussed with the patient prior to the start of care. If the patient is on Medicare and/or Medicaid, prior authorization of some treatments may still be necessary.
- Patient’s education level. This is best obtained by asking the patient about the level of formal education they have completed.
- What is the patient’s preferred choice for learning new material? Remind the patient that they can choose one of several options.
- Cultural or religious beliefs. Some wound care products contain substances that may not be allowed in certain religions (e.g., dressings derived from pork). The source of animal-based products (e.g., honey- or pork-based dressings or ostomy products containing gelatin) should also be discussed with patients who are vegan.
PSYCHOLOGICAL HISTORY
- How does the patient rate their satisfaction with their life at the moment? Pay close attention not only to the words the patient uses but also to body language, facial expressions, eye contact, and general emotional affect (angry, depressed, sense of hopelessness). For example, “This wound is never going away; I’ll take it to the grave with me.”
- How has the patient coped in the past with illness and loss?
- Is the patient receiving treatment from a psychiatrist or a psychologist presently or in the past? Research findings show that individuals with chronic wounds experience more mental health issues than those without chronic wounds and are less able to cope with stressful events (Baranoski & Ayello, 2020).
- Has the patient experienced chronic wounds in the past? For patients with diabetes and venous or arterial disease, recurrent wounds are a common phenomenon. How did the patient cope with such wounds? How do they perceive that the current wound is different? As a patient’s age and chronic health conditions (such as diabetes or COPD) take a toll on their stamina, a new wound can have a more debilitating effect on their life than previous wounds.
- What are the patient’s beliefs on wound care and healing? Culture and background play an important role in everyone’s life, and many cultures have their own healing practices. At the assessment stage, respectful listening is essential, not dismissing the patient’s beliefs (e.g., “My grandmother always said that you have to leave a wound open to air for it to heal properly”). Current wound care practices can be discussed with the patient during the formulation of the treatment plan (e.g., “Nearly all grandmothers thought that way, since it was the accepted care for that time period”).
Nutritional Assessment
The importance of optimal nutrition in wound healing cannot be overemphasized. Therefore, the patient’s current nutritional intake must be assessed, along with lifestyle issues that may impede adequate intake, such as inability to shop independently, lack of transportation, diminished financial resources, etc. The main components of a nutritional assessment are:
- Weight during the initial assessment. Determine the patient’s weight history, e.g., has the patient experienced significant weight loss in the past six months? It is important to recognize that overweight or obesity is not an indication of sufficient nutrition.
- Capacity to eat independently. Does the patient have chewing or swallowing difficulties, physical limitations, or diminished mental status? Do they rely on the assistance of others to prepare and serve food? Is this assistance available to them on a consistent basis?
- Appropriateness of the patient’s total nutritional intake, including fluid intake.
- Medication regime, for possible negative impact on intake and nutrition.
Nutritional assessment is an ongoing process, and changes may occur in a patient’s nutritional status during the course of wound treatment. Wound healing causes a general increase in metabolism and greater demand for calories and protein.
Nutrients also play significant roles in different stages of the wound-healing process.
- Inflammatory phase. During this stage, calcium and vitamin K are needed to trigger the coagulation process and fibrin for clot development.
- Proliferation phase. Amino acids are critical to the successful completion of this phase. Arginine assists with the management of collagen deposition; it also supports neovascularization and helps with wound contraction. Vitamin B complex has an important part in metabolism and stimulates cell proliferation. Lipids supply energy for wound healing and cell production. Zinc also plays a role in cell proliferation, while iron is involved in the creation of hemoglobin, which is necessary for tissue perfusion in all stages of wound healing.
- Remodeling. Vitamin C is essential for collagen production, and vitamin E aids in the reduction of scar formation.
Water is a vital component in the wound-healing process but often does not get the attention it deserves. Water assists with the transfer and maturing of epidermal cells and provides important basics to the cytoplasm of skin cells (Baranoski & Ayello, 2020; Ju et al., 2023).
SIGNS OF MALNUTRITION
During the nutritional assessment, the clinician observes for these signs of malnutrition:
- Dull, brittle hair
- Dry eyes
- Pale mucous membranes
- Fissures at the corners of the mouth
- Swollen gums that bleed easily
- Tongue soreness, diminished sense of taste
- Missing teeth or poorly fitting dentures
- Brittle or spoon-shaped nails
- Dry, flaking skin
- Lack of subcutaneous tissue beneath the skin
- Muscle cramps, pain, or muscle wasting
- Lethargy
- Poor memory
(Baranoski & Ayello, 2020)
When the clinician recognizes that a patient is nutritionally compromised or at risk for inadequate nutrition, a consultation with a registered dietitian nutritionist (RDN) is crucial (WOCN, 2022).
Decreased albumin and prealbumin levels were in the past regarded as biochemical markers for malnutrition. However, research has shown that albumin and prealbumin levels are affected by the inflammatory process and not by nutritional status. They are now considered to be a better gauge of the severity of inflammation than of nutritional intake (Baranoski & Ayello, 2020; WOCN, 2022).
The recommendation from the Academy of Nutrition and Dietetics and the American Society of Parenteral and Enteral Nutrition is that malnutrition in adults is determined when two or more of the following measures exist:
- Inadequate energy intake. Comparing current intake to approximated needs is a standard to measure malnutrition.
- Clarification of weight loss, taking into account existing health conditions and patient hydration status.
- Loss of subcutaneous fat. Areas assessed on examination include the presence or absence of orbital fat, or fat overlying the rib cage.
- Loss of muscle mass, including deltoid, scapula, quadriceps, and other areas.
- Presence of edema. Localized or generalized edema can sometimes conceal weight loss.
- Decreased functional capacity, determined by handgrip strength.
The rate of malnutrition differs according to age, disease process, and location of the patient. In older adults (over 65 years), malnutrition occurs in 5% to 10% of those living in a community setting, 20% to 40% of patients in a hospital setting, and up to 50% of those in a nursing home (Shah et al., 2018; WOCN, 2022; Cederholm & Bosaeus, 2024).
Treatment for malnutrition will be based on recommendations from the RDN. It may also require a swallowing evaluation done by the speech pathologist and recommendations for adaptive equipment from the occupational therapist.
As far as possible, an individualized dietary plan should be implemented for each patient. For patients on restricted diets with poor intake, a more liberal plan is considered. For example, offering six small meals a day rather than the traditional three large meals may be more beneficial. Alternating the type and texture of high-protein, high-calorie snacks offered to the patient between meals provides variety and helps to prevent flavor exhaustion (WOCN, 2022).
(See also “Nutrition” later in this course.)
WOUND HISTORY FOR CHRONIC WOUNDS
The wound history for a chronic wound is similar to that for an acute wound, although there are some differences. Questions may include:
- How did the wound occur?
- How long has it been present? There is a direct correlation between the length of time a wound has been open and its ability to heal; wounds of longer duration are less likely to heal.
- What treatments have been used and how successful have they been? What are the patient’s understanding and perceptions of the wound care they have received so far?
- What difficulties has the patient encountered in caring for the wound at home?
- Is there pain associated with the wound? When does it occur? What does the patient do to relieve the pain? Are they taking pain medications, and if so, how effective are they? Have the patient describe the pain in their own words and then ask them to put a numerical value to their worst pain level and to the pain they are experiencing at that moment.
Wound Assessment
A thorough physical examination of the wound is vital regardless of the etiology of the wound. Every wound assessment is important, but the initial assessment is particularly so. It is the baseline against which all future assessments will be compared, it provides the basis on which the treatment plan will be devised, and it serves as an important indicator in evaluating the outcomes of care.
A wound assessment can be done by an RN, an advanced practice nurse, a physical therapist, or a physician with the required knowledge and experience in wound care. A wound assessment provides objective findings to assist in classification of the wound by its etiology, which is an important factor in deciding overall patient care. Each new assessment will be compared to the previous assessment and to the baseline assessment. It is imperative that changes in the wound, especially any subtle signs of deterioration, are identified and addressed immediately (Bryant & Nix, 2024; Baranoski & Ayello, 2020; WOCN, 2022).
The steps of wound assessment are described below.
LOCATION
The clinician accurately describes the anatomic location of the wound, using identifiers such as proximal, distal, medial, lateral, anterior, posterior, right, or left. Depending on facility protocols, diagrams can be useful in precisely identifying wound location. If there is more than one wound in the same proximity, it is important to label and number each wound for clarity (e.g., “wound 1a” and “wound 1b”) (Baranoski & Ayello, 2020).
SHAPE
The shape of the wound can be useful in identifying its etiology. For example, chronic wounds on a lower extremity that are round and with a “punched-out” appearance are typically caused by problems with arterial circulation to the affected leg, while those that are irregular in shape are associated with venous disease.
SIZE
Wounds are measured in centimeters using a disposable plastic or paper ruler. An accurate measurement of wound size on the initial assessment is essential.
There are two commonly used techniques for measuring wounds, and which one is used depends on the policies of the facility:
- The first method uses the “face of the clock” and measures the length of the wound straight up and down, from 12 o’clock to 6 o’clock. The width of the wound is then measured straight across, from 3 o’clock to 9 o’clock.
- The second method of wound measurement uses the longest aspect of the wound (top to bottom) and the widest aspect perpendicular to the length.
Another method of measuring wounds is planimetry. A tracing of the wound is done using metric graph paper with a 4-cm or 8-cm grid. The completed squares inside the outline of the wound edges are counted to provide an estimated area of the wound in square centimeters (Baranoski & Ayello, 2020).
Wound area can also be obtained by using stereophotogrammetry (SPG), a noninvasive method using a digital camera and computer software. A digital photo of the wound is uploaded to the computer, and the software calculates the length, width, and area of the wound. Using this method, it is possible to obtain a color picture of the wound with up-to-date measurements done at each wound assessment. SPG has been shown to provide concise and dependable wound measurements, especially of irregular wounds (Baranoski & Ayello, 2020). Advances in technology for noncontact wound area measurement are increasing in popularity. In clinical studies, the accuracy of the new technology demonstrated a very positive correlation when compared to customary methods for wound measurement (Tambella et al., 2025).
There is debate among clinicians as to which is the most accurate method. What is most important is to employ one method of wound measurement consistently over time, as this will best capture changes in the dimensions of the wound.
The results of several studies show an important relationship between the degree of change in wound size at four weeks and the percentage of healing achieved at 12 and 20 weeks. That is, the greater the percentage decrease in wound size at four weeks, the greater the possibility of complete wound healing.
DEPTH
Wound depth is gauged by gently placing a sterile cotton-tipped applicator into the wound bed and marking the point at which it is even with the surface of the skin. It is important to keep in mind that many wounds have areas of varying depth, and measurements may need to be taken at different locations. The deepest area of the wound is used to classify the depth.
UNDERMINING
Undermining is an area under intact skin where the tissues have separated and there is a “shelf” present. It is not visible from the outside. The face-of-the-clock method is typically used to describe the location of undermining. Starting at the top of the wound (toward the head, or the 12 o’clock location), a sterile cotton-tipped applicator is inserted to the full depth of the undermining and slowly moved in a clockwise direction to determine how far the undermining extends.
Measurements are documented in the following manner: “Undermining to a depth of 5 cm, extending from 12 o’clock to 3 o’clock, deepest at 2 o’clock = 5 cm.” The clinician gently probes along the entire wound edge to determine if there are other areas of undermining. Frequently, undermining will occur at different locations and to different depths and can be interspersed with areas of normal wound edges.
TUNNELING
Unlike undermining, tunneling is a narrow channel (tunnel) that can be located anywhere along the wound edges or in the base of the wound. It is measured in the same way as undermining. Since tunneling is an area of dead (empty) space, there is a risk for abscess formation (Baranoski & Ayello, 2020). A newly discovered tunnel in a surgical wound or a trauma wound left open to heal by secondary or tertiary intention must be reported to the surgeon and not probed by the clinician until the surgeon has had an opportunity to evaluate it.
WOUND BED
A gentle cleansing with normal saline and gauze is usually sufficient prior to assessing the wound bed. The type and amount of tissue in the wound must be described accurately. If there is more than one type of tissue in the wound, this will be documented in approximate percentages, for example, “The wound base is 50% black necrotic tissue and 50% grayish/green slough.”
TISSUE TYPES
Eschar is dead tissue; it is usually associated with deeper tissue damage. It can be dry or moist, with colors ranging from black, brown, tan, or yellow to gray. Eschar can be adherent to the wound bed like a leathery coat. It presents a medium for bacterial growth and impedes wound healing. In most instances, eschar is debrided (removed) from the wound, but there are exceptions to this rule (discussed later in this course).
Slough is soft, moist, devitalized tissue present on the wound bed. In appearance, it is a mucus-like, stringy, fibrin material with a yellowish/green color. It may crisscross the wound bed in a wiry pattern or be found in moist clumps at different locations in the wound bed. Slough is composed of cell debris, fibrin, intact leukocytes, microorganisms, and serous exudate. Slough is not found in stage 1 and stage 2 pressure injuries (Bryant & Nix, 2024; Baranoski & Ayello, 2020).
The terms eschar and slough are both used to describe different levels of necrosis.
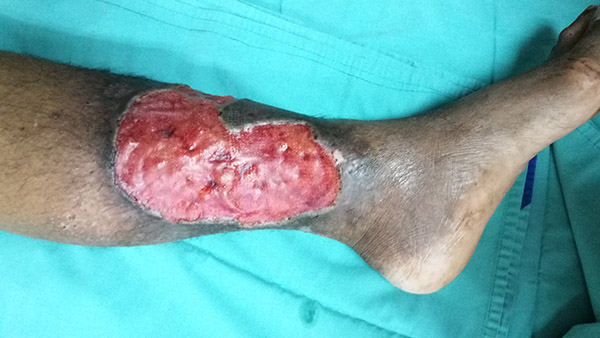
Varicose ulcer with granulation tissue. (Source: Casa Nayafana/Shutterstock.com.)
Granulation is beefy-red tissue with a “cobblestone” appearance, comprised of small blood vessels and connective tissue. A distinction is made between a wound that is actively growing new tissue and one that has plateaued or stalled. The latter is characterized by pink, shiny, smooth tissue and is no longer healing.
WOUND EDGES
Assessment of the wound edges must not be overlooked. Wound edges should be clear and distinct from the wound bed as well as open and proliferative, which means that they are red, moist, and flexible. Wound edges must be open to facilitate the ingrowth of new blood vessels and tissue in the wound bed and for the wound to contract and become smaller. Wound edges that are closed are a common finding in chronic wounds. These wound edges are thickened and rolled under and feel hard to the touch.
DRAINAGE
Wound drainage is a natural and necessary component of the wound-healing process. Wound drainage serves many purposes, including dispersion of immune mediators and growth factors, and stimulating the separation of dead or damaged tissue from the wound bed. In a dry wound, there is no drainage. A moist wound has sufficient drainage to keep the wound bed moist, which is critical for healing. However, a large volume of malodorous wound drainage is a sign of infection (Harding et al., 2019). The following terms are used to describe wound drainage:
- Serous: clear to yellow drainage with no blood or pus present
- Serosanguineous: watery drainage that is pink to pale red in color
- Sanguineous: bright red blood drainage
- Seropurulent: thin, watery drainage that is white to cream in color
- Purulent: thick, cloudy drainage that varies in color from tan to dark brownish
(Shah et al., 2018; Baranoski & Ayello, 2020)
WOUND ODOR
Odor is present in nearly all wounds. Wound odor is usually associated with the amount of drainage in the wound, the presence or absence of necrotic tissue, and infective organisms. Necrotic wounds have a strong malodor, and wounds infected with Pseudomonas have a distinctive sweet, fruity smell. An ammonia-like smell is typical of a Proteus infection in the wound (WOCN, 2022; Baranoski & Ayello, 2020).
PERIWOUND SKIN
The skin surfaces around the wound are carefully assessed. Ideally, these areas should be clean, dry, and intact, indicating that they have not been adversely affected by the wound’s presence. Periwound skin can experience changes that may indicate that the current wound treatment is not effective, the absorptive dressing is not preventing leakage of wound drainage onto the intact skin area, or the presence of infection or pressure.
The color of the periwound area can be described as:
- Increased redness/erythema
- Pale/pallor
- White/gray
- Blue/purple
The texture of the area can be:
- Dry/flaky
- Moist
- Indurated/hard
- Excoriated (linear scratch marks)
- Denuded (loss of epidermis)
- Boggy/soft to touch
- Macerated/waterlogged
Periwound skin temperature can be assessed as:
- Normal (same temperature as skin surfaces not in the immediate wound area)
- Warm
- Hot
- Cool
CASE
Mr. Jackson arrived to the emergency room (ER) with a facial injury. The wound, situated below his right eye, was bleeding profusely. Mr. Jackson was pale and clammy, exhibiting signs of shock and complaining of severe pain. The ER doctor ordered IV administration of pain medication to be started. After completing this task, the nurse, Miguel, set about gently cleaning the wound with normal saline and sterile gauze.
Miguel determined that the wound base was clean and free from debris. The wound measured 4 cm in length, was shallow, and had ragged wound edges. He covered it with gauze moistened with saline and a small pad while waiting for a surgical consultation.
Miguel continued to monitor the patient’s vital signs, which stabilized, and the pain level, which the patient described as “tolerable.” The nurse then proceeded to take a history of the wound, determining when, where, and how it happened. It occurred about 30 minutes before the patient arrived in the ER, in the chicken coop in his backyard, when he tried to restrain a neighbor’s dog from attacking his chickens. The dog turned on Mr. Jackson and attacked him.
Since the patient was wounded in an area with potentially contaminated soil, Miguel inquired about the patient’s tetanus status, to which Mr. Jackson stated that he had received a tetanus shot about six months earlier. Miguel also asked if the patient knew whether the dog had received rabies shots and was told that the dog had not. The nurse put a red asterisk beside this to inform the doctor. The nurse then took a brief history of the patient’s past and current medical history, which included back surgery and a diagnosis of hypertension.
Arriving to the ER, the surgeon assessed the wound and decided it could be closed by primary intention in the operating room. Following the procedure, Miguel reinforced the surgeons’ instructions to Mr. Jackson not to remove the wound dressing before he returns to the surgical clinic in five days’ time; to keep the dressing clean and dry; and if he develops fever, pain, or wound drainage, to call the surgeon’s office. The patient verbalized understanding of the instructions given.
Miguel also advised Mr. Jackson that the dog who attacked him should be observed for 10 days for signs and symptoms of rabies, such as restlessness, irritability, aggression, foaming at the mouth, or excessive saliva or drooling. If the animal is healthy during this period, it is considered not to have rabies, and the patient will not need rabies shots (Mayo Clinic, 2025; WebMD, 2025).
Pain Assessment
Pain assessment is a vital part of all wound care. Management of wound pain is individualized and takes into account patient preferences. When completing the pain assessment, the clinician considers the following elements:
- Location. Is the pain felt in the wound bed, the surrounding area, or both?
- Distribution. Is the pain localized or does it spread to other parts of the body?
- Patient’s description. How does the patient describe the pain in their own words? If they find it difficult to come up with descriptive terms, some terms can be offered, such as “aching,” “sharp,” “dull,” “throbbing,” or “pins and needles.”
- Intensity. A 0-to-10 scoring method is the most frequently used, in which 0 is no pain and 10 is the worst pain possible.
- Procedural and resting pain. Does the pain increase or decrease with wound care, and how long does it last after the procedure is completed?
- Management strategies. What interventions make the pain better or worse? This may include medications, relaxation techniques such as guided imagery, etc.
(WOCN, 2022)
Wound Photography
Photography can be used to track the progress of a wound and as an aid to written documentation. It is particularly useful in difficult-to-describe wounds such as those with irregular shapes, rolled wound edges, and involvement of the periwound areas. Photography allows for consultation with numerous specialties to determine complex differential diagnoses. It lessens the overexposure of the wound by many healthcare professionals. Photography also aids when interacting with payment sources to show reason and support for treatments used. Some facilities choose to use photos and others do not.
The question arises, how often should photos of the wound be taken? The answer depends on individual facility policies, but at a minimum, photos are taken the first time the wound is assessed, once healing has occurred, and when the patient is transferred to another care setting. Some facilities have policies in place that require weekly wound photography, and this again can help to evaluate the effectiveness of the current wound care.
Prior patient consent is required for wound photography, and the use and confidentiality of the photos must be thoroughly explained to the patient. A written facility policy on wound photography will address:
- Patient consent (may be part of the conditions of admission, but some facilities add a separate consent for wound photos)
- Frequency of photography
- Staff authorized to take wound photos
- Methods of identifying the patient, for example, placing the patient’s initials, medical record number, date, and time on a measuring guide placed proximately to the wound and included in the photo
- Storage of the photos in the patient’s records and who will have access to them
(Baranoski & Ayello, 2020; Bryant & Nix, 2024)
Assessment Frequency
After the initial assessment, the frequency of subsequent assessments depends on the patient’s overall condition, the status of the wound, and the care setting. For example, a wound that is infected and draining heavily may require daily or twice-daily assessment. As the condition of the wound changes, the frequency of assessments will also change. For example, a wound that is free of infection and displaying the development of healthy granulation tissue can be assessed once or twice a week.
CASE
The clinician, David, is assessing a wound on a 94-year-old male patient, Mr. Rodriguez, who was recently admitted from home. After reviewing the patient’s history, David is aware that the patient has limited mobility and has been cared for at home by his elderly spouse. He is confused, malnourished, and dehydrated. When Mr. Rodriguez was admitted to the hospital, the nurse who conducted the admission assessment discovered a pressure injury on the patient’s sacrum.
In order to complete his wound assessment, David requires assistance to reposition the patient on his right side. He washes his hands, dons clean gloves, and removes the pad covering the sacral wound. Mr. Rodriguez moans slightly when David removes the dressing, but he does not respond when David asks if he is in pain.
David takes a few moments to look at the wound. Its position over the sacrum indicates that it is a pressure injury. The wound edges are regular in shape and appear to be open. The surrounding skin surfaces are intact, but there is some redness in the vicinity of the wound, which David determines is due to unrelieved pressure. He gently palpates the area around the wound. There is no warmth, induration, or boggy texture felt. The wound bed appears moist, and David notes that there is a moderate amount of serosanguineous drainage on the dressing he removed.
Using 4×4 gauze, David gently cleans the wound bed. He notes that it is pale pink with a shiny, slick surface, which indicates the probability that a biofilm is present. There is no detectible wound odor. Muscle tissue and bone are not visible, and there are no areas of tunneling. There are scattered strands of loosely adherent grayish/white slough in the wound bed.
David estimates that the slough accounts for approximately 30% to 35% of the wound surface. Using a disposable measuring guide, he measures the wound using the face-of-the-clock method. He measures the length as 10 cm from 12 o’clock to 6 o’clock and the width as 6 cm from 3 o’clock to 9 o’clock. He documents the measurements, while also noting the method that he used to take the measurements and the position the patient was in at the time. David is aware that this information is important to include so that the same process will be followed with subsequent wound assessments in order to obtain accurate indications of any changes.
Using a sterile cotton tip, David then locates the deepest area of the wound bed, and using a felt pen, he places a mark on the cotton tip at skin level. He documents the wound depth as 2.5 cm. With a sterile cotton tip, he carefully probes the wound edges. Using the face of the clock as a guide, he discovers an area of undermining beginning at 7 o’clock and extending to 11 o’clock. At its deepest location, this area is 4 cm. David documents his findings, indicating that shear also likely played a part in the developing the pressure injury.
Mr. Rodriguez’s spouse has signed a consent for wound photography, and so David takes three photos of the wound, including a close-up of the wound surface and two from a wider angle, one from an anterior angle and one from the posterior angle.
David completes his assessment by documenting all his findings and indicating that this is a stage 3 pressure injury.
(See also “Pressure Injuries” later in this course.)
Developing the Plan of Care for Wound Management
The plan of care is a collaborative effort between the patient, physician, nurse, rehab therapists, case manager/discharge planner, and other specialists included on the wound care team. It is developed once all initial assessments have been completed. The plan of care is based on the goals of therapy: What does the patient want to achieve, and what can the members of the wound care team contribute to help achieve those goals?
Problems can arise when there is a discrepancy between what the patient wants and what can be realistically accomplished. Often the patient will express goals in general terms: “I want it all to go away so I can get back to living normal again.” This sentiment is frequently expressed by patients with chronic conditions who feel physically and emotionally worn down by pain and physical limitations.
In these situations, it is important to acknowledge what the patient is feeling and to work with them in establishing achievable short-term goals, for example, “Compression stockings will be worn every day for the next week and will result in a visible decrease in swelling.” Or in the long term, “Leg circumference will return to normal, and wearing compression stockings will become part of the daily routine.”
Patients are more apt to adhere to therapies when they can see and feel that gains are being made. This can be difficult and challenging with chronic wounds, where improvement can be slow and complex. However, goals can be broader than the actual wound healing. For instance, the physical therapist and occupational therapist can set goals with the patient to prevent contractures, increase mobility, and increase self-care.
Stress Management
A high level of stress is a common feature among those with chronic wounds. Patients with chronic wounds experience more depression, worry, and social isolation than those without chronic wounds. The physiologic response to stress includes increased cortisol production, which has several negative effects on wound healing, including delay in collagen synthesis. Interventions that can be employed to help patients deal with the stress of chronic wounds include identifying the patient’s primary concern, examining problem-solving strategies, setting goals, and routinely evaluating progress (Baranoski & Ayello, 2020; Bryant & Nix, 2024).
WOUND-CLEANSING TECHNIQUES
All debris, foreign bodies, and devitalized tissue must be removed from the wound bed in order for the healing process to begin. It is essential to select the correct cleaning agent and the correct technique for cleaning based on the characteristics of the wound. The goal of cleansing is to remove as much devitalized tissue as possible without damaging healthy tissue in the wound.
Wound Irrigation
Normal saline or water from a standard treated drinking supply can be used to gently clean the surface of the wound. Studies indicate that tap water can be used for cleaning most pressure ulcers/injuries, and in the home setting it is an effective and readily available method of wound cleansing. Research shows that tap water is a safe and cost-saving way to clean wounds (Holman, 2023).
Commercial wound-cleansing products contain surfactants capable of breaking down the bonds that attach contaminants to the wound bed, and they provide a higher level of wound-cleaning efficiency than saline or tap water. These products should be used at the strength recommended by the manufacturer.
Surfactants work well in wounds in which there is a high level of bacterial infection (Baranoski & Ayello, 2020). Current research shows that using wound cleansers containing surfactants has a positive effect on managing wound biofilms and is linked to improved rates of wound closure. The activity of surfactants can be boosted by physical disruption of the wound by the use of interventions such as sharp wound debridement or low-frequency ultrasound (Bryant & Nix, 2024).
Wound irrigation is an effective means of wound cleaning. It decreases the bacterial count in the wound and loosens devitalized tissue and debris while flushing them from the wound. The recommended pressure for wound irrigation is 4 to 15 pounds per square inch (psi) as measured using a 19-gauge angiocatheter and a 35-mL syringe. This has been found to adequately clean the wound without harming healthy tissue and embedding debris into the wound base. A pressure less than 5 psi is not successful in wound cleansing (WOCN, 2022; Baranoski & Ayello, 2020).
Pulsatile lavage is another alternative for wound irrigation. It delivers the cleaning agent to the wound bed from a powered device and is usually used in conjunction with suction.
Whirlpool was once a standard method for wound cleansing, and it was the recommended care for chronic wounds with slough and necrotic tissue. Pressurized streams of water were pumped into the whirlpool bath via jets. However, concerns about contamination and cross-infection have made whirlpool therapy a less-favored practice in wound care today.
CLEAN TECHNIQUE
Evidence to date shows that there is no difference in the incidence of wound infections between the use of clean and sterile technique in wound care. Clean technique has proven to be the most cost-effective in wound care, saving on time and resources (Staebel, 2023).
“Clean” means free of dirt, marks, or stains. This technique is also referred to as nonsterile technique. The components of clean technique are:
- Thorough handwashing
- Preparing a clean field and preserving a clean environment
- Using clean gloves and sterile instruments
- Preventing direct contamination of supplies
(Morgan, 2019)
Cleansing Agents
Cleaning of heavily contaminated wounds will require more than saline or tap water. Antiseptic solutions are usually required to kill bacteria in the wound, but these agents are also capable of destroying healthy wound tissue, so they must be used judiciously. Antiseptic use should be short-term and closely monitored.
Dakin’s solution (sodium hypochlorite) half strength 0.25% used for short periods of time (approximately 7 days) has been proven to be effective against most bacteria found in chronic wounds without damaging viable wound tissue; it also decreases wound odor.
Quarter-strength acetic acid can be used as wound irrigation or a soak to clean wounds. It has been shown to eradicate Pseudomonas aeruginosa from wounds. Sterile 4×4 gauze can be soaked in acetic acid then placed on the wound bed for approximately 15 minutes. However, acetic acid is also recognized for its cytotoxic action and can impede wound healing. Once the blue-green drainage associated with Pseudomonas infection and soft yellow slough have been eradicated from the wound, it is recommended to discontinue use of acetic acid (Chen & Zhou, 2022).
Debridement
Debridement is removal of dead tissue from the wound, and it is an important factor in wound bed preparation. When and how to debride is determined based on the type of wound, the patient’s overall status, the presence or absence of infection, and the goals of wound care (i.e., maintenance of the current wound status or progression toward healing). For example, debridement would not be the treatment of choice for intact, dry eschar covering the wound of a terminally ill patient.
Unless infection is present, debridement should not be done until the patient’s ability to heal has been thoroughly assessed. Patients with lower extremity wounds, regardless of the etiology, should have their circulatory status evaluated. The bottom line is that wounds will not heal without a good blood supply, and before de-roofing (removing) eschar and causing an open wound, the clinician must have a realistic expectation that the wound will heal.
In hospice patients, however, a wound may be debrided with no expectation the wound will heal. It is debrided to relieve pain and pressure by allowing the wound to drain and to provide improved access to cleanse the wound and decrease odor.
Debridement can be divided into two broad categories: selective and nonselective. Selective is the most beneficial to the patient, since it only removes nonviable material from the wound.
Nonselective debridement, as the name suggests, removes healthy granulating tissue as well as devitalized tissue from the wound bed. Saline wet-to-dry dressings are the best-known method of nonselective debridement but are no longer regarded as state-of-the-art care, and providers are moving away from this practice. Such dressings are painful for the patient and labor-intensive, since dressing changes need to be done three to four times daily.
Selective debridement methods are as follows:
SURGICAL DEBRIDEMENT
A physician or advanced-practice clinician can perform sharp surgical debridement. Sharp debridement is required when there is tunneling in the wound that needs to be opened and to address areas of undermining, if present. For large wounds, the procedure is normally performed in the operating room under anesthesia. Smaller wounds can be debrided at bedside or in an outpatient wound clinic using topical or local anesthesia.
The clinician must be aware of the importance of adequate pain management before, during, and after debridement. The procedure is fully explained to the patient and consent obtained. Prior to beginning the procedure, it is also important to ascertain whether the patient is on anticoagulant therapy and to review their most recent international normalized ratio (INR) results (Baranoski & Ayello, 2020).
REMOVAL OF EXCESS GRANULATION TISSUE
Granulation tissue is the loose collection of fibroblasts, inflammatory cells, and new blood vessels that forms in the bed of open wounds during the regrowth (proliferation) phase of healing. Healthy granulation tissue looks beefy red with bumpy, irregular surfaces. Epithelial cells use granulation tissue as a surface to move along as they re-cover the wound. The regrowth phase usually lasts four to 24 days. (When wounds are left open to heal by indirect closure, the regrowth phase is prolonged.)
Excess granulation tissue (also called exuberant granulation tissue or hypergranulation) can form in any wound. Sometimes, especially in wounds for which growth factors or other healing stimulants have been used, granulation tissue will overgrow the top of the wound and become a barrier to the growing epithelial cells. For a wound to heal properly, excess granulation tissue should be removed. The growth of hypergranulation tissue in a wound can also indicate the presence of fungal or bacterial infection (BioStem Technologies, 2024).
![Photograph showing hypergranulation tissue]](805/xwound-granulation-tissue.jpg.pagespeed.ic.Wh9C6bX_IQ.jpg)
Excess (hyper) granulation during healing on a cut finger. Note the beefy red, cobblestone appearance and small blood vessels that characterize granulation tissue. (Source: Linuxfox00, public domain image.)
Debridement can be a painful process, and prior to any tissue removal, the patient should be medicated for pain. Topical anesthesia can also be applied to the wound. When administering oral pain medications, acetaminophen or nonsteroidal anti-inflammatory medications (NSAIDs) are the treatment of first choice. It is important to keep in mind that NSAIDs have the potential for serious side effects in older patients, such as diminished renal function, gastrointestinal bleeding, and exacerbation of congestive heart failure. Wounds requiring extensive debridement are done in the operating room under anesthesia (Baranoski & Ayello, 2020).
To clear the wound, the physician, nurse, or physical therapist with specific training will scrape out the granulation tissue down to the bed of the wound, level with the surrounding skin. Then, the wound is irrigated.
Excess granulation tissue can also be treated with silver nitrate by a clinician trained in wound care. This will turn the surface of the wound a whitish/gray color and allow the excess granulation tissue to slough off. More than one treatment may be necessary.
Hypergranulation can be corrected by changing the cause, which can be added growth factors, excessive moisture in the wound, etc. In a clinical setting, the wound is protected with sterile gauze and/or covered with an appropriate dressing per physician orders. The application of vapor-permeable (breathable) foam dressings has also been found to be helpful in treating hypergranulation tissue (Mitchell & Llumigusin, 2021).
CONSERVATIVE SHARP WOUND DEBRIDEMENT
Certified wound care nurses and physical therapists with advanced wound care training can (where legally permitted) perform conservative sharp wound debridement. This is a sterile procedure whereby loosely connected necrotic tissue is removed from the wound using sterile scissors or scalpel and forceps.
It is important that clinicians check with their state board to ensure this intervention is within their scope of practice. A facility policy and procedure guidelines must also be in place for conservative sharp wound debridement.
ENZYMATIC DEBRIDEMENT
Enzymatic debridement is often used when surgical debridement is not feasible, for example, in patients on anticoagulant therapy and for whom there is a risk of bleeding. It is a safe and selective method of debridement. Enzymes selectively remove devitalized tissue and prevent damage to healthy tissue.
Collagenase (SANTYL) is the only enzymatic debriding agent available in the United States at this time. It is a derivative of Clostridium bacteria, and it cleanses the wound bed by dissolving the collagen bonds that secure the necrotic tissue to the wound bed. Collagenase works from the bottom up, with changes occurring beneath the wound’s surface. Visible changes in the wound may not appear for several days to weeks, depending on the size and depth of the wound.
Before each application of collagenase, the wound must be completely cleaned to remove residual enzymatic ointment and unattached wound debris. Collagenase is then applied in a thin layer to the necrotic tissue in the wound bed daily and covered with a moist normal saline dressing to prevent the wound from drying out. The manufacturer recommends a nickel-size thickness of the ointment on the wound bed. Collagenase can also be applied to the edges of eschar that has started to separate from the wound bed. This can be successfully done using a swab to gently push the agent under the edges of the eschar to promote further separation.
Hard, intact eschar should be crosshatched before applying collagenase to allow the agent to make contact with the wound bed. Crosshatching is a technique that cuts fine lines horizontally and vertically into the surface of the eschar using a sharp blade. This procedure may be performed by a physician, nurse practitioner, RN with wound certification, or physical therapist, depending on state licensing board regulations and facility policy (Baranoski & Ayello, 2020; Bryant & Nix, 2024).
AUTOLYTIC DEBRIDEMENT
This is a natural form of debridement that utilizes the body’s own white blood cells to clear necrotic tissue from the wound bed. It is a safe, although slow, form of wound cleaning. For autolytic debridement to work, the patient must have a normal white blood cell count and there must be adequate circulation to the wound bed. Autolytic debridement is not recommended for immunocompromised patients due to their low white blood cell count.
A moisture-retentive dressing (such as hydrocolloid, transparent film, or hydrogel) is applied to the wound bed and left in place for up to 72 hours. During this period, the white blood cells, neutrophils, and macrophages in the wound bed cause the necrotic tissue to loosen from the wound bed and become soft and stringy (Baranoski & Ayello, 2020; Bryant & Nix, 2024).
Autolytic debridement can cause the wound to become wider and deeper and to have an accumulation of exudate in the wound bed that produces a malodor. This is a normal part of the autolytic process, which the clinician should explain to the patient and caretakers, who often become concerned that the wound is infected.
BIOLOGIC/BIOSURGICAL DEBRIDEMENT
Also referred to as maggot therapy, this form of debridement employs sterile maggots applied to the wound bed. These larvae produce a mixture of enzymes and broad-spectrum antimicrobials. This method is faster than autolytic or applied enzyme debridement, but some level of discomfort can be noted. It is frequently used in wounds containing infected necrotic tissue but for which surgical debridement is contraindicated.
Medical-grade maggots are applied directly to the wound bed, around five to eight maggots per cm2. The maggots may be applied either in small pouches resembling teabags or “free-range.” Research indicates that free-range maggots are more effective at wound debridement than those enclosed in pouches or small sacks. Care needs to be taken to ensure that the maggots do not come in contact with healthy skin surfaces, as the proteolytic enzymes secreted by the maggots can injure intact skin (Baranoski & Ayello, 2020; Bryant & Nix, 2024).
The wound is then covered with a dressing to keep the maggots in place. It is essential for the cover dressing to allow oxygen to reach the wound bed and to facilitate adequate drainage so that the maggot therapy remains viable (alive). It is possible to purchase specially designed dressings for the enclosure of maggot therapy from the larvae distributor (WOCN, 2022).
Maggot therapy is contraindicated where blood vessels are exposed, in acute infections, in wounds requiring frequent inspections (maggot therapy usually lasts one to three days but may last up to four to five days), in wounds with necrosis of bones or tendons, or in areas with low circulation that impairs healing. A possible serious side effect is bleeding, and the clinician must carefully monitor the patient for this, especially those who are on anticoagulant therapy.
Since many patients are uncomfortable with even the sterile maggots provided by medical supply companies, this treatment must be fully discussed with the patient. Some facilities require a signed patient consent prior to using maggot therapy.
Maggot debridement therapy on a diabetic foot ulcer. (Source: Alexsey Nosenko. CC BY-3.0.)
| Name | Mechanism of Action | Advantages |
|---|---|---|
| Conservative sharp debridement | Loosely connected necrotic tissue is removed from the wound bed using sterile scissors or scalpel and forceps. | Quick and safe way to remove dead tissue |
| Enzymatic debridement (collagenase) | Collagenase dissolves the collagen bonds that secure necrotic tissue to the wound bed. | Used when surgical debridement is not feasible, i.e., a patient on anticoagulant therapy with a risk of bleeding |
| Autolytic debridement | A natural form of debridement, it utilizes the body’s own white blood cells to clear necrotic tissue from the wound. | Safe, although slow |
| Biologic/bio-surgical debridement (maggot therapy) | Maggot larvae produce a mixture of enzymes and broad-spectrum antimicrobials to remove necrotic tissue from the wound bed. | Faster than autolytic or enzymatic debridement |
WOUND DRESSINGS
A dressing at its most basic is a covering applied over an open wound to form a barrier between the wound and the external environment. There are multiple forms of dressings available to the wound clinician, and they all serve the following important functions:
- To protect the wound from infection and trauma
- To promote a moist wound environment that is conducive to healing
- To absorb excess drainage from the wound bed
- To protect the intact skin surfaces surrounding the wound
Choosing the Correct Dressing
A wound dressing is only one component of wound healing, but it is an important one. Deciding on the most appropriate dressing for a particular wound is a team effort. The guiding principle is to maintain an environment conducive to moist wound healing. In simple terms, “a dry cell is a dead cell” and will hinder rather than aid the progression of wound healing (Baranoski & Ayello, 2020; Bryant & Nix, 2024).
During the process of wound healing, the type of wound dressing used may have to be changed more than once to address the changing characteristics of the wound. Some recommendations for clinicians to keep in mind when deciding on a wound dressing include:
- Moist does not mean “soupy.” If the wound has too much drainage, then an absorptive dressing will be required.
- If the wound is dry, then moisture must be added.
- If there is undermining or tunneling, packing is required.
- Periwound areas must be protected from damage.
Other issues to consider when choosing a wound dressing are the frequency of dressing changes, the availability of supplies, and the time and personnel required to perform the dressing change. All of these are factors in a realistic and well-laid-out plan of care for the patient.
FREQUENCY OF DRESSING CHANGES
When looking at the frequency of dressing changes, one of the first questions to consider is how often the wound must be assessed. The answer to this question is highly individualized. Patients with a systemic condition and an infectious wound or a wound at increased risk for infection require close monitoring. The decision may be made to perform daily or twice-daily dressing changes. Most, if not all, of these patients will be in a facility. For patients at home, home health nursing and family support are required.
With chronic wounds that are progressing and infection free, once-a-week dressing changes are normally recommended. Many of these patients will be living at home, and some may be in nursing homes or assisted-living facilities. The patient, family members, or facility staff will require instructions on daily monitoring of the dressing and on signs and symptoms to be reported immediately to the clinician.
AVAILABILITY OF SUPPLIES
Availability of supplies is usually not a problem in an acute-care facility or nursing home. However, for patients in assisted-living facilities and those living at home, the question of who will provide the wound care supplies and how they will be paid for must be taken into account. The case manager or social worker on the wound care team will address the major concerns surrounding the procurement of supplies, but all team members must be aware of the insurance and financial restraints that may limit the options.
TIME AND PERSONNEL REQUIRED
Time and personnel are considerations regardless of the care setting. For example, a highly complex, open abdominal wound on an obese patient with diabetes who is in the intensive care unit and requires conscious sedation for sterile dressing changes will require the presence of the anesthesiologist, one to two clinicians skilled in wound care, and other staff members to help with positioning the patient, opening supplies, and maintaining a workable environment. From start to finish, the time commitment for this procedure could take up to three hours. A dressing choice to consider in this situation may be negative-pressure wound therapy—which would require dressing changes only every 48 to 72 hours depending on the wound status—rather than the larger commitment it would take in time and staff to apply daily dressings (see “Negative-Pressure Wound Therapy” later in this course).
Time must be considered not only for the clinician but for the patient and/or caregiver. Patients who visit an outpatient wound clinic may have to take time off from work, and their caregiver may have to take time off to bring them to the clinic. In many instances, patients and family will state that they can only come once a week, and the wound care team must take this into account when developing the treatment plan.
Dressing Types
There are thousands of wound care products on the market, and choosing among them can be a daunting task. However, every facility, clinic, and home health agency involved in wound care will carry a wound care formulary. Who decides what products are made available? Ideally, this is a joint decision between all interested parties, such as administration, the purchasing department, and wound care clinicians.
There are frequent changes in the many different companies’ dressings, names, and types, and so it is important to gather up-to-date information on dressing options before selecting a particular dressing. Some major dressing companies include Hollister, Convatec, Medline, 3M, Healthpoint, Johnson & Johnson, and DeRoyal. (This is not a complete listing and is not intended as a recommendation of one brand over another.)
Several major types of dressings are discussed below.
HOW TO PURCHASE WOUND CARE PRODUCTS
There are thousands of wound care products on the market, and more being added all the time. It can be a daunting task to decide which products to use. Some basic steps to follow are:
- Talk to the purchasing department at the facility. They may already have contracts in place with certain providers, in which case the choice will be limited to products from these companies.
- Most large wound care companies carry a wide range of products. Request a catalog of wound care supplies and, referring to the above table, identify the available wound cleansers and dressings that best meet the needs of the facility’s patients. Research the products you are interested in.
- Get approval from the purchasing department and set up an appointment with company representatives to learn more about their products. Prepare questions prior to the meeting. Explain to the rep that the meeting with them is for informational/educational purposes only, since buying decisions are typically made by the facility’s purchasing department.
- For a specialized product that is not carried by the facility’s provider (e.g., honey dressings), it may be necessary to explain the therapeutic value of the product to the purchasing department, which will typically contact the supplier and negotiate the purchase.
ALGINATES
Usually referred to as calcium alginates, these dressings are made from lightweight seaweed. Alginate dressings can absorb up to 20 times their weight in wound exudate, and they are an ideal choice for moderately to highly draining wounds. They are available in flat dressings of various sizes and also as a rope dressing (Baranoski & Ayello, 2020; Bryant & Nix, 2024).

Alginate dressing. (Source: Korrupt, CC BY-SA 4.0.)
Forms
There are two forms of alginate dressing. One will turn into a gel after it comes in contact with the wound drainage, and the other retains its original shape while it absorbs drainage. The choice of alginate used is usually based on clinician preference.
Advantages
Alginate dressings can assist in hemostasis, making them a first-line option for bleeding wounds. Since the dressing remains soft and moist, it does not stick to the wound tissue and does not cause pain to the patient upon removal. Some dressing brands now offer the option of a silver alginate, which provides the antimicrobial action of silver to the wound bed.
Frequency of Dressing Changes
Alginate dressings can normally remain in the wound for up to 72 hours. However, the frequency of dressing change is determined by the amount of drainage and the frequency of wound assessment indicated in the patient’s wound care plan.
Clinical Guidelines for Use
For deep wounds, a single layer of alginate dressing is applied directly to the wound bed and then covered with layers of fluffed gauze to fill the cavity of the wound. Using layers of alginate dressings to completely fill the wound will not increase the rate of healing and is not cost-effective. Alginate rope should not be placed in narrow tunnels, as there is a possibility that small pieces of the dressing could be left behind. Once the alginate dressing is removed from the wound, the wound bed must be cleaned thoroughly to completely remove the dressing residual (Bryant & Nix, 2024; WOCN, 2022).
HYDROCOLLOIDS
Hydrocolloid dressings help to prevent secondary infections. They are made from a gelatinous substance and have a self-adhesive surface. They provide for limited absorption of wound drainage and are suitable for shallow, dry wounds.

Hydrocolloid dressing applied to a finger. (Source: Leonart LMT, CC BY 4.0.)
Forms
Hydrocolloids are wafer-like, flexible, water-impermeable dressings that conform well to different wound locations. They are available in dressing, paste, and powder forms.
Advantages
Hydrocolloids are simple to apply. Because of their conformity, range of sizes, and varied shapes, they can easily be applied to wounds on most parts of the body. Due to their occlusive nature, they promote a moist wound environment, maintain wound temperature, and protect the wound from contamination (e.g., a coccyx wound in an incontinent patient). These dressings also prevent friction.
Frequency of Dressing Change
The recommended frequency of dressing change is twice weekly. If the wound must be assessed more frequently, a different dressing should be considered.
Clinical Guidelines for Use
Hydrocolloids can be used to protect fragile skin and are often cut into strips to be applied as a “picture frame” along the periwound area to protect it from trauma and drainage and as a surface for attaching a secondary adhesive dressing. They are not an appropriate dressing for infected wounds.
To properly apply a hydrocolloid dressing, the clinician ensures it is larger than the wound size. Optimal dressing adherence is achieved when the dressing extends a minimum of 2.5 cm onto the skin surfaces around the wound.
Hydrocolloids produce an odor that is noticeable when the dressing is removed, and the gelatinous material of the inner layer can be mistaken for purulent drainage. The clinician must educate the patient, family, and other staff about this and instruct them to adequately flush and clean the wound after removing the dressing and then assess for signs of infection (Baranoski & Ayello, 2020; Bryant & Nix, 2024).
HYDROGELS
These are hydrating dressings (i.e., they donate water to the wound bed) and are suitable for shallow wounds with scant drainage. They can also be used along with other agents such as topical medications and antibacterial substances.
Forms
They are available as solid gel dressings, impregnated gauze dressings, and amorphous hydrogels composed of gelatin, polysaccharides, and polymers. The liquid gel is applied directly to the wound bed and covered with moistened, fluffed gauze.
Advantages
These are a cost-effective and easy-to-apply dressing. The hydrogel sheet dressing produces a cooling effect on the wound, which can help with pain management. These dressings can also be safely used during radiation therapy. They are a recommended dressing for use on donor sites, superficial surgical wounds, and chronic wounds (in which they can be used to promote autolytic debridement).
Frequency of Dressing Change
There is flexibility in how often hydrogel dressings can be changed, depending on the characteristics of the wound. Such dressings can be replaced once or twice daily or left in place for up to three days.
Clinical Guidelines for Use
The clinician must avoid overpacking the wound and instruct caregivers likewise, since this may cause pressure on the wound bed, damage newly forming tissue, and impede wound healing.
Hydrogel sheet dressings may also cause maceration of the periwound area, and the dressing must be cut to fit within the wound area without overlapping onto intact skin. Applying a liquid barrier film to the periwound area provides an extra layer of protection.
When using the liquid gel form of the dressing, the clinician applies at least 1/8-inch thickness along the surface of the complete wound bed and covers this with the fluffed, moistened gauze.
If the dressing has dried out, it must be moistened prior to removal in order to prevent pain and discomfort and to avoid damaging healthy wound tissue. The use of this dressing should then be reassessed as to frequency and cover dressings used to prevent it from drying out (Bryant & Nix, 2024; Baranoski & Ayello, 2020).
HYDROFIBER
The main component in hydrofiber dressings is carboxymethylcellulose, which is responsible for its absorptive ability. These dressings are sometimes confused with alginates because of their ability to absorb wound drainage (WOCN, 2022).
Forms
They are available in sheet and ribbon dressings and in plain and antimicrobial forms.
Advantages
These dressings have been shown to reduce bacterial burden in wounds due to their ability to absorb exudate-containing bacteria.
Frequency of Dressing Change
The frequency of dressing changes depends on the amount of wound drainage. Heavily draining wounds may require daily dressing changes, while in wounds with moderate amounts of drainage, the dressing can be left in place for two to three days.
Clinical Guidelines for Use
Hydrofiber dressings cannot be used in dry wounds. They also require a secondary dressing to hold them in place. If the dressing becomes overpowered by wound drainage, there will be leakage onto the periwound area (WOCN, 2022).
FOAM
Foam dressings are capable of absorbing large amounts of wound drainage while maintaining a moist wound environment. Most foam dressings are made from polyurethane, with a matrix of small open cells that absorb drainage from the wound bed (Bryant & Nix 2024; WOCN, 2022).

Polyurethane foam dressings shaped for a heel (left) and toe (right). (Source: Korrupt, CC BY-SA 4.0.)
Forms
Foam dressings come in many shapes and sizes, including special shapes that can be used on elbows, heels, and the sacrum. They are available in a plain form and also impregnated with antimicrobial agents (Baranoski & Ayello, 2020). Foam dressings can be either adhesive or nonadhesive. They come in various thicknesses, ranging from 7 mm to less than 1 mm.
Advantages
Foam dressings are highly versatile and have been shown to decrease wound pain, especially when being removed from the wound bed. They can be used successfully under compression therapy with venous ulcers and as a secondary dressing to supplement absorption in heavily draining wounds. Although they do not relieve pressure, foam dressings can be used to protect against shear injuries.
A traditional foam dressing will usually require a secondary dressing to hold it in place. However, newer thin foam dressings usually have an adhesive wound surface layer and outer layer of transparent film that provides a waterproof surface. Many foam dressings also come with an adhesive border (Bryant & Nix, 2024; Baranoski & Ayello, 2020).
Frequency of Dressing Change
Depending on the type of wound and the amount of drainage present, foam dressings can be left in place for variable lengths of time, ranging from one day up to one week.
Clinical Guidelines for Use
Due to their versatility, foam dressings are widely used in wound care. However, they should not be used on dry wounds or wounds with very little drainage. Manufacturers frequently indicate the absorption capacity of foam dressings, and the clinician must follow these guidelines when choosing foam dressings for a particular wound. Foam dressings can be cut and shaped to align with body contours. Care must be taken to apply the correct side of the dressing to the wound bed; this is often indicated on the dressing itself with the instructions “this side up.”
COMPOSITE
Composite dressings combine more than one physical property in a single dressing and can serve multiple functions. They provide adhesion, absorption of wound drainage, and a protective barrier against bacterial infection (Bryant & Nix, 2024; Baranoski & Ayello, 2020).
Forms
Composite dressings are multilayer dressings, and they conform to anatomic curvature. They come in several different shapes and sizes.
Advantages
Composite dressings are easy to apply and remove. The adhesive border around the edge of the dressing secures it to the periwound area, removing the need for a secondary dressing. Composite dressings can be used in conjunction with other topical wound therapies, such as topical medications applied to the wound bed. Composite dressings can enable autolytic or mechanical debridement of the wound (Baranoski & Ayello, 2020; Bryant & Nix, 2024).
Frequency of Dressing Change
According to the condition of the wound, dressing changes can vary from daily to three times a week.
Clinical Guidelines for Use
Since composite dressings come in several different sizes, the correct size must be chosen for the wound. These dressings cannot be cut, since this will compromise the structure of the dressing. The correct size will allow the dressing to extend for one inch onto the intact periwound area.
CONTACT LAYER
Contact layers are thin, nonadherent layers that are placed directly onto the wound bed. They have an open-weave or perforated structure that allows drainage to pass through the layer to be absorbed by the dressing placed over it.
Forms
Contact layers come in various sizes and types. Many are gauze-based dressings permeated with petrolatum or oil; some are perforated, silicone-impregnated sheets; others are perforated cloth-like sheets.
Advantages
Contact layers protect the wound surface from trauma and help maintain a moist wound environment. They conform well to the wound surface. They are easy to apply and remove from the wound bed, and they assist in pain-free dressing changes. Contact layers can also be used in conjunction with topical medications applied to the wound (Baranoski & Ayello, 2020; Bryant & Nix, 2024).
Frequency of Dressing Change
Contact layers can be left in place for one week. They do not usually need to be removed with each dressing change.
Clinical Guidelines for Use
Contact layers are often used on surface wounds of the extremities, but they can also be used to line the base of deeper wounds to prevent filler dressings from sticking to the wound bed. Contact layers are not recommended for dry wounds, for areas of tunneling or undermining, or for wounds where the drainage has a thick consistency (WOCN, 2022; Bryant & Nix, 2024).
ANTIMICROBIAL DRESSINGS
Antimicrobial dressings comprise a wide selection of types, including:
- Cadexomer iodine dressings
- Silver dressings
- Honey dressings
- Hydrofera Blue dressings
These products are effective against a broad spectrum of microorganisms that cause wound infection and biofilm formation (Bryant & Nix, 2024; Baranoski & Ayello, 2020).
Forms
Antimicrobial dressings come in several different forms, including sheet dressings, pads, rope, powder, creams, and ointments (Baranoski & Ayello, 2020; Bryant & Nix, 2024).
Advantages
These dressings provide absorption of wound drainage and maintain a moist wound bed. They provide the wound with a constant delivery of antimicrobial agents that eradicate bacteria from the wound. Rope forms of these dressings can be used to wick drainage from areas of tunneling. Honey dressings help to reduce wound odor. Antimicrobial dressings can be used in conjunction with compression for venous ulcers of the lower extremities. They can be easily removed from the wound and decrease discomfort during dressing changes.
Frequency of Dressing Change
The frequency of dressing change depends on the properties of each type of dressing and the amount of wound drainage. For instance, silver dressings are usually changed every 72 hours, while honey dressings can be left in place for up to seven days.
Clinical Guidelines for Use
Guidelines are specific to the type of antimicrobial dressing being used:
- Cadexomer iodine dressings release a constant amount of iodine to the wound and can be left undisturbed in the wound bed for 72 hours. They are contraindicated in patients who are allergic to iodine, shellfish, or dyes (WOCN, 2022).
- Manuka honey dressings can remain in the wound for up to seven days. However, dressing changes may need to be done more frequently if there is a high volume of wound drainage. Honey has shown to have a broad range of antimicrobial action, to prevent the growth of biofilm, and to decrease biofilm colony creation (WOCN, 2022).
- Hydrofera Blue dressings are a combination of methylene blue crystal and gentian violet, both with a long history of use in healthcare. They are active against several organisms, including methicillin-resistant S. aureus (MRSA) (Wound Source, 2025; WOCN, 2022). They can be used in conjunction with collagenase, used under compression therapy, and left in a wound for up to seven days.
- Silver has been used for centuries in healthcare, and in recent times it has been found to be a potent agent in wound care. Silver dressings come in foam, alginate, contact layer, powder, and rope forms. They function in one of two ways: the dressing donates silver to the wound bed or the silver remains in the dressing material, which absorbs the wound drainage and destroys the bacteria contained in it (Baranoski & Ayello, 2020; Munter et al., 2024). Silver dressings can be left in place for up to seven days and used under compression therapy.
COLLAGEN
Collagen dressings help to stabilize the chemical balance in the wound by decreasing the level of proteases (which destroy the newly forming collagen fibers in the wound bed). Collagen dressings are derived from either type 1 bovine collagen, avian collagen, or type 3 porcine collagen (Bryant & Nix, 2024).
Forms
Collagen comes in flat dressings, gels, pads, particles, and freezer-dried sheets (Bryant & Nix, 2024; Baranoski & Ayello, 2020).
Advantages
Collagen dressings are easy to apply and can be used for either partial- or full-thickness wounds. They are absorbent dressings, while at the same time they maintain a moist wound environment. Collagen dressings are comfortable, can be used with topical wound agents, and are easy to apply.
Frequency of Dressing Changes
The frequency of dressing changes will depend on the state of the wound—how far along it is in the healing process and the amount of wound drainage. Since these are relatively expensive dressings that usually do not require daily changes, they are typically left in place for three to seven days.
Clinical Guidelines for Use
Collagen dressings can be used on a wide range of wounds, including donor sites, surgical wounds, wounds with tunneling and undermining, and chronic wounds. They are not recommended for dry wounds, for wounds with necrotic tissue present, or for patients who are sensitive to bovine or pork products (some patients may also have a religious or ethical objection to the use of such animal products). Collagen products require a secondary dressing, and some may need to be rehydrated before being removed from the wound (Baranoski & Ayello, 2020).
TRANSPARENT ADHESIVE DRESSINGS
These are thin, plastic dressings with an adhesive surface that sticks to the wound margins without adhering to the wound itself. They are most frequently used as a cover dressing, for example, with an alginate dressing. They can also be used to cover IV sites and as a primary dressing for both shallow and dry wounds (Bryant & Nix, 2024; Baranoski & Ayello, 2020).
Forms
Transparent dressings come in a range of shapes and sizes that adapt easily to different anatomic sites.
Advantages
Although they are not able to absorb wound drainage, transparent dressings facilitate moist vapor transfer and atmospheric gas exchange. At the same time, they provide a waterproof covering for the wound and protect against external bacterial infection; for this reason, they are a good choice for wounds at high risk of stool and urine contamination. They are also a good choice when autolytic wound debridement is required. Since they are unable to absorb drainage, they retain moisture on the wound surface (often referred to as a greenhouse effect); this aids in the process of autolytic debridement (Bryant & Nix, 2024; Baranoski & Ayello, 2020).
Frequency of Dressing Changes
Transparent dressings are usually changed every three days, although they can be left in place for up to seven days (Bryant & Nix, 2024; WOCN, 2022).
Clinical Guidelines for Use
Transparent dressings are not recommended for wounds that require frequent monitoring, such as infected wounds. These dressings cannot be used as a primary dressing for wounds that have tunneling or undermining. Transparent dressings should provide a one-inch overlap onto the periwound area, and if necessary, a liquid skin barrier is applied onto the periwound area to provide protection from skin stripping when the dressing is removed (Bryant & Nix, 2024; WOCN, 2022).
ADVANCED WOUND CARE DRESSINGS
The increasing number of chronic and complex wounds is leading to the creation of advanced wound care dressings. These dressings act specifically on the wound environment and assist in eliminating obstacles to healing, such as insufficient moisture and heightened concentrations of proteases. For instance, oxidized regenerated cellulose (ORC)/collagen dressings assist in preserving a moist wound environment conducive to wound healing (Chowdhry et al., 2021).
Matrix metalloproteinases (MMPs) belong to the larger family of proteases, enzymes that break down the peptide links of proteins, which play an essential role in the early inflammatory phase of wound healing. Continuing high levels of MMPs is a hallmark finding in chronic wounds. Application of dressings that target MMPs reduces their concentration in the wound bed and can help to move the wound back into a healing stage (WOCN, 2022).
| Name (Type) | Use(s) | Advantages |
|---|---|---|
| Alginates (absorbent, made from light seaweed) | In moderately to heavily draining wounds |
|
| Hydrocolloids (occlusive) | For autolytic debridement; to protect periwound area from trauma and drainage |
|
| Hydrogels (hydrating; donate water to the wound bed) | In shallow wounds with scant drainage |
|
| Hydrofiber (absorbent; made from carboxymethylcellulose) | In moderately to heavily draining wounds |
|
| Foam (absorbent, can be adhesive or nonadhesive) | In moderately to heavily draining wounds; under compression; to protect against shear injuries |
|
| Composite (combination) | To provide adhesion, absorption of wound drainage, and protective barrier against bacterial infection |
|
| Contact layer (nonadherent layers placed directly onto wound bed) | To allow drainage to pass through to absorptive dressing above |
|
| Antimicrobial (cadexomer iodine, silver, honey, Hydrofera Blue) | Against a broad spectrum of microorganisms that cause wound infection and biofilm formation |
|
| Collagen (derived from type 1 bovine, avian, or type 3 porcine collagens) | To stabilize the chemical balance in the wound by decreasing the level of proteases, which destroy the newly forming collagen fibers in the wound bed |
|
PROPHYLACTIC USE OF DRESSINGS IN THE ICU
Around 5 million U.S. patients are admitted to intensive care units (ICUs) on a yearly basis, and research shows that deep tissue injury is the most pervasive pressure injury found among them (Cox et al., 2022).
While performance criteria for prophylactic pressure injury prevention dressings have not been established, the use of silicone foam dressings most likely reduces the incidence of pressure injury, particularly in the sacrum area and heels. Hydrocolloids, transparent dressings, and hydrogel dressings can also be used. When deciding which dressing to use, clinicians are advised to consider these factors:
- Anatomic site
- Dressing size needed
- Avoiding pressure between the dressing and medical devices
- Not causing dislodgement of devices due to the dressing
- Best management of microclimate, particularly around and under tubes
- Dressing that can be safely applied and removed
- Dressing that remains in place
(Bryant & Nix, 2024)
ANSWERING PATIENT QUESTIONS
Q: Wound care supplies are expensive; can any of them be laundered and/or reused?
A: Wound care dressings are for single use and cannot be cleaned and reused. However, in some instances where a bandage (e.g., ACE bandage, stockinette bandage) is being used to hold a dressing in place, it may be possible to launder it by washing it in mild soapy water and gently drying it. This can help to reduce the cost of wound supplies. It is advisable to discuss this further with your healthcare provider or wound care nurse.
Changing Dressings
In general, changing a dressing daily allows for assessing the condition of the wound and progress of the healing process. The frequency of dressing changes depends on the status of the wound and the amount of wound drainage.
Since wounds require being at body temperature for healing to occur, it is important to be aware that any time spent in changing a dressing, or even cleansing the wound, will cool down the wound, which can then take several hours to come back up to body temperature after being re-covered. This may slow the healing process. Therefore, it is important to be organized and prepared prior to beginning the procedure and to choose a dressing that will minimize the need for frequent changes.
WHEN TO CHANGE
The first thing to determine is the time when the dressing change will be performed. Planning ahead and adopting a patient-centered approach is important. Most patients have many other activities going on during a hospital stay, and the same is true in nursing homes and assisted-living facilities. For example, in a hospital setting, it is not a good idea to show up for a dressing change just as a patient returns from physical therapy and is already tired and ready for a nap. It is also important for home health nurses to collaborate with patients and families when scheduling visits.
PAIN MANAGEMENT
Once a dressing change time has been decided on, the next issue is pain management. Will the dressing change be painful? Does the patient have pain medication ordered? Clinicians usually request that the physician order a dose of pain medication specifically for dressing changes. The medication must be given so that there is adequate time for it to take effect prior to beginning the procedure, usually 30 to 60 minutes before the dressing change. Topical pain medications such as lidocaine can be considered for point-of-use pain management. If the wound dressing has dried out, it can be soaked and gently removed to lessen pain (Baranoski & Ayello, 2020; Bryant & Nix, 2024).
DRESSING CHANGE PROCEDURE
Following is an example of the steps a clinician will follow when changing a dressing. Always follow facility protocol. When the patient has more than one wound, each wound is regarded as a separate treatment.
- Identify the patient according to facility protocol.
- Explain how the dressing change will be performed and position the patient to expose the wound area.
- Ensure that all supplies are available on a clean, dry working surface, conveniently positioned near the patient. If a sterile dressing is being applied, then a sterile work area must be created. (However, most wound care dressings are done using clean technique.)
- Wash hands, then gently remove the old dressing and dispose of it according to facility protocol.
- Don new gloves and perform a wound assessment.
- Clean the wound and apply a new dressing per instructions in the wound care plan.
- Pack narrow tunnels loosely with ribbon gauze or other sterile rope dressing as ordered. The end of the gauze is placed in the wound to ensure that the packing is removed with the next dressing change. The goal of packing is to remove drainage and to allow the tunnel to collapse from the distal end to the wound bed. The dressing material used in the wound bed can be placed in areas of undermining if the depth is such that removal of the dressing material can be adequately done.
- Cover the wound with a secondary dressing, e.g., a border foam dressing.
- Assist the patient into a comfortable position.
- Discard all used supplies per facility protocol.
Immobilizing Wounds Near Joints
Wounds occurring over a joint or in close proximity to one may need to be immobilized, since repeatedly moving a wound by contracting nearby muscles will slow wound healing and increase the size of the eventual scar.
Plastic or aluminum splints can sometimes be added to the outer bandages of a wound. Otherwise, a separate splint may be placed along the joint. At times, a plaster cast may be needed. Also available are joint immobilizers, which can be soft (e.g., a sling) or rigid (e.g., knee brace).
Physical therapists have extensive knowledge of anatomy and correct positioning. As part of the wound care team, they can provide interventions that will reduce swelling in the affected area and provide correct immobilization to aid in tissue regeneration without impairing joint function. The goal is to optimize correct positioning of the joint, avoid joint contractures, and maintain neurovascular functioning while promoting wound healing. A splint should be correctly sized and applied and carefully monitored to support appropriate joint positioning and avoid loss of joint mobility. For example:
- Knees are supported in a position of 10 degrees flexion.
- Ankles are dorsiflexed and supported in a position of 90 degrees.
- The shoulder is maintained parallel to the chest.
- The elbow is supported at 90 degrees flexion.
- The wrist is sustained in a neutral position.
- Fingers are flexed.
(DeYulis & Hinson, 2023)
WOUND INFECTION
Open wounds present an unrestricted environment for organisms to live and reproduce. Infections always obstruct wound healing, and infection is the most common impediment to wound healing.
Bacteria are present on the surface of all open wounds (which is referred to as colonization), and this low level of contamination does not need to be treated. However, wounds that have been contaminated with significant numbers of bacteria will most likely result in infection. The degree of infection is dependent on several factors, including the type or types of microorganisms present and how virulent they are.
Within hours of an injury, neutrophils and macrophages migrate into the wound and begin removing debris. Large amounts of bacteria, however, cannot be removed within the normal reaction phase. When contamination persists, the influx of white blood cells continues, too. But these neutrophils die after 24 hours, and when they are continuing to infiltrate the wound because of persistent contamination, the dead neutrophils accumulate and begin to clog the wound in the form of pus. Pus slows the formation of granulation tissue and the reepithelialization of the wound, giving bacteria still more time to multiply. Furthermore, many bacteria secrete toxins that add to the tissue damage in the wound when it has become infected.
Types of Infectious Organisms
Bacteria exist in several different shapes and sizes: round, cylinder, and rods. The most widely known classification of bacteria is as either gram positive or gram negative, determined by a staining technique first developed by the Danish scientist Hans Christian Gram in 1884. Gram staining is still widely used today to quickly distinguish between gram-positive organisms (including species of Enterococcus, Staphylococcus, and Streptococcus) and gram-negative organisms (including Pseudomonas, Escherichia coli, and Klebsiella).
Another classification of bacteria important to the clinician is aerobic vs. anaerobic. Aerobic organisms live and thrive in an oxygen-rich environment, whereas anaerobic organisms do not need oxygen to survive and in some instances will die when exposed to it. Anaerobic organisms are a particular threat in wounds with areas of deep tunneling. In any wound where there is a likelihood of anaerobic organisms, a cover dressing that is impervious to oxygen should not be used. Identification of such organisms is made on tissue biopsy or through the use of an anaerobic culture kit.
Free-floating (planktonic) bacteria are what clinicians are most familiar with, and these organisms are most easily cleared from the wound. In many cases, they can be eradicated by the patient’s own immune system or the introduction of antimicrobials into the wound treatment. In more severe cases, antibiotic therapy may be required (Bryant & Nix, 2024; Baranoski & Ayello, 2020).
BIOFILM AND INFECTION
One of the most persistent and difficult-to-treat forms of wound infection is biofilm formation (see above under “The Healing Process and Chronic Wounds”). Biofilms are collections of organisms comprised of bacteria, fungi, and other microorganisms existing together in a synergistic community, enveloped in a polymeric matrix that attaches tightly to the wound surface. Biofilms are more prevalent in chronic wounds (3 in 5) than in acute wounds (1 in 12) (Baranoski & Ayello, 2020; Bryant & Nix, 2024).
The organisms in a biofilm have the capacity to decrease their metabolism, which in turn decreases their need for oxygen and nutrients, permitting them to become dormant and to reactivate later. Biofilm formation will increase the level of wound inflammation and also support the production of anaerobic organisms in the wound. Biofilms can negatively impact the wound-healing process without exhibiting the normal symptoms of infection.
Research shows that biofilms develop in four phases:
- Biofilms form from a small group of bacteria and other microorganisms that have fastened themselves to the wound surface. This early phase of development provides clinicians with the best opportunity to halt biofilm development by cleaning and debriding the wound.
- The biofilm forms a stronger attachment to the wound bed, creating an organized, symbiotic community.
- The biofilm produces an extracellular viscous material that forms a protective barrier for the biofilm. At this stage, the biofilm may be detected as a tacky, shiny film on the wound surface.
- If the biofilm is not interrupted or removed, the microcolonies continue to progress, become more established, and present with increased resistance to biocides. The result is additional biofilm creation and extension.
From beginning to end, the process of biofilm formation happens over two to four days (Bryant & Nix, 2024; Spector, 2025).
Biofilms can proliferate 2 mm below the wound surface and infiltrate surrounding healthy tissue (Shah et al., 2018; WOCN, 2022). Currently, there are no tests available to clinicians to detect biofilms in wounds.
Effective treatment for biofilm eradication is weekly sharp debridement of the wound followed by immediate application of a broad-spectrum antimicrobial agent to prevent organisms freed during debridement from creating a new biofilm. Cadexomer iodine and silver agents have been shown to be successful in treating biofilms when used as a second step following debridement.
Research indicates that biofilms can reform in wounds as quickly as 24 hours after debridement. The recommendation is to perform serial debridements no more frequently than once every seven days (Spector, 2025; Baranoski & Ayello, 2020).
Signs and Symptoms of Infection
The classic signs of wound infection include:
- Fever
- Pus
- Abscess
- Abnormal smell (malodor)
- Cellulitis
- Persistent inflammation with an exudate
- Warmth and redness
- Delayed healing
- Continued or increasing pain
- Edema
- Weak, crumbly granulation tissue that bleeds easily
(University Hospitals Sussex, 2025)
If any of the above signs of wound infections are present, a wound culture is obtained to determine the appropriate course of treatment (Bryant & Nix, 2024).
Infection is usually easily detected in acute wounds, and many of the above symptoms will be recognizable. But in chronic wounds, infection can be difficult to detect, and the presenting symptoms may be subtle. For example, the first indication of infection in a diabetic foot ulcer is oftentimes unexplained elevated blood sugars. The patient may complain that “for no reason my fasting blood sugar is over 200 mg/dL every morning.”
Increased wound drainage can be another symptom of infection in a chronic wound. If a certain amount of redness has always been present in the periwound area, cellulitis may not be detected. The clinician must look closely for changes in the quality of the granulation tissue that appear “liver-like,” which is evidenced by a dark red color and more friable tissue (falls apart easily). It is important to keep in mind that the majority of patients with these signs will not run an elevated temperature, and so a normal temperature reading cannot be taken as a sign that the patient is infection free.
In chronic wounds in which bone is exposed or the clinician can probe down to bone, there is a high probability that tissue and bone infection (osteomyelitis) are present (Bryant & Nix, 2024).
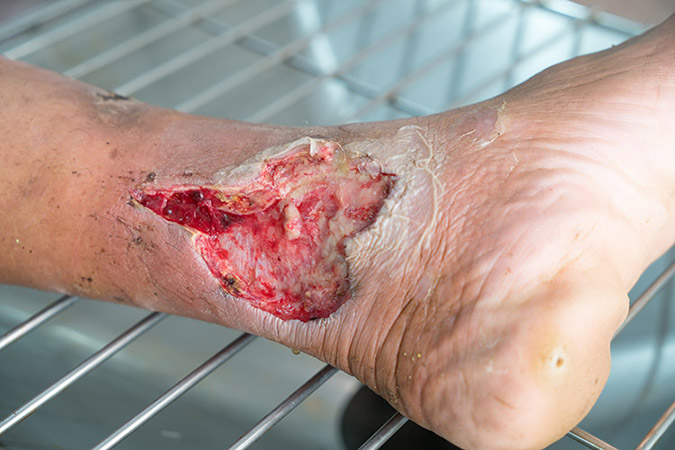
Infected open wound. (Source: Chatuphot/Shutterstock.com.)
ANSWERING PATIENT QUESTIONS
Q: How do I know if a wound is infected?
A: Seek medical attention if you have any of these signs and symptoms of an infection:
- Fever
- Pus in the wound
- Increasing redness of the wound or the area around it
- Swelling
- Persistent or increasing pain
- Redness or red streaks spreading out from the wound
Diagnosing Infection
Wound cultures are most commonly used to confirm the presence of infection. They include:
- Swab culture
- Tissue culture
- Aspiration culture
- Bone culture
It is important to remember that necrotic tissue and slough should not be collected as part of a wound culture.
SWAB CULTURE
Swab culture is one of the most frequently used methods to collect wound cultures, but it is not the most reliable. The surface of all chronic wounds is contaminated, and this will be evident in the culture. But this may not give a true picture of what is happening at a deeper level in the wound tissue.
The technique used to collect the culture is extremely important. First, the wound bed is thoroughly cleaned with sterile normal saline. Next, using an area approximately 1 cm2, the culture swab is pressed against the wound surface with sufficient pressure to produce wound exudate. The swab is then rotated to capture this exudate (Bryant & Nix, 2024).
TISSUE CULTURE
Tissue culture (also referred to as punch biopsy) is considered the gold standard for accurately collecting wound culture. One disadvantage of this procedure is that a punch biopsy can only be performed by a physician, nurse practitioner, or physician assistant, which limits the settings in which it can be obtained. Punch biopsies are performed using sterile technique. Injectable, local anesthesia is administered to numb the area, which is then cleansed with sterile normal saline. Using a 3-mm curette and sterile scissors, the clinician removes a tissue sample from the wound bed, which is then placed in a sterile container and transported to the laboratory (Bryant & Nix, 2024).
ASPIRATION CULTURE
This technique is often used to collect fluid from an abscess. It is done under sterile technique with local anesthesia. The procedure is performed by a physician, nurse practitioner, or physician assistant using a 10-mL syringe with a 22-gauge needle. The needle is inserted into the tissue adjacent to the wound and fluid is withdrawn. After the needle is removed from the area, the syringe is capped and sent to the lab for analysis (WOCN, 2022).
BONE CULTURE
If bone is visible in the wound, the physician may elect to cut away a sample of the bone and send it for culture. This is a sterile technique, but it does not require local anesthesia because only bone fragments are being removed.
If a bone biopsy is not possible but osteomyelitis is suspected, then an X-ray or MRI of the affected area will be necessary. Although some insurance carriers may insist that an X-ray be used for diagnosis, clinicians must consider that an X-ray is not the most reliable means of confirming the presence of osteomyelitis and that an MRI may still be required (WOCN, 2022; Bryant & Nix, 2024).
Treating Infection
Prompt recognition of wound infection and early intervention can help prevent microorganisms from spreading to deeper tissues and possibly bone, reducing the risk of systemic infection. Topical antibiotics are not as widely used in wound care as they once were due to the increase in bacteria-resistant organisms and the possibility of hypersensitivity reactions (Baranoski & Ayello, 2020). For critically colonized wounds, antimicrobial dressings are the first choice of treatment.
The frequency of dressing changes may need to be increased during periods of infection to carefully monitor the wound and to address an increase in wound drainage that usually accompanies wound infection.
Where systemic infections are present, a specialist may be required. Such infections must be addressed promptly in order to prevent adverse consequences, such as the risk of limb amputation for a diabetic patient. Once culture results are available, appropriate antibiotic therapy will be ordered. Mild infections are usually treated with oral antibiotics. However, more severe infections such as osteomyelitis require intravenous antibiotics, and surgical removal of the infected bone may be done in conjunction with antibiotic therapy (WOCN, 2022).
Some wounds that have been sutured closed over extensive subcutaneous tissue dissection and debridement can develop a temporary inflammatory reaction in which they become red and edematous even though they are not infected. If a clinician suspects this problem, the physician is contacted and may order that one or two stitches or staples be removed to lessen the tension. Any fluids in the wound are then gently expressed or aspirated, followed by packing the area with sterile saline-moistened gauze and then covering it with the appropriate dressing. In this case, the wound is cleansed daily and dressings packed/applied per physician instructions. This type of inflammatory reaction will decrease within 48 hours.
Preventing Infection
Preventing infection, environmental contamination, and cross-infection are the responsibility of all clinicians involved in wound care. Any break in the skin surface provides a portal of entry for bacteria and other microorganisms into the body, and clinicians must be cognizant of this when performing dressing changes regardless of the location—facility, clinic, or home. In the home setting, the clinician is also responsible for educating caregivers about the means of preventing infection.
Patients with acute and chronic wounds are at particular risk for healthcare-associated infections, which remain a major threat to patient safety. The Centers for Disease Control and Prevention reports that on any given day, 1 in 31 hospitalized patients and 1 in 43 nursing home occupants has at a minimum one healthcare-associated infection (HAI) (CDC, 2023). The key to elimination of HAIs is full adherence to recommendations across the continuum of care.
Preventing the spread of infectious organisms includes the following actions:
HAND HYGIENE
Since most infections occur with direct patient contact, proper hand hygiene (handwashing or using alcohol-based rubs) remains the single most effective way to prevent infection to and from patients. In order to prevent infection:
- Wash hands or use an alcohol-based product immediately after gloves are removed, between patient contacts, and when otherwise indicated.
- Wash hands between tasks and procedures on the same patient to prevent cross-contamination of different body sites.
- Avoid unnecessary touching of surfaces near the patient to prevent contaminating clean hands and to prevent transmission of pathogens from contaminated hands to surfaces.
- Do not wear artificial fingernails or extenders.
STANDARD PRECAUTIONS
Standard Precautions are used with every patient. The degree of Standard Precautions implemented will be determined by the complexity of care. For interactions such as changing a dry dressing covering an intact surgical wound, only gloves may be needed. During other interactions (e.g., wound cleansing, irrigation, and wound debridement), use of gloves, gown, face shield, or mask and goggles may be required, and these should be readily available.
BARRIERS AND PERSONAL PROTECTIVE EQUIPMENT
Personal protective equipment (PPE) is specialized clothing and/or equipment worn by a healthcare worker for protection against a hazard. PPE provides barriers to the transmission of infectious organisms during wound care, thereby protecting both the healthcare professional and the patient.
Types of PPE include:
- Gloves, both sterile and nonsterile
- Gowns of varying permeability
- Face shields
- Goggles
- Masks
- Head coverings
- Booties
Clinicians must follow these rules when using PPE:
- Know how to use the equipment.
- Always wear PPE in exposure situations.
- Remove and replace PPE that is torn, punctured, or has lost its ability to function.
- Remove clothing that becomes contaminated with blood or other potentially infectious material as soon as possible.
- Remove PPE before leaving the work area.
- Handle contaminated laundry as little as possible.
- Place contaminated PPE in appropriately labeled bags or containers until disposed of, decontaminated, or laundered.
- Know where these bags or containers are located in the work area.
(APIC, 2022)
ANSWERING PATIENT QUESTIONS
Q: Do I need to wear gloves when doing wound care at home?
A: It is better to wear gloves when doing wound care to decrease the risk of infecting the wound, but sterile gloves are not necessary. Gloves should be discarded after each dressing change.
Documentation
Clear, concise, and accurate documentation is an essential element of each wound care contact in all settings. Documentation allows all those involved in patient care to know the wound status and provides for good communication among all clinicians. It is also of paramount importance for reimbursement and in the case of litigation.
Documentation of the care given includes the following components:
- Date and time
- Interventions performed
- Wound characteristics, including the amount and type of drainage
- Wound odor
- Patient’s pain level during and after the treatment
- Interventions to relieve pain and the effectiveness of the interventions
- Patient’s level of anxiety before, during, and after treatment
- Patient’s reported level of comfort with applied dressings
- Supplies used
- Name and credentials of the clinician providing the care
Only approved abbreviations should be used and objective findings accurately described.
IMPEDIMENTS TO WOUND HEALING
Unfortunately, not all wounds heal in a timely or predictable manner. There are multiple factors that negatively impact wound healing. Some of these are related to the patient’s overall health status, and others are environmental. One of the most serious impediments to wound healing is infection (discussed above). Other impediments to wound healing are discussed below.
Reinjury
Reinjury can slow or stop wound healing. Pushes and pulls that would have no effect on healthy parts of the body can reopen a healing wound even when it is protected by a well-made dressing. Similarly, if there is significant skin tension surrounding the wound (e.g., over a bent knee), the healing wound will not be able to seal tightly (see below).
Ischemia/Hypoxia
Oxygen is required in all phases of wound healing, from inflammation to reepithelialization, and any condition that results in decreased oxygen supply (hypoxia) to the wound bed will impede wound progress.
Ischemia of a wound can arise from too much physical tension across the wound; ineffective oxygenation of the blood (e.g., anemia, lung problems, smoking); or reduced circulation (e.g., atherosclerosis, heart failure, kidney failure, vasoconstriction, too much pressure on the wound). Differences in the available blood supplies account, in part, for the fact that facial wounds tend to heal better than foot wounds.
The importance of local circulation to wound healing is reflected in the healthcare maxim “wounds that don’t bleed don’t heal.” Circulation brings oxygen, which aids healing. A wound that continues to bleed, however, is not proceeding to the next step in healing. A crumbling (friable) wound bed is not healing and may indicate infection.
Local Skin Tension
Skin and its underlying tissues are normally under tension. Most skin in the body is being stretched, at least slightly, by the adjacent skin and the underlying structures, but the actual tension at any one location varies along the surface of the body. Movement changes skin tension: bending a joint stretches the overlying skin, while contracting a muscle tends to reduce tension in the overlying skin. Skin creases and skin wrinkles are indications of lines of least tension; on the face, the lines of facial expression are also lines of least tension. As a rule, the lines of least skin tension are perpendicular to the long axis of underlying muscles.
Skin tension is negligible along skin creases, moderate over relaxed joints and muscles, and high over bent joints (knees and elbows) and the skull. During a cutting, ripping, or puncturing injury, the tension from the adjacent intact skin pulls the free edges of the wound apart. In places where the wounded skin is under greater tension, the wound gapes more widely and heals more slowly, and the resulting scar is relatively large.
Obesity increases tension on the abdomen and difficulty due to movement of the panniculus (overhanging folds of subcutaneous fat, which may weigh several pounds), particularly when the panniculus moves to the sides and away from the center line. Thus, obesity delays wound healing due to mechanical forces.
Patient Factors
DISEASES
Certain diseases are noted for causing poor wound healing. The most common of the problem diseases is diabetes mellitus. Scars formed from wounds in people with diabetes have less collagen, and the collagen that is laid down is more brittle than normal. Diabetes also damages blood vessels and makes the skin more prone to ischemia. The reduced circulation is especially notable in the feet, and foot wounds are notorious for not healing well in patients with diabetes (see also “Diabetic Foot Ulcers” later in this course).
To make matters worse, diabetes leads to peripheral neuropathy. Patients lose sensation in their fingers and toes, so diabetic injuries tend to go unnoticed in the extremities. Finally, people with diabetes have a weakened inflammatory response and are more susceptible than other people to developing tissue infections.
NUTRITION
Nutrition plays a vital role in all stages of wound healing. For instance, malnourished people begin to break down their proteins as a source of energy, and this slows healing. Specific vitamin deficiencies also lead to poor wound healing, such as vitamin K deficiency, which impairs blood clotting.
Patients with wounds are advised to have a nutrition consult, especially for complex wounds, for wounds that are slow to heal, and when there are any concerns about the patient’s nutritional status. Such a consult addresses the following concerns, among others:
- Carbohydrates are the body’s source of energy. Glucose is the primary source of fuel for collagen synthesis, which is needed for wound healing. The types of carbohydrates consumed should be mainly complex carbohydrates, such as fruits, vegetables, and whole grains. These are also good sources of fiber, minerals, and vitamins.
- Protein is required for the restoration and production of enzymes that contribute to wound healing, cell duplication, and collagen and connective tissue creation. Increased protein levels are associated with better wound-healing rates. The European Pressure Injury Advisory Panel, National Pressure Injury Advisory Panel, and Pan Pacific Pressure Injury Advisory Panel (EPIAP/NPIAP/PPPIA, 2019) International Guideline recommends 1.25 to 1.5 grams of protein per kilogram of body weight daily for persons with a pressure injury and for those who are malnourished or at risk for malnourishment. However, the patient’s renal function and coexisting conditions must be taken into account when regulating protein intake. Several amino acids, such as L-arginine, glutamine, and cysteine, play vital roles in wound healing.
- Fats provide a concentrated source of energy as well as a standby source of energy in the form of deposited triglycerides in the adipose tissue. Healthy sources of fat include vegetable, olive, and nut oils, along with fatty fish, nuts and nut butters, and dairy products. Fats are required for cell membrane formation, and the demand for fatty acids appears to increase in the presence of a wound. Lipid constituents are required for tissue growth, collagen, and extracellular matrix synthesis.
- Micronutrients is another term for vitamins and minerals. These are critical for wound healing. Although several micronutrients play a role in the wound-healing process, vitamin A, vitamin C, and zinc have been identified as the most important.
- During the inflammatory phase of wound healing, vitamin A is responsible for increasing the quantity of macrophages and monocytes in the wound bed. Thus, vitamin A deficiency impedes the transformation of monocytes into macrophages, which can slow or halt healing. Vitamin A also plays a part in activating cellular differentiation in fibroblasts and collagen production, and it promotes epithelialization. Vitamin A is found in eggs, fish, and dark green vegetables.
- Vitamin C is an antioxidant (an element that inhibits or reduces damage caused to cells by free radicals), which is crucial for collagen production. It also plays a vital part in the creation of new blood vessels and assists with iron absorption. Vitamin C is also a needed component in immune function. Vitamin C deficiency prolongs wound-healing time, increases the risk for infection, and leads to weak collagen, which is the basis of scurvy. Good sources of Vitamin C include citrus fruits, leafy green vegetables, and tomatoes.
- Zinc is needed for protein metabolism, collagen production, and cell proliferation. Wound drainage can lead to a swift decrease in zinc, with resultant deficiency. This can cause diminished collagen strength, reduction in the rate of epithelial cell production, and poor wound healing. There is no clinical evidence to support zinc supplementation. High levels of zinc can impede wound healing. Good dietary sources of zinc include meats, seafood, beans, nuts, whole grains, and dairy products.
(Baranoski & Ayello, 2020; Rosenthal, 2020; WebMD, 2024)
HYPERGLYCEMIA
Tight glycemic control is important in wound healing and preventing complications. Hyperglycemia can negatively impact the inflammatory phase of wound healing and the formation of granulation tissue. It can also result in decreased tensile strength of collagen and epithelial resurfacing. To maintain adequate wound healing, it is recommended to maintain blood glucose between 140 and 180 mg/dL (WOCN, 2022).
SMOKING
Tobacco contains a mixture of chemicals, many of which are highly toxic. Patients who smoke have poor wound healing in addition to suffering a number of other skin problems (wrinkling, premature skin aging, higher risks of squamous cell carcinoma, psoriasis, and hair loss). Smoking causes vascular constriction, which decreases circulation and leads to chronic wounds. Research has also demonstrated that patients who smoke experience more wound pain than those who do not smoke. This increase in pain sensation is due to chemicals in tobacco negatively affecting the body’s perception of pain signals (WOCN, 2022).
DEHYDRATION
Patients who are dehydrated may have impaired kidney function and reduced blood volume, leading to decreased blood pressure and perfusion, which can slow wound healing.
Healthcare Impediments
Medical care of wounds is an attempt to overcome obstacles to natural healing. In the course of managing a wound, clinicians reduce the amount of contamination, minimize the area that must be filled by new tissue, maintain moist granulation tissue, and protect the healing area. However, efforts at facilitating wound healing sometimes introduce new impediments, such as decreased mobility, increased wound pain, and restrictions on activities of daily living.
PROBLEMATIC DRUGS, SOLUTIONS, AND OINTMENTS
Chemotherapy drugs negatively affect wound healing during the treatment period and immediately afterward. However, since they are an essential part of cancer treatment for many patients, it is important that the wound care team work closely with the patient’s oncologist to develop a safe therapeutic plan for wound care.
Steroids have a negative impact on wound healing when they are taken in doses greater than 30 mg per day. Glucocorticoids (e.g., prednisone) limit the proliferation of fibroblasts and the production of collagen, thus making scars relatively weak. Vitamin A applied topically to the wound bed has been shown to help counteract the local effects of steroids on wound healing while not interfering with their systemic therapeutic value.
The antiseptic solutions 10% povidone-iodine, 3% hydrogen peroxide, and 0.5% chlorhexidine can slow wound healing by destroying healthy cells as well as infected ones. Antiseptics should always be used judiciously in wound care and for a limited period of time, usually 7 to 10 days.
Creams and ointments can also be impediments to wound healing. Silver sulfadiazine, used in infected wounds, must be thoroughly cleaned from the wound bed during dressing changes. Although early studies showed statistically faster healing rates when Neosporin ointment was used, bacitracin is more commonly used as an antibiotic ointment due to sensitivities to Neosporin. Moisturizing creams such as Eucerin and topical steroids such as triamcinolone should not be used in open wounds. Patients must be cautioned about the use of over-the-counter products in wound care and that nothing should be applied to the wound without first discussing it with their wound care practitioner (WOCN, 2022; Bryant & Nix, 2024).
X-RAYS
Ionizing radiation damages actively dividing cells. In wounds, the regrowing epithelium, newly growing blood vessels, and fibroblasts that form new connective tissue are likely to be damaged by a large dose of ionizing radiation. Normal X-ray imaging is usually not a problem. Cancer therapies, however, give relatively high doses of ionizing radiation, and in areas of the body exposed to radiation therapy, wounds heal poorly and infections are more common.
ANSWERING PATIENT QUESTIONS
Q: Can antibiotic ointment be used indefinitely on a wound?
A: It is not advisable to use antibiotic ointment in a wound indefinitely, since this can cause allergic reactions and result in bacterial resistance.
Q: Can I apply aloe lotion to help heal my wound?
A: Rubbing herbal medicines made from the aloe vera plant on wounds is a common home remedy. Scientific studies show that aloe preparations do not help infected wounds to heal. The wound should be covered with a dressing to keep it from drying.
Q: I am using hydrogen peroxide to treat my wound, so why is it not healing?
A: Hydrogen peroxide can be used to clean a wound where dirt and grit are present. But once the wound is clean, hydrogen peroxide should not be used, since it can also destroy healthy tissue.
TREATING SPECIFIC TYPES OF CHRONIC WOUNDS
Chronic wounds are slow and can be difficult to heal. They are often caused by underlying disease processes such as chronic inflammation, venous and arterial insufficiency, pressure ulcers/injuries, diabetic foot ulcers, atypical wounds where the underlying cause can be difficult to pinpoint, and end-of-life wounds. Addressing any underlying causes, such as pressure relief and pressure redistribution in pressure injury treatment, is a must if progress is to be made in healing chronic wounds (WOCN, 2022).
Chronic wounds can have a detrimental effect on quality of life and be a major source of anxiety, pain, and depression for patients. It is estimated that chronic wounds adversely affect the quality of life for almost 2.5% of the U.S. population (Sen, 2023). Older adults have the largest prevalence rate of chronic wounds, with over 85% of chronic wounds occurring in adults over the age of 65 (Gregory, 2025).
Since the causes of chronic wounds are diverse, there is no ideal dressing that addresses the needs of all chronic wounds. Treatment recommendations for some types of chronic wounds, including pressure injuries, diabetic foot ulcers, vascular ulcers, and wounds caused by xylazine use, are described below.
Pressure Injuries
(The following content is adapted in part from the Wild Iris Medical Education course “Pressure Injury Prevention and Treatment.”)
In 2016 the National Pressure Injury Advisory Panel replaced the term pressure ulcer with pressure injury. The NPIAP published an updated definition of pressure injuries in 2025, which continues to define pressure injuries as “localized damage to the skin and/or underlying tissues, usually over a boney prominence or related to a medical or other devices” caused by “prolonged pressure” or a combination of pressure and shear forces (NPIAP, 2025).
Pressure injuries can and do occur across the lifespan. They are a recurrent problem in the pediatric population, patients who are immunocompromised, the elderly, spinal cord injury patients, and others with limited mobility at all ages (Bryant & Nix, 2024; Baranoski & Ayello, 2020).
Pressure injuries affect over three million individuals each year, and the emotional and physical consequences are tremendous. Pressure injuries are a source of intense pain. They can lead to sepsis, delay recovery, prolong hospitalization, and result in death.
Pressure injuries can be caused by exposure to severe pressure, extended periods of pressure, or pressure in conjunction with shear force. Other factors associated with pressure injury occurrence include nutritional status, tissue perfusion, microclimate, and coexisting diseases.
Most pressure injuries occur on the lower body, with the coccyx area being the most common site, followed by the heels and lower extremities (WOCN, 2022; Baranoski & Ayello, 2020; Melter & Mamou, 2023).
CAUSES
Unrelieved pressure is the most common cause of pressure injury development. There are several elements that determine when pressure has reached a stage to cause damage:
- Intensity of the pressure
- Duration of pressure
- Capacity of the skin and tissues to withstand pressure
Research shows that higher pressure levels will cause skin breakdown and tissue damage in a shorter period of time (minutes to hours), depending on the circumstances. However, so far, no exact level of pressure has been identified as a cutoff point for ischemia and injury, which are instead regarded as being dependent on other factors such as the overall physical condition and age of the patient and concomitant conditions.
Studies indicate that duration of pressure and tissue injury depend on several factors, such as the level of pressure applied and the strategies used to relieve pressure. Even moderately low levels of pressure can lead to blood vessel compression and tissue death.
RISK ASSESSMENT
The best-known and most widely used tool for pressure injury assessment is the Braden Scale. It assesses six areas of risk for pressure injury development:
- Sensory status
- Mobility
- Activity level
- Exposure to moisture
- Nutritional status
- Degree of friction and shear present
Each of these six areas is scored from 1 (highest risk) to 4 (least risk). The lower the score on the Braden Scale, the greater the likelihood of pressure injury formation. For example, a patient with a score of 9 or less would be considered at high risk for a pressure injury, whereas someone with a score of 18 would be considered not at risk.
Risk factors related to pressure injury development include:
- Immobility
- Age
- Comorbidities
- Smoking
- History of a previous pressure ulcer/injury
- Obesity
- Edema
- Surgical procedures that last three hours or more
- Length of time on a stretcher
- Medications
- Nutrition status
- Long illness
- Patient noncompliance with pressure-relief interventions
- Elevated body temperature
- Psychological factors
STAGING
Wound care clinicians must accurately stage a pressure injury to ensure appropriate interventions, monitor progression or regression of the ulcer, and utilize a uniform language and set of terms that are understood by all those involved in the patient care regardless of the setting. Pressure injury staging is a skill that takes time to learn, and novice clinicians will benefit from mentoring by experienced practitioners in wound care.
Two basic principles apply to staging pressure injuries:
- A wound bed that is covered with necrotic tissue cannot be staged until enough of the wound bed has been exposed to determine the extent of the damage.
- Pressure injuries are not “down staged” (i.e., a stage 4 pressure injury that has improved is not then described as a stage 3 pressure injury; it is described as a healing stage 4 pressure injury).
| Stage/Name | Description | Example |
|---|---|---|
| (Baranoski & Ayello, 2020; WOCN, 2022; Bryant & Nix, 2024; photos © Gordian Medical, Inc., dba American Medical Technologies and © AAWC, used with permission.) | ||
| Stage 1 | Intact skin with nonblanchable redness; can be painful, firm, soft, and warmer or cooler compared to adjacent areas |
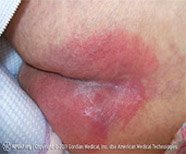
|
| Stage 2 | Partial-thickness loss of the dermis; shallow, open ulcer with a red-pink wound bed without slough |
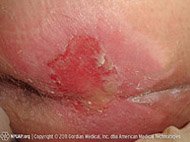
|
| Stage 3 | Full-thickness tissue loss; bones, tendons, or muscles are not exposed; slough and/or eschar may be present but do not obscure the depth of tissue loss |
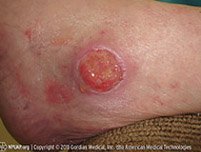
|
| Stage 4 | Full-thickness tissue loss with exposed bone, tendon, or muscle; depth varies by anatomic location; shallow over the bridge of the nose, ear, occiput, and malleolus due to lack of subcutaneous tissue |
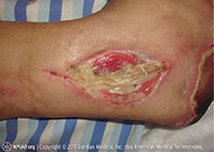
|
| Deep tissue pressure injury (DTPI) | Intact or nonintact skin with localized area of persistent nonblanchable, deep red, maroon, purple discoloration or epidermal separation revealing a dark wound bed or blood-filled blister; must be confirmed that the purple skin (appearing as ecchymoses or bruising) is due to pressure or shear and not a response to medication or trauma |
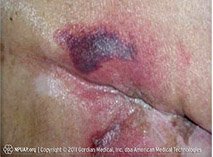
|
| Unstageable | Full-thickness skin and tissue loss in which the extent of tissue damage within the pressure injury cannot be confirmed because it is obscured by slough or eschar; refers to the inability to visualize the wound base rather than the clinician’s inability to determine the injury stage |
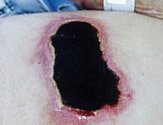
|
| Medical device–related pressure injury (MDRPI) | Results from the use of devices designed and applied for diagnostic or therapeutic purposes; injury generally conforms to the pattern or shape of the device | |
| Mucosal membrane pressure injury | Occurs on mucous membranes exposed to a medical device; most common sites are the buccal mucosa, lips, tongue, and nares, caused by the presence of nasal cannula, and endotracheal tubes; not staged owing to the composition of the tissue involved | |
PREVENTION
Prevention includes putting in place protocols for identifying patients at risk for pressure injury development. This includes using a risk assessment tool (e.g., the Braden Scale, described above), training staff in the use of the assessment tool, and deciding how often assessments will be done. The latter will vary according to the practice setting and regulatory guidelines.
A pressure injury prevention protocol includes the following:
- Regular skin assessment
- Routine skin care
- Interventions for patients with limited mobility
- Nutrition assessment
- Appropriate support surface while the patient is in bed or in a chair
- Routine repositioning
- Moisture control
- Measures to decrease exposure to friction and shear
- Proactive intervention to prevent medical device–related pressure injury
TREATMENT
Treatment is dependent on the stage of the pressure injury, the patient’s goals for treatment, and the patient’s capacity for healing. Treatment options for pressure injury prevention and care include:
- A facility-wide program for pressure injury prevention, which includes skincare education for all levels of staff
- Risk assessment and regular screening
- Routine repositioning individualized to the patient’s needs, with skin assessment
- Support surfaces
- Heel and elbow protectors
- Nutritional management and hydration
- Ulcer assessment and staging
- Individualized program of wound care
- Debridement of devitalized tissue
- Dressing selection to maintain moist wound therapy and encourage cellular proliferation
- Maintaining muscle strength and mobility
(Bryant & Nix, 2024; Baranoski & Ayello, 2020; WOCN, 2022)
CASE
Mrs. Perry is a 78-year-old female admitted to the hospital from home with a stage 4 pressure injury on her coccyx. Mrs. Perry’s other diagnoses include mild dementia, kidney disease, hypertension, and poor vision. She lives in a single-level house with her 82-year-old husband. Mr. Perry reports that he’s in good health apart from arthritis and stiffness, which makes it difficult for him to get around. The Perrys have two adult children who live out of state.
The nurse, Eva, documents that Mrs. Perry’s stage 4 pressure injury measures 6 cm by 5 cm, with depth varying from 4 cm to 5 cm. There is a moderate amount of drainage, and the periwound area has increased redness but is intact. Approximately 50% of the wound bed is covered with loose fibrinous tissue, with exposed tendon and muscle in the remaining 50% of the wound. Eva also reports that Mrs. Perry has signs and symptoms of malnutrition and dehydration, including dry, brittle skin. Mr. Perry states that he kept a dry pad over his wife’s coccyx wound at home.
Silvia, the nurse practitioner (NP), performs a punch biopsy of the wound to rule out infection. She orders collagenase enzymatic debridement to be applied to the wound bed, with daily dressing changes. Silvia also orders the following consultations for Mrs. Perry: physical therapy, occupational therapy, dietitian, and case manager for discharge planning.
The physical therapist, Tim, recommends a low air loss mattress for the patient’s bed, an air-cell cushion for the bedside chair, and ambulation for short distances using a rolling walker and standby assistance. The occupational therapist, Kristi, reports that Mrs. Perry is able to feed herself but requires verbal cueing. She also needs hands-on assistance with toileting and bathing. Kristi recommends a bedside commode. The dietitian, Anh, suggests liberalizing Mrs. Perry’s strict renal diet to include more of the patient’s food choices and six small meals a day.
The team members agree that a one and one half– to two-hour side-to-side turning schedule is appropriate for Mrs. Perry. Mr. Perry requests his wife be allowed to sleep at night with as little disturbance as possible.
Biopsy results show that the wound is colonized with multiple organisms but with no osteomyelitis present. Silvia performs a sharp debridement of the wound using EMLA cream to provide local anesthesia. After the sharp debridement, she applies cadexomer iodine dressing to the wound bed, with a secondary cover dressing. Silvia asks the nurse, Eva, to change the dressing every three days and to inform her of any changes in the wound bed.
At a team conference a week later, Silvia reports that after a second sharp debridement the wound appears to be healthy. Eva states that the wound size has not decreased, although there is less wound drainage, and suggests changing the wound treatment to a collagen dressing and discontinuing cadexomer iodine dressings.
The physical therapist, Tim, reports that Mrs. Perry can ambulate for longer periods of time and requires hands-on assistance of one person to transfer in and out of bed. The occupational therapist, Kristi, reports that use of the bedside commode has made toileting easier for the patient and that a long-handled sponge has increased Mrs. Perry’s independence in bathing. The dietitian, Anh, reports that the results from a three-day calorie count show a marked improvement in the patient’s nutritional intake.
Mr. Perry wants his wife home as soon as possible and refuses to consider nursing home placement. Silvia raises the possibility of a short-term stay in a skilled-nursing facility to help with ongoing wound healing and therapies. Mrs. Perry and her husband are open to this suggestion and will work with the case manager to find a facility close their home. Both Kristi and Tim suggest a home evaluation to determine what adaptive equipment and modifications will be needed prior to Mrs. Perry’s discharge home. Eva starts preparing the discharge summary for review of the team members at their next meeting.
Diabetic Foot Ulcers
A diabetic foot ulcer (DFU) is defined as a full-thickness wound that penetrates through the skin and dermis, the most frequent sites for a diabetic foot ulcer being the plantar surface of the foot and the toes. Diabetic foot ulcers occur in about 15% of individuals with diabetes (Armstrong & Lavery, 2024; APMA, 2025).
A DFU is the most frequently occurring complication associated with diabetes and one that healthcare providers will encounter across the continuum of care. DFUs are complex, chronic wounds that are often disabling and greatly impact the morbidity and mortality of patients. Data show that 40% of persons with a healed DFU will develop another ulcer within a year and 60% within three years and that 14% to 24% of patients who develop a DFU will need an amputation (Conyers & Swoboda, 2023; APMA, 2025). Patients who develop a DFU are also at higher risk of early death, heart attack, and fatal stroke than people with diabetes who do not develop such ulcers.
RISK FACTORS
There are several risk factors for diabetic foot ulcers. Uncontrolled blood sugars are one of the main underlying causes. Other contributing factors include neuropathy (a condition that results in sensory loss to the foot, also described as a “loss of protective sensation,” or LOPS). This loss of protective sensation leaves the patient vulnerable to injury (e.g., the patient with diabetes who steps on a nail and feels nothing).
If care is not taken to monitor feet and to wear proper footwear, problems with the feet may occur in patients with diabetes. If peripheral neuropathy develops, even the smallest unnoticed foot wound may become a DFU.
Foot deformities related to changes in the structure of the foot due to diabetes are also a leading cause of ulceration. Charcot foot, a destructive process that leads to multiple fractures of the small bones in the foot and joint deformity, is another key factor in the development of DFUs.
ASSESSMENT
Diabetic foot ulcers must be thoroughly assessed. The site of the ulcer assists in determining the primary cause and the best way of relieving pressure to the area.
Diabetic/neuropathic ulcers are usually found on the plantar (sole) aspect of the foot, under the heel, over the metatarsal heads, or on the toes. Margins of these wounds are even, and the wound bed can be deep. There may be granular tissue present unless the patient has peripheral vascular disease (PVD). Exudate from the wound is low to moderate. A characteristic callous surrounding the wound bed may be present and indicates that the patient has not off-loaded but has continued to walk on the foot. The foot may be warm but with diminished or absent feeling. There may be atrophy of subcutaneous fat. (Armstrong & Lavery, 2024).

Diabetic foot ulcer with periwound callus formation. (Source: Mark A. Dreyer, DPM, FACFAS, CC BY 4.0.)
INFECTION RISK IN DIABETIC ULCERS
Diabetic foot ulcers are prone to infection, including osteomyelitis, and this is one of the leading reasons for lower extremity amputations in patients with diabetes. A DFU may not exhibit the classical signs of infection, and the most important signs to look for are:
- Increased wound drainage
- Unexplained increase in blood sugar levels (patient reports of fasting blood sugars of 200 mg/dL and greater)
- Increase in wound odor
- Increase in wound size and depth
- Patient complaints of lethargy and “not feeling good”
TREATMENT
Once a diabetic foot ulcer is present, aggressive treatment is required. The gold standard in the treatment of DFUs is off-loading. This refers to relieving pressure from the area of ulceration and evenly distributing it across the surface of the foot. This even distribution of pressure is extremely important to prevent a further area of ulceration.
Off-loading can be achieved by several means: total contact casting, soft casting, off-loading shoes, removable cast walkers, and therapeutic insoles. The physical therapist on the wound care team is instrumental in ensuring that the patient can safely ambulate when off-loading. The patient who has total contact casting applied will require instruction in walking with crutches or another appropriate assistive device.
Dry skin is common in people with diabetes. Dry skin can crack open, allowing bacteria to enter. Applying lotions to the feet is good for treating dryness, but lotions should never be applied between the toes, because moisture between toes can lead to skin breakdown and/or fungal infections. Small pieces of cotton wool or lamb’s wool can be placed between the toes to keep them dry and should be changed at least daily. Daily foot inspection is important, especially between toes.
Wound treatment includes:
- Debridement of necrotic tissue
- Wound bed preparation (cleaning and irrigation)
- Dressing to maintain a moist wound environment
- Advanced therapies (discussed later in this course), such as:
- Bioengineered skin substitutes
- Hyperbaric oxygen therapy
- Electrical stimulation
- Negative-pressure wound therapy
- Growth factors
PATIENT ADHERENCE
Patients with diabetes are often unconcerned about wound care. A small hole in the sole of their foot that they cannot feel may not register as something with potentially serious consequences to them, and clinicians must understand that time and sensitivity is required in building a therapeutic relationship with many patients with diabetes.
(See also the Wild Iris Medical Education course “Diabetes Care: Prevention and Clinical Care of Diabetic Foot Ulcers.”)
ANSWERING PATIENT QUESTIONS
Q: I have a small wound on my ankle. It doesn’t look infected, but it refuses to heal. What can I do?
A: Make an appointment with your primary care provider to have the wound evaluated. There are reasons why a wound does not heal, including poor circulation.
SKIN CHANGES ASSOCIATED WITH COVID-19 INFECTION
In some instances, skin changes may be the first or only sign of COVID-19 infection, or they may develop during infection. The etiology behind COVID-related skin changes is not fully understood at this time; it may be related to the immune response or vascular change. The relationship between severe COVID-19 infection with iatrogenic pressure injury development is still a concern (Panahi, 2022).
Skin changes on or near bony prominences have been identified in patients with COVID-19 infection. These include areas of deep purple discoloration and necrotic tissue. The challenge for wound care clinicians is to differentiate these lesions from pressure injuries. This begins with careful and thorough assessment of the patient and their overall condition. A further consideration is that COVID skin response may increase the likelihood of developing pressure injury.
Livedoid vasculopathy (a condition characterized by augmented thrombotic activity, diminished fibrinolytic activity, and concurrent endothelial damage) has been found in patients with COVID-19 infection. These vaso-occlusive lesions most frequently occur on the legs and feet and are found in patients who have moderate to severe COVID-19 infections. They present as reddish, often with a punched-out appearance, in an irregular pattern. The lesions can become necrotic and are frequently painful and are related to a greater risk for the development of serious pneumonia, the need for intensive care treatment, and higher mortality rates (Panahi, 2022).
It is important for the clinician to do a full assessment of all skin conditions and lesions that may occur in patients with COVID infection to rule out other etiologies before determining that they are COVID related (Majmundar & Baxi, 2022; Pontieri-Lewis et al., 2021).
Venous Ulcers
Almost 50% of people in the United States have some form of vein malfunction. Data indicates that between 1% and 22% of those over the age of 60 experience lower extremity skin wounds. This data may underestimate the extent of the problem, as many people care for leg ulcers at home and do not seek medical attention. Venous leg ulcers are the most frequently occurring lower extremity ulcer and account for around 70% of total leg ulcers (Baranoski & Ayello 2020; Bryant & Nix, 2024).
Venous ulcers arise from pooling of blood in the veins as a result of venous insufficiency, in which blood leaks backwards in the veins, stagnating in the lower extremities. The most commonly held theory for the cause of this problem is damage to the valves of the lower extremity veins. Ulcerations occur spontaneously or from minor trauma.
RISK FACTORS
Venous ulcers occur in patients with chronic venous disease. With the increase in the population of older adults, there has been a concurrent increase in the rate of venous ulcers. However, venous disease and venous ulceration can also occur in much younger adults, even those in their 20s.
Risk factors for venous ulcers include:
- Venous hypertension
- Obesity
- History of DVT (deep vein thrombosis)
- Decreased activity
- Pregnancy
- Heart failure
(Baranoski & Ayello, 2020; Bryant & Nix, 2024)
WOUND ASSESSMENT
Lower extremity wounds have several different etiologies, and making a differential diagnosis is important. To put in place the appropriate treatment plan, the wound must be correctly identified.
The location of the wound is an important element in accurately diagnosing the underlying cause. The usual location of a venous ulcer is from the ankle up to the knee in what is often described as the gaiter area. Venous ulcers can also occur on the dorsum of the foot. The patient may present with one or several ulcers.
Venous ulcers are normally shallow, and drainage amounts can vary from moderate to high. The wound bed is typically ruddy red with irregular wound edges. Islands of eschar may be present, but black necrotic tissue is seldom seen except if infection or trauma are present. The skin surrounding the wound may be scaling and weeping but can also be thin and dry. If left untreated, venous ulcers will grow larger. In patients with advanced venous stasis, firm edema may be present and will resist compression.
A common indicator of venous disease is the presence of hemosiderin staining (a brownish discoloration of the skin surfaces of the affected extremity). The discoloration is in the gaiter area of the leg and is permanent. It is something the clinician should look for during the wound assessment (Bryant & Nix, 2024).
Malignancy in venous ulcers was previously regarded as uncommon, but recent studies have demonstrated an increased incident of basal cell carcinoma (BCC) associated with venous ulcers. The typical BCC ulcer is characterized as pearly ulceration with elevated wound margins and a smooth surface. However, when BCC develops inside a venous ulcer, its typical characteristics are not present. Indicators of possible malignancy include the presence of granulation tissue with translucent, glossy wound edges (Tchanque-Fossuo et al., 2018; Toussaint et al., 2021).
The pain associated with venous ulcers is frequently described as dull, heavy, continuously aching, and being relieved by elevation. The pain is usually worse at the end of the day, especially for individuals who spend several hours on their feet but have little opportunity for ambulation.
The CEAP system (an acronym for clinical, etiologic, anatomic, and pathophysiologic) provides a subjective classification of venous disease (see table below).
| Classification | Description |
|---|---|
| Clinical | |
| (Baranoski & Ayello, 2020) | |
| C0 | No visible or palpable signs of venous disease |
| C1 | Telangiectases or reticular veins |
| C2 | Varicose veins |
| C3 | Edema |
| C4A | Pigmentation and eczema |
| C4B | Lipodermatosclerosis and atrophie blanche (white atrophy) |
| C5 | Healed venous ulcer |
| C6 | Active venous ulcer |
| Etiologic | |
| Ec | Congenital |
| Ep | Primary |
| Es | Secondary (postthrombotic) |
| Anatomic | |
| S | Superficial veins |
| P | Perforator veins |
| D | Deep veins |
| Pathophysiologic (Basic) | |
| Pr | Reflux |
| Po | Obstruction |
| Pr,o | Reflux and obstruction |
| Pn | No venous pathophysiology identifiable |
COMPRESSION TREATMENT
The gold standard of wound care treatment for venous ulcers is compression. It is virtually impossible to heal a venous ulcer until the swelling is reduced. Along with compression, leg elevation plays a big part in reducing edema. Healing time can take up to several months, and recurrence is a major problem. Compression consists of wrapping the leg in a particular fashion (depending on the product used) so that compression is greatest at the ankle and least at the calf.
Unna boots are one of the most widely known and widely used forms of compression, and they work well for patients who are ambulatory. Multilayer compression wraps provide more consistent compression to patients who are chairbound or have limited mobility. Multilayer wraps also provide higher levels of compression when needed. Research demonstrates that four-layer compression wraps and short-stretch bandages achieve higher healing rates than Unna boots (Baranoski & Ayello, 2020; Bryant & Nix, 2024).
The recommended criteria for compression in venous ulcers are as follows:
- Mild, <20 mmHg
- Moderate, 21–40 mmHg
- Strong, 41–60 mmHg
- Very strong, ≥61 the-assessment-and-management-of-hypergranulation
(Baranoski & Ayello, 2020)
However, many patients have mixed venous and arterial disease, and before compression therapy is applied, the wound care team must be aware of the patient’s circulation status. Compression is contraindicated in the presence of arterial disease. Depending on the severity of the arterial disease, modified and low levels of compression may be used. A general consideration is that any compression is better than none, but patients with severe arterial disease cannot tolerate any compression.
One of the most common measurements of arterial circulation is the ankle-brachial index (ABI). Therapeutic levels of compression can be used in patients with an ABI of 0.8–1.0. An ABI measurement of ≥1.1 is usually an indication of noncompressible arteries and is common in patients with diabetes. This should not be taken as a normal reading, and these patients will require further diagnostic studies to evaluate circulation.
Compression therapy is also contraindicated in patients with uncompensated heart failure, coexisting venous thrombosis in the affected leg, and severe peripheral vascular disease.
If the wound is heavily infected, compression will not be used until infection is resolved.
DRESSINGS
The dressing used under compression will depend on the status of the wound. A contact layer next to the wound bed, covered with a highly absorbent dressing, is a good choice for a wound with copious drainage.
If the wound bed is red and granulated, it is important to maintain a moist but not wet environment. If bacterial levels are high, silver products, topical antiseptics, cadexomer iodine, or antimicrobial dressings may be used (WOCN, 2022). Skin replacements are also used successfully on venous ulcers. If the exudate is minimal, foam-type dressings may be used; but if the exudate is heavy, absorptive dressings (such as alginate or other specialty absorptive dressings) may be used.
The frequency of dressing and compression wrap changes will depend on the amount of drainage. Compression can be left in place for up to a week; however, if the wound is draining large amounts, dressings will have to be changed more frequently (two to three times per week).
Since the periwound area may already be dry, fragile, and crusted, it is important to protect it from further damage. Gentle cleansing technique is important, along with the application of a skin sealant. These come in either individualized skin wipes or as spray on. Most brands have both alcohol and nonalcohol preparations. Nonalcohol barriers are recommended for denuded skin to prevent a burning sensation and discomfort (Bryant & Nix, 2024; Baranoski & Ayello, 2020).
CASE
Mr. Hernandez, age 57, presents to the wound clinic with an ulcer on his right lower leg. Mr. Hernandez works as a baker and spends up to 10 hours daily on his feet. He is married and lives in a duplex home with his wife and daughter. He smokes one to two packs of cigarettes daily and has done so for over 30 years. Mr. Hernandez is 5’10” tall and weighs 220 lbs. Apart from seasonal allergies, Mr. Hernandez has no other health problems.
During the assessment, Mr. Hernandez states to the nurse, Chantal, that the wound “has been there for several weeks and nothing I do makes it better.” He also tells the nurse, “I wasn’t always this heavy, but in the evenings I’m so tired and my leg hurts so much that I don’t want to do anything but watch TV and eat.”
Chantal then performs an assessment of the wound. It is located on the anterior surface, mid-calf area of the right lower extremity. The wound is shallow, irregularly shaped, and with a moderate amount of drainage. The wound bed has a ruddy red appearance, and the periwound area has scaling and weeping. The nurse also notes brown skin discoloration on both lower extremities between the calf and the ankle; the discoloration is more pronounced on the right leg than the left leg.
Jaime, the nurse practitioner (NP), reviews the nurse’s findings and meets with Mr. Hernandez. He diagnoses the patient with a venous ulcer and grades it as a C6 using the CEAP classification system. Jaime performs an ABI test and determines that Mr. Hernandez does not have any arterial disease. He orders silver dressing to the wound, with a secondary absorbent cover dressing and a four-layer compression wrap. He asks Mr. Hernandez to return to the wound clinic in three days for the first dressing change and then weekly after that. Jaime also requests physical therapy, occupational therapy, and a dietitian to work with the patient.
Before he leaves the clinic, the nurse educates Mr. Hernandez on the signs and symptoms indicating that the compression wrap is too tight and possibly interfering with arterial circulation. She also provides him with contact information for the clinic.
The physical therapist discusses with Mr. Hernandez ways to incorporate short periods of frequent ambulation and periodic positional changes into his work schedule as well as a home exercise program. The occupational therapist introduces the topic of a smoking cessation program. Mr. Hernandez is resistant to the idea but says he is willing to “cut back” on the number of cigarettes he smokes. The occupational therapist also explains the importance of keeping his legs elevated as much as is comfortably possible whenever he is sitting or lying down.
His wife and daughter accompany Mr. Hernandez to his next appointment. He states that the compression wrap “feels strange” but that he had no problems with it. The nurse, Chantal, notes that the periwound area has less weeping. Mr. Hernandez’s daughter talks to the physical therapist about ways to motivate her father to exercise more. Mrs. Hernandez meets with the dietitian and looks at options to decrease his calorie intake while keeping his favorite foods in his diet.
By the third week of care the wound has decreased in size, and the wound bed is 100% healthy granulation tissue. Mr. Hernandez reports less leg pain, and he’s motivated to stick with the home exercise program because his daughter does the exercises along with him. The NP, Jaime, discontinues the silver dressing and orders a contact layer dressing to be applied, with the secondary cover dressing underneath the compression wraps.
By week six the ulcer is healed, and Mr. Hernandez is delighted that he no longer has to wear compression wraps. Jaime discusses with him the high risk of further ulceration unless he wears compression stockings on a permanent basis. Mr. Hernandez is concerned that the stockings will be too difficult for him to put on, and he refuses to allow his wife or daughter to do this for him, stating, “I’m not an invalid.” The occupational therapist works with Mr. Hernandez on strategies to make it easier to put on and remove the compression stockings himself, such as using a stocking aid. The nurse provides Mr. and Mrs. Hernandez with instructions for compression stocking care. The physical therapist gives them a list of online vendors where compression stockings may be purchased at reasonable rates.
ANSWERING PATIENT QUESTIONS
Q: When is the best time to put on compression stockings?
A: The best time to put on compression stockings is first thing in the morning, as soon as you wake. This prevents swelling. Once swelling begins, it is much more difficult to put on compression stockings. Make sure that you are securely sitting on the side of the bed or on a sturdy chair before you start putting on the stockings.
Q: My legs are swollen and the doctor wants me to wear compression stockings. Can I use the TED hose I got at the hospital?
A: No. TED hose are not recommended for compression. They are used in hospital settings, but they do not exert high enough pressures to be effective for long-term compression.
Arterial Ulcers
Arterial ulcers (ischemic ulcers) are caused by a severe lack of blood flow to the tissues (Baranoski & Ayello, 2020). The affected arteries become blocked or narrowed, which reduces blood flow to the extremities first, especially the lower extremities. Arterial ulcers are the result of trauma to the skin in areas of ischemia (oxygen and nutrient deprivation) and decreased circulation.
The gold standard of care for these ulcers is revascularization. Without interventions to reestablish an adequate blood supply, arterial ulcers will not heal.
RISK FACTORS
Arterial ulcers result from lower extremity arterial disease (LEAD) and occur less frequently than venous ulcers; however, they are a common occurrence in older adults. Additional predisposing factors are diabetes and advanced age. Data indicate that 8.5 million adults in the United States suffer from LEAD. The highest incidence has been found in those over age 65, non-Hispanic Black people, and women (WOCN, 2022; Bryant & Nix, 2024).
WOUND ASSESSMENT
Arterial ulcers are found on the forefoot and toes, locations that are farthest away from the heart. Infection is often present in these wounds but may not be detected in the presence of significant ischemia. The clinician must be watchful for mild redness around the wound (WOCN, 2022; (Baranoski & Ayello 2020; Bryant & Nix, 2024).
Wounds characteristic of arterial ulcers are even margins with a “punched-out” look. The wound bed is pale and deep. Tissue around the wound is blanched or purpuric (having some purplish mottling). Exudate from these wounds is minimal, if any. Toenails on the affected foot are usually thickened, and there is loss of hair to the thin, shiny skin on the ankle and foot.
Part of the assessment for patients with arterial ulcers includes palpation of the pedal pulses—the dorsalis pedis pulse (located on the dorsum of the foot) and the posterior tibial pulse (located on the medial aspect of the leg, behind the ankle). Temperature to the lower extremities will be decreased, and toe/pedal pulses will be diminished or absent. However, the presence of these pulses does not rule out arterial disease and should not preclude other testing.
Ankle-brachial index (see also “Compression Treatment” under “Venous Ulcers” above) is an indirect measurement of blood flow to the extremity and is recommended as part of a routine assessment for all patients suspected of having LEAD. Toe pressure should be performed in patients in whom an ABI reading cannot be obtained or where the accuracy of the reading is suspect (i.e., ABI >1.3) (WOCN, 2022; Baranoski & Ayello, 2020).
Duplex ultrasounds are used to identify the location of an arterial blockage and are a noninvasive test frequently used to diagnosis LEAD.
Intermittent claudication is a classic sign of arterial disease and is reported as calf pain that occurs each time the patient walks a set distance, for example, the patient whose legs are “always hurting after walking to the mailbox and back to the house.” Resting pain and leg pain at night that wakes up the patient are signs of advanced LEAD. Peripheral artery disease (PAD) may cause the patient to hang a foot off the edge of the bed or to sit up with legs down to stop postural pain. The lower extremities will pale on elevation but display dependent rubor (redness) when down.
At the last stage of PAD, pain is severe, as is the arterial blockage. Cellulitis (infection of the connective tissue of the skin) may develop. As the tissue dies, necrosis begins at the tip of the toes. Gangrene then develops. A distinct line between gangrenous and healthy tissue, as a result of the inflammation caused by irritation from the dead tissue, is an important diagnostic feature of dry gangrene. Gangrene is dark brown to black, with tissue forming a hard mass. If untreated, the gangrenous portions eventually will separate and the toe will be lost. If unstable (wet) gangrene is present, it will progress, causing extreme pain and destroying more tissue (WOCN, 2022; Baranoski & Ayello, 2020).
TREATMENT
The debridement of stable, noninfected necrotic tissue should not be undertaken until the perfusion status of the limb is clinically determined.
Wound care follows the universal goal of maintaining a moist healing environment, management of wound drainage, and protection of periwound skin surfaces. Due to the high degree of infection in ischemic wounds and the difficulty in ascertaining whether the wound is infected or not, hydrocolloid dressings are not recommended for ischemic wounds. Dressings that allow for visualization of the wound are recommended.
Since walking and exercise causes pain, many patients with LEAD may restrict mobility and will require education and therapy to preserve their level of functioning. The physical therapist can help develop an individualized exercise program that is practical to the patient’s needs and circumstances. Exercise benefits circulation to the lower extremities, and studies show that regular exercise helps to decrease the severity of intermittent claudication.
Atypical Lower Extremity Wounds
Although venous and arterial ulcers are the most frequently occurring lower extremity wounds, there are other causes of lower extremity wounds. These atypical wounds are not always easy to diagnosis, and many times they are initially mistaken for venous or arterial ulcers. Four of the more frequently encountered atypical wounds are discussed below.
VASCULITIS
This condition results in inflammation of blood vessels; it can affect small, medium, and large vessels. Vasculitis is an autoimmune process and is found more often in older adults. It is usually a recurrent condition and many times happens along with systemic lupus or rheumatoid arthritis. Reports indicate that up to 20% of patients with rheumatoid arthritis develop rheumatoid nodules (solidified swellings under the skin), which can progress to ulceration (Eustice, 2022; WOCN, 2022; Bryant & Nix 2024).
When small blood vessels are involved, vasculitis manifests in pinpoint areas of bleeding beneath the skin. The clinician will observe clusters of tiny red to purple spots on the skin, most often occurring on the patient’s legs. However, lesions and wounds associated with vasculitis can occur on any part of the body.
If larger blood vessels are involved, the end result will be ulceration and necrosis. On the lower extremities, the most common sites for these ulcers are on the lateral malleolus, which makes them hard to distinguish from venous ulcers.
A complete assessment will include a biopsy of the lesion, usually from more than one location, for histologic evaluation; blood work for ANA (antinuclear antibodies) and rheumatoid factor; and urinalysis to determine if there is renal involvement. Histologic identification of vasculitis is crucial due to its resemblance to other clinical conditions. Biopsy findings will include endothelial cell swelling and fibrinoid necrosis of the blood vessel walls (Vasculitis Foundation, 2022; WOCN, 2022).
Treatment consists of managing the underlying disease process, whether this is lupus or rheumatoid arthritis. In these instances, the wound care team works closely with the patient’s rheumatologist and other healthcare professionals involved in treating the systemic disease process.
Local wound treatment involves removing necrotic tissue (autolytic debridement is often used in these cases), maintenance of a moist wound environment, drainage management, and protection from injury. Infection is an issue with these wounds, and the clinician will monitor the wound carefully for any signs of infection, however subtle, and ensure that prompt treatment is instigated.
PYODERMA GANGRENOSUM
Pyoderma gangrenosum is a chronic inflammatory skin condition (Baranoski & Ayello, 2020; Bryant & Nix, 2024). It is frequently associated with inflammatory diseases such as rheumatoid arthritis and inflammatory bowel disease.
The initial wound presentation is a pustule that progresses to ulceration. These wounds are extremely painful and can occur anywhere on the body, but they are frequently found on the lower extremities. A characteristic sign of pyoderma ulcers is a purplish halo surrounding the wound. The wound bed may present with soft necrotic tissue, purulent drainage, and deep dermal destruction. Although the wound may start off small, it grows rapidly in size and may occur in a cluster of wounds.
Many times the diagnosis is based on exclusion of other wound types, the clinical presentation of the wound, and the patient history. Approximately half of patients with pyoderma have a concomitant inflammatory condition. A hallmark sign of pyoderma gangrenosum is pathergy, a state in which minor trauma will cause marked deterioration in the wound (Bryant & Nix, 2024).
Treatment requires the use of gentle, nontraumatic wound care measures. Sharp debridement is not indicated, since this may trigger the process of pathergy. Autolytic debridement is usually the best process to employ, and antimicrobial dressings are useful in controlling bacterial counts.
The wound cannot be treated in isolation; the systemic condition underlying the process also must be addressed. Corticosteroids are the mainstay treatment and are usually started in high dosages of 100 mg to 200 mg of prednisone daily. The wound care team works closely with the medical team managing the systemic disease in order to implement a holistic approach to care (Bryant & Nix, 2024).
CALCIPHYLAXIS
Calciphylaxis is a rare condition occurring mainly in patients with kidney disease who are receiving hemodialysis. It is also known as calcific uremic arteriolopathy. The current annual prevalence is 4%, but it is expected this will increase with the concomitant increase in chronic kidney disease due largely to increasing rates of diabetes. Calciphylaxis occurs more frequently in women and White people than in other groups. Risk factors for the condition include:
- Hyperparathyroidism
- Presence of obesity
- Diagnosis of diabetes
- Hypercoagulability
- Coumadin therapy
- Hypoalbuminemia
- Trauma
Of this list of risk factors, the most pertinent is hyperparathyroidism, which results in increased levels of calcium phosphate resulting in vascular, cutaneous, and subcutaneous thrombosis and subsequent tissue death. Other causative elements include hypercalcemia and hyperphosphatemia (Baranoski & Ayello, 2020; WOCN, 2022; Bryant & Nix, 2024).
Calciphylaxis presentation is initially as purplish nodules or plaques. The condition usually evolves into irregularly shaped areas of black, gangrenous plaques, which then develop into indurated ulcers. The ulcers are characteristically deep and may penetrate into muscle, with a star-shaped appearance. The ulcers may be present on the fingers, toes, heels, and torso. Calciphylaxis is extremely painful, and pain management is of prime importance for these patients (Shah et al., 2018; WOCN, 2022).
Treatment includes enzymatic or autolytic debridement, resorting to surgical debridement only if infection is present. No single type of wound dressing is most advantageous for treating calciphylaxis. The recommendation is to ensure thorough wound care, including the possible use of hyperbaric oxygen therapy (HBOT) to increase oxygen concentration to the ulcers and help with healing. Treatment with intravenous sodium thiosulfate (STS) has enhanced prospects for patients with calciphylaxis. STS has the capacity to stimulate vasodilation, which greatly reduces the pain experienced by the patient.
Calciphylaxis is frequently fatal, with a mortality rate at the end of one year of over 60%. The risk of mortality from calciphylaxis is related to the body area involved, with ulceration on the torso having the highest mortality rate compared to involvement of the lower extremities (Baranoski & Ayello, 2020; WOCN, 2022; Bryant & Nix, 2024).
SICKLE CELL ULCERS
Sickle cell disease is a hereditary genetic condition that affects hemoglobin in red blood cells. In persons with sickle cell disease, the hemoglobin has an abnormal crescent shape and is rigid and tacky. This results in altered red blood cells that are unable to move through small blood vessels, leading to vessel occlusion. Sickle cell ulcers develop as a complication of sickle cell disease. Sickle cell disease is principally found in the Black population, and the presence of leg ulcers in young Black males may indicate undiagnosed sickle cell disease.
The development of sickle cell ulcers is caused by the interplay of several factors, including vascular obstruction, bacterial infection, and trauma. Venous insufficiency is commonly found in patients with sickle cell ulcers. The most common sites for sickle cell ulcers are the medial and lateral malleolar regions of the lower extremities. Other areas that can be affected are the anterior tibia, Achilles tendon, and dorsal surface of the foot.
Sickle cell ulcers are painful and challenging to treat. Their presentation is as hard-to-heal wounds that frequently reoccur, often associated with ankle stiffness. These ulcers are highly susceptible to infection and are frequently covered with biofilm.
Treatment includes debridement. Sharp debridement is most often used when biofilm is present, but due to the severe pain associated with these ulcers, ultrasonic debridement is recommended as an alternative treatment. Applying collagenase to the wound bed as an adjunct to other forms of debridement has been found to decrease biofilm reformation. Dressing choices for treating sickle cell ulcers include silver and cadexomer iodine. Compression wraps are usually needed due to edema caused by venous insufficiency (Bryant & Nix, 2024).
A pain management regime must be in place before any intervention is instigated. One study found that up to 90% of patients with sickle cell ulcers required pain management interventions to deal with ulcer pain and that more than one therapeutic approach to pain management was frequently required. EMLA cream can be used as a topical anesthetic. Research data have also demonstrated that EMLA cream is an effective antibacterial capable of destroying several strains of staphylococcus (including methicillin-resistant and methicillin-sensitive forms) when left in contact with the wound bed for approximately one hour. Other types of pain management, including opioid therapy, may be required to achieve pain control.
Patients with deep, painful ulcers that do not respond to treatment are assessed for possible osteomyelitis, and radiologic evaluation is recommended.
Since sickle cell ulcers are difficult to heal, prevention is of paramount importance, and overall disease management is crucial. Strategies to decrease the risk of these ulcers include compression to manage venous insufficiency and prevent and control edema. Trauma to the lower extremities is a causative factor in the development of sickle cell ulcers, and interventions to decrease trauma injury should be employed, including protective wraps and shin guards (Baranoski & Ayello, 2020; WOCN, 2022).
Wounds Caused by Xylazine Use
Xylazine is an FDA-approved medication used for sedation in veterinary medicine. It is not approved for use with humans. In the early 2000s it started appearing in the United States as an additive to illicit street drugs. (Street names for xylazine include tranq or tranq dope.)
Xylazine-associated wounds are a distinctive clinical finding. The underlying process that causes their development is not yet fully understood. A proposed sequela is the development of peripheral vasoconstriction, diminished perfusion and tissue necrosis, and small vessel disease. The presence of xylazine-associated wounds increases the likelihood of other adverse events, including bacteremia, endocarditis, the need for limb amputation, and death (DPH City of Philadelphia, 2024).
The first stage in the development of xylazine-associated wounds is the formation of blisters, usually around the injection sites; however, they may occur in other areas of the body. The blisters can merge and become deeper wounds, with full-thickness tissue involvement and the exposure of tendon and bone. These wounds are often painful, and pain management is usually part of the treatment plan (NIDA, 2024).
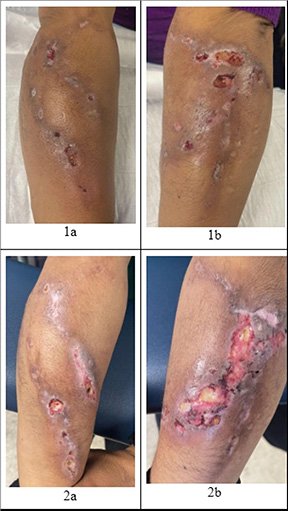
Xylazine-induced wounds. (Source: Warp et al., 2024. CC BY 4.0.)
The goals of treatment for these wounds are:
- Preserving healthy tissue
- Debridement of nonviable tissue
- Diminishing the possibility of infection
Enzymatic or autolytic debridement is recommended as a first treatment approach to preserve healthy tissue and protect bone and tendon from exposure. However, if there is worry that deeper infection may be present, an abscess formation, or the patient presents with generalized illness, sharp surgical debridement will be considered. Systemic antibiotic therapy may also be required.
Wound care includes protection of the periwound areas from wound drainage, which can be achieved with a nonstinging skin prep. A primary dressing is applied to the wound bed, such as Xeroform gauze, and this is covered with a highly absorbent secondary dressing, for example, abdominal pads. The dressing is secured in place with an ACE bandage or tubular net dressing.
It is important to recognize that continuing drug use is not a contraindication to wound treatment. However, patients should be referred to addiction medicine evaluation and therapy (Penn Medicine, 2025). It has also been found that wound healing takes longer in patients who continue to use illicit drugs containing xylazine (NIDA, 2024).
OTHER TYPES OF WOUND CARE
Healthcare professionals work in multiple clinical settings, and the ability to provide adequate wound care to diverse patient populations is an essential part of the services provided. Other types of wound care that clinicians may provide include:
- Simple surgical wound care
- Complex surgical wound care
- Pediatric wound care
- Bariatric wound care
- Spinal cord injuries and wound care
- End-of-life wound care
(WOCN, 2022; Baranoski & Ayello, 2020)
Simple Surgical Wound Care
Noncomplicated surgical wounds progress to healing in a timely manner. These wounds normally have a sterile, nonadherent dressing applied in surgery, and these dressings will be left in place for the first 48 hours after the procedure. However, if dressings become soiled from drainage or are loosened or removed for any reason, the clinician must re-dress the wound with a sterile dressing using a sterile technique.
It is imperative that the clinician check the patient’s orders; often the surgeon will perform the first dressing change or request that the clinician inform the surgeon when the dressing change will be performed so that the surgeon can be present to assess the healing status of the wound.
Normally, the process of epithelial resurfacing is complete after about 72 hours and a dressing is no longer required. At that point, the patient is free to shower. If epithelialization is not yet complete, a sterile dressing is reapplied and the condition of the wound carefully monitored (WOCN, 2022; Baranoski & Ayello, 2020).
Complex Surgical Wound Care
An open abdominal wound is one example of a complex surgical wound. Such a wound cannot be closed at the time of surgery for a variety of reasons, such as abdominal compartment syndrome, bowel edema as a consequence of peritonitis, or wound infection. In other instances, the wound is closed at the time of surgery but later dehisces.
The operating surgeon will drive the management of these wounds, but the expertise of the wound care team is called upon to help with treatment options while the wound remains open. In the majority of cases, when surgeons ask wound care clinicians to assist with wound care, the surgeons are open to—and expect—recommendations based on the clinicians’ expertise and experience in dealing with wounds.
For the clinician, the first step in assessing complex surgical wounds is a thorough knowledge of the surgical procedure that was performed, the reason necessitating surgery, complications that prevented immediate wound closure, comorbid diseases, and the surgeon’s plan for the wound. Most of this information can be obtained from the patient’s chart, but it is a good policy to meet with the surgeon and discuss any questions and recommendations.
During the physical wound assessment of an open abdominal wound, the clinician identifies abdominal structures, areas of exposed mesh (if present), sutures, and viable and nonviable tissue. Careful assessment must be made for the presence of enteric fistulas. The wound care clinician must know whether or not new anastomoses were created during surgery because a high percentage of fistulas develop due to leaks at anastomosis sites.
The majority of open abdominal wounds are candidates for negative-pressure wound therapy (NPWT) (see “Negative-Pressure Wound Therapy” later in this course), but this may not be the first treatment option due to the state of the wound or surgeon preference. Other dressing choices include a contact layer that will not adhere to the wound bed. Depending on the size of the wound, the contact layer can be covered with alginate dressings or saline-moistened gauze.
Since these wounds normally require daily or twice-daily dressing changes, applying a wound manager over the wound may be of benefit. A wound manager is a large pouch with a hydrocolloid border that secures it to the intact periwound area. The pouch is transparent, which allows visualization of the wound, with a center flap that can be opened and resealed to provide easy access for wound care.
Another type of complex wound the clinician may be asked to assist with is an open chest wound, for example, after a failed coronary artery bypass graft. The wound care team will work closely with the surgeon in monitoring the progress of the wound. The guiding principles for care include stabilizing the open margins of the wound, protecting the myocardium, and promoting granulation tissue (WOCN, 2022; Baranoski & Ayello, 2020).
BATHING AND INFECTION RISK
Recent studies have shown that there is no statistically substantial difference in infection levels between patients who showered or bathed in the first 48 hours post surgery compared to those who postponed showering or bathing to beyond 48 hours. However, many surgeons prefer that patients do not immerse surgical wounds in water until there is full closure. The patient is advised to discuss this with their surgeon and follow the recommendations given (WOCN, 2022).
Pediatric Wound Care
In neonates, infants, and older children, differences in skin composition and texture are taken into consideration when caring for wounds. Premature infants have a thinner stratum corneum layer, which decreases the barrier function of the skin and increases the likelihood of injury. For these infants, skin maturation is delayed. Full-term infants have a thinner dermis than adults, giving the skin a very soft texture. In both premature and full-term infants, skin tears pose a particularly high risk. The occipital area has the highest risk for skin breakdown in the pediatric population and is the most frequent site for the development of immobility-associated pressure injury in children from birth to 3 years. The Braden QD Scale is used to measure pressure injury risk assessment in the pediatric population.
Other areas of the body where pressure injury occurs in this age group are the ears and the nose. The nose is especially susceptible to medical device–related pressure injury related to the use of ventilation masks. In fact, research has found that half of pressure injuries among pediatric patients are caused by equipment pressing on the skin.
Clinicians work closely with respiratory therapists in the repositioning of respiratory equipment to ensure pressure relief and to prevent injury. Interventions include repositioning oxygen masks and prongs on an hourly basis while performing a skin check. Applying a hydrocolloid or silicone dressing under medical equipment can also prevent injury. Pediatric patients are not left lying on tubing or cables, and the surface under and around the patient is kept free from anything that might cause pressure injury.
Another area requiring special attention in this population is insensate skin associated with spinal cord injury or myelomeningocele, which introduces high risks for pressure.
In many instances, a wound in an infant or child is a complication of another diagnosis or a surgical procedure. Thus, the clinician must keep in mind that the child and parents may be dealing with several evolving healthcare issues at the same time. Wound care may need to be continued postdischarge, and the bulk of that care may fall on the parents. The clinician will work with the family to develop a plan of care that is clear and practical.
One of the major concerns that parents have is the appearance of the healed wound and the risk of scar formation. In early childhood, wounds that move through the inflammatory phase without delay heal with minimal scarring.
Choosing the “best” wound cleanser and dressing for the pediatric population can be challenging. The safety and efficacy of wound care products have often been tested only in adult populations and not in pediatric populations. Products that include alcohol, dyes, or fragrances are avoided.
However, the same basic principles that guide wound care in adults are relevant to neonate and pediatric populations: to provide a moist wound environment free of devitalized tissue and contaminants. Dressings that incorporate a soft silicone adhesive are a good choice for neonates and infants with fragile skin, and hydrogels work well to maintain a moist wound environment (Baranoski & Ayello, 2020; Byrant & Nix, 2024).
Bariatric Wound Care
The prevalence of obesity among both adults and children is on the increase worldwide, and clinicians working in wound care will continue to see an increase in the numbers of patients with obesity. Greater than two thirds of the U.S. population is overweight or obese, including at least 8 million individuals with class 3 obesity; many of these are children (Baranoski & Ayello, 2020). Obese individuals are more susceptible to chronic health conditions such as diabetes, heart disease, and stroke.
A patient with obesity can develop a wound without being aware of it. It may be difficult for the patient to bathe, clean, and dry adequately in areas with excessive skin folds such as the back of the neck, under breasts, armpits, beneath a large abdominal pannus, and inner thighs. A lack of proper hygiene along with a buildup of moisture make these areas vulnerable to moisture-associated skin damage (MASD).
For people with obesity, especially class 3 obesity, there is a risk of developing necrotizing fasciitis (also known as Fournier’s gangrene), with elevated risk among individuals with diabetes mellitus. Necrotizing fasciitis is a serious and potentially fatal skin infection that may result due to excessive body moisture located in deep skin folds and beneath a bulky superimposing abdominal pannus. When the condition advances to skin necrosis and cutaneous gangrene, the patient will require extensive surgical debridement of the affected area. Successful outcomes depend on early detection and immediate treatment. Prevention is of primary importance and includes thorough cleansing of all skin folds along with measures to keep the skin dry (Beitz & Kennedy-Evans, 2023; Bryant & Nix, 2024).
Hospitalized patients with obesity are at especially high risk for developing pressure injuries, and moisture, friction, and shear injuries are highly problematic for this group. A complete assessment is required to identify existing wounds and areas of high risk.
Skincare education for patients who are obese addresses the following points:
- A complete daily bath is not necessary, but areas under skin folds must be cleaned on a daily basis.
- Soaps and body washes should be free from skin irritants.
- Gentle cleaning is important; do not scrub to wash and pat dry.
- Disposable wipes that are pH balanced and do not require rinsing can be used.
- Placing folded cotton material between skin folds can maintain dryness and decrease irritation.
- Using a cool hair dryer on weeping areas can help to promote dryness.
Selecting the correct equipment, such as a bariatric bed or extra-large wheelchair, to meet the needs of patients who are obese is an essential part of providing safe care for such patients. These needs must be addressed across the continuum of care and in the home setting. Physical therapists and occupational therapists are experts in this area and are involved in determining appropriate bariatric equipment. This may include a home evaluation as well as working closely with the social worker or case manager to identify funding options for recommended equipment.
Wounds under an abdominal pannus require good care. Adhesive foam dressings are a good choice. The use of a pannus sling reduces pressure and promotes patient comfort. Choosing the correct sling for each patient requires the input of several team members, including PT and OT. Slings are available that can be used with either an overhead or a floor lift (Baranoski & Ayello, 2020).
Regardless of the etiology of the wound, patients who are overweight or obese face unique problems with healing. These include diminished circulation to the wound bed (increased adipose tissue decreases blood flow to the skin), difficulty performing self-care, inability of caregivers to provide adequate assistance due to the patient’s size, and the potential for immobility and decline in function.
Patients with Spinal Cord Injuries
Studies show that pressure injuries are the most widely occurring complication of spinal cord injury (SCI) and one of the most devastating. Pressure injuries rank second as the causative factor for rehospitalization for individuals enrolled in the U.S. Spinal Cord Injury database. In the first year post–spinal cord injury, pressure injuries are responsible for 17% of rehospitalization, and at the five-year mark 23% of rehospitalizations (Baranoski & Ayello, 2020).
Decreased mobility and muscle atrophy are the leading causes of pressure injury development in this population. There is also a problem with diminished blood flow to the areas of the body below the level of injury, which results in lessened oxygen supply to tissues. These factors combine to delay healing when ulcers do develop (WOCN, 2022).
Preventive care focuses not only on pressure relief but also on psychological and social elements. A spinal cord injury is a sudden, overwhelming, life-changing event and usually occurs in an age group in which the concepts of skin checks and pressure relief are uncommon. Sustaining wellness involves a daily, lifetime commitment to lifestyle adaptations. This is a daunting task and requires consistent, empathetic care from clinicians as they assist patients to become knowledgeable proponents of their own care.
To be successful, pressure injury preventive care for SCI patients must be based on a multidisciplinary, coordinated approach. Holistic care for these patients includes a focus on the setting in which the patient lives and the elements in this setting that may support and impede care. Important teaching points for patients with SCI include:
- Performing daily skin inspection
- Ensuring good personal hygiene
- Performing frequent repositioning and weight shifts
- Using suitable pressure-relieving surfaces for bed and wheelchair
- Ensuring that support surfaces are properly maintained and replaced at recommended time frames
- Ensuring adequate nutritional intake
- Avoiding weight gain
- Sustaining a healthy lifestyle with no alcohol, tobacco, or illicit drug use
(Baranoski & Ayello, 2020; WOCN, 2022)
Learning correct transfer technique is one of the first steps in preventing skin tears, bruising, and wound development in these patients. If self-transfers are achievable, the clinician works with the patient on a technique that avoids sliding and bumping against parts of the wheelchair or landing surface and ensures the patient lands softly on the surface they are transferring to. Physical therapy works with the patient on developing and refining these skills and emphasizes the importance of consistently using correct technique with every transfer.
It is important to recognize that skin damage can generate an episode of autonomic dysreflexia (AD) in patients with SCI above the level of T5. Autonomic dysreflexia is overstimulation of the sympathetic nervous system to perceived noxious stimuli below the level of injury. It is characterized by extreme elevation in the patient’s blood pressure and is a medical emergency. As far as possible, identifying and eliminating triggers that can potentiate the risk for AD is an important clinical consideration. Common triggers for autonomic dysreflexia include irritation to the skin, pressure injury, abrasions, cuts and bruises, a full bladder, and ingrown toenails (WOCN, 2022).
Wounds in Geriatric Patients
Older adults are at increased risk for chronic wounds due to the presence of other conditions such as diabetes and decreased circulation. Wound healing in the older adult population is supported by eliminating the causative factors, following the principles of moist wound healing, and managing comorbid conditions.
SKIN CHANGES AND WOUNDS
Skin changes, both intrinsic and extrinsic, occur with aging. Intrinsic changes are the physiologic alternations that occur as part of the process of aging, and include:
- Reduction in the number of fibroblasts and decrease in the functioning ability of the remaining fibroblasts, resulting in delayed collagen synthesis and prolonged wound healing
- Decrease in the number of macrophages in the inflammatory phase of wound healing
- Fewer melanocytes to protect the skin from ultraviolet radiation
- Decrease in Langerhans cells, leading to comprised immunity
- Decreased adhesion between the epidermis and the dermis
- Fewer vitamin D receptors and a reduction in the production of vitamin D
- Thinning of the adipose layer
- Reduced blood flow to the skin, resulting in delayed wound healing
(WOCN, 2022)
Extrinsic factors are related to the environment and how it interacts with aging skin. The most important of these are:
- Ultraviolet light from the sun, which can penetrate farther into the dermis of aging skin
- Cigarette smoke, which is highly damaging to aging skin
- Hydration status (since older individuals, especially those in residential care, are vulnerable to dehydration due to a combination of factors that include decreased mobility, cognitive changes, and the need for outside assistance)
- Nutrition (since older patients at increased risk for nutritional deficits due to a combination of factors, and therefore at greater risk for pressure ulcer development and prolonged wound healing)
(WOCN, 2022)
SKIN TEARS
Older persons are at particular risk for skin tears, which usually occur during bathing, repositioning, dressing, and transfers. The most commonly reported causes of skin tears are wheelchair injuries, bumping into items such as furniture, injury during patient transfers, and falls. Those who are totally dependent on others to meet their care needs are at the highest risk for skin tears. Thus, clinicians must use gentle “handling” when working with older individuals.
A skin tear can be a significant injury depending on the patient’s age and overall health status. Infection and poor circulation can increase the seriousness of a skin tear. (Patients who are critically ill and neonates are also at high risk for skin tears.)
Skin tears can occur on any part of the body. Even small bumps can lead to an extensive skin tear, which is a traumatic injury and can result in either a partial- or full-thickness wound. Skin tears cause considerable pain to patients and are largely preventable (Baranoski & Ayello, 2020).
The International Skin Tear Advisory Panel (ISTAP) defines skin tears as “traumatic wounds caused by mechanical forces” and which can involve the removal of adhesives. The severity of a skin tear is dependent on the depth; however, they do not spread through the subcutaneous layer of the skin. The ISTAP skin tear classification system has been validated as useful and easy for clinicians to use:
- Type 1: No loss of skin; a linear or flap tear that can be repositioned to cover the wound
- Type 2: Partial flap loss, cannot be repositioned to cover the wound
- Type 3: Total flap loss, exposes the complete wound bed
(ISTAP, 2025)
ISTAP has also introduced a skin-tear reduction program that addresses the following areas:
- Patient’s general health, including enhanced nutrition and hydration
- Mobility, including suitable selection and use of assistive devices and eliminating clutter in surroundings
- Skin, including individualized skincare routines and avoidance by clinicians of sharp fingers and jewelry when providing patient care
- Healthcare setting, including introducing a skin-tear reduction program
(ISTAP, 2025)
Intervention when a skin tear occurs first requires controlling bleeding. This involves gentle cleaning with normal saline or a wound surfactant. Next, the skin edges are approximated and secured with Steri-Strips to allow healing. The area can be covered with a self-adhesive foam dressing to add extra protection. Wrapping (not tightly) with a gauze bandage or applying a cotton circular bandage also adds a layer of protection. Skin tears must be monitored closely for signs and symptoms of infection and delayed healing (WOCN, 2022; ISTAP, 2025).
Minor Burns
Major burn wounds are treated in specialized burn units (and are beyond the scope of this course). Smaller burns (classified as minor burns) are those that typically comprise <15% of the total body surface area (TBSA) for an adult and <10% TBSA for a child and do not include joint surfaces (WOCN, 2022). There are several sources of burn injuries:
- Chemical
- Electrical
- Radiation
- Thermal
A burn wound may involve the epidermis only, part or all of the dermis, or the subcutaneous tissue, extending down to muscle and bone (WOCN, 2022).
A burn that involves the epidermis only is red and painful (e.g., sunburn). There is no loss of skin and no development of blisters. Epidermal burns normally heal within a few days without complications. The patient is advised to clean the area gently, apply moisturizing lotion, wear loose-fitting clothing, and take mild painkillers as needed.
Partial-thickness burns can include the upper layer of the dermis, but they do not extend beyond the dermis. The clinical presentation of such a burn is a swollen, red, and painful area. Blisters and weeping are present. Moist wound care is used, and care must be taken to prevent dressings from adhering to the surface of the burn area. Small blisters <2 cm in diameter should be left intact. Larger blisters are excised in order to complete an assessment of the wound bed and depth.
A deep partial-thickness injury includes more of the dermal layer and may or may not have blistering. It is whitish/gray in color, dry, and nonblanchable. Deep partial-thickness wounds are treated with moist dressings. The usual healing time is less than three weeks, but these wounds are susceptible to injury from minor trauma for several months due to a decrease in cohesion between the epidermis and dermis.
A full-thickness burn will be covered with dry, waxy eschar. These burns are usually treated in a special burn unit.
Burn wounds evolve, and what may at first appear as a partial-thickness burn may over the course of two to three days deteriorate into a much deeper wound.
Children under the age of 4 and adults over the age of 65 are the two major groups at risk for burn injuries. Burns to children and the elderly can be the result of abuse, and clinicians should assess whether there is any discrepancy between the clinical findings on physical examination of the patient and the stated history of the burn (WOCN, 2022). For example, a red flag alerting the clinician to a nonaccidental cause of injury may include a burn that looks older than the reported time of occurrence or symmetrical burns to the extremities that could indicate that the patient’s hands or feet were forcibly held under hot water (Bryant & Nix, 2024).
End-of-Life Wound Care
In end-of-life wound care, the aim is usually one of wound maintenance, not wound healing. The primary goals are pain management, containment of drainage, odor relief, and maintaining the patient’s quality of life and dignity. Pain control and incontinence management are also critical. The wound care team coordinates all treatments around pain management and ensures that patients who require analgesics receive them about 30 minutes before repositioning or dressing changes.
The types of wounds that occur at the end of life vary and commonly include:
- Pressure injuries
- Malignant-related fungating wounds
- Moisture-associated skin damage (see also above under “Bariatric Wound Care”)
PRESSURE INJURIES AT END OF LIFE
Several factors combine to make patients in hospice care more vulnerable to pressure injuries, including poor physical condition, decreased food and fluid intake, an inability to feel pressure or pain, immobility, and compromised immunity. Positioning to avoid pain can also contribute to pressure injury formation; many patients who are terminally ill are unable to lie supine due to pain levels. Repositioning, even with assistance, may cause extreme pain and lead to impaired perfusion of already fragile skin surfaces. Choosing the correct support surface to relieve pressure and decrease pain is paramount in providing comfort for these patients.
(See also “Pressure Injuries” earlier in this course.)
KENNEDY TERMINAL ULCERS
Kennedy terminal ulcers were named after Karen Lou Kennedy, a nurse practitioner credited with creating one of the first skincare teams in a long-term care facility in the United States. Although Kennedy terminal ulcers first came to attention in 1989 in this country, they were actually described over 100 hundred years ago by French neurologist Dr. Jean-Martin Charcot, who referred to them as acute bed sores.
These ulcers can develop within a few hours and rapidly deteriorate and are indicative of impending death. They can be distressing for families and nursing staff alike. Families may perceive them as a lack of care for their loved one, and staff may at first be surprised that skin that was normal a few hours ago now has a large area of purple/black discoloration. It is helpful to explain to everyone involved that as the end of life approaches, the body begins to shut down, hypoperfusion of the skin leaves it vulnerable to the development of terminal ulcers, and the continued lack of sufficient perfusion causes these ulcers to quickly increase in size.
Apart from their sudden onset, other distinctive features of Kennedy terminal ulcers are:
- Location over the sacrococcygeal region
- Pear, horseshoe, or butterfly shape
- Irregular ulcer margins
- Continued worsening and progression to necrotic tissue even with the most appropriate care
The principles of care for Kennedy terminal ulcers include drainage management and comfort care. Debridement is not an appropriate intervention.
(Baranoski & Ayello, 2020)
FUNGATING WOUNDS
Fungating wounds develop when malignancy, either local or metastatic, spreads into the skin and its supporting structures (Baranoski & Ayello, 2020).
For patients, the most distressing symptom of these wounds is odor, and many patients report that this greatly compromises their quality of life. Due to shame and embarrassment, they isolate themselves from social interaction, leading to feelings of hopelessness and depression. Wound odor can be reduced by decreasing the amount of necrotic tissue and bacteria in the wound. Bacteria load can be decreased by irrigating the wound with tap water or by taking a shower and allowing water to flow across the wound bed.
The application of topical crushed metronidazole has proven to be successful in controlling odor. Charcoal dressings and honey dressings have also been shown to reduce odor and decrease pain at dressing changes. Due to large volumes of wound drainage, highly absorbent secondary dressings will also be required. Dakin’s solution can also be used to control wound odor. A gauze dressing soaked in Dakin’s solution 0.25% is placed in the wound for a limited amount of time. However, there are reports of Dakin’s dressings resulting in some discomfort (Baranoski & Ayello, 2020; Bryant & Nix, 2024).
CASE
Ms. Williams is a 68-year-old female patient in hospice care. She has metastatic right breast cancer. She has been doing fairly well at home with care from her family, and her pain is well controlled. However, the wound odor and drainage are causing her concern and limiting her interaction with family and friends. The hospice nurse, Dante, therefore reaches out to a wound care specialist for suggestions.
The wound care specialist, Shondra, visits the patient along with the hospice nurse. After explaining why she is there and listening to the patient’s concerns, Shondra asks Ms. Williams for permission to examine the wound. The anterior surface of the patient’s right breast is covered with a large fungating wound, and there are areas of leathery black necrotic tissue, greenish slough, and pale pink tissue. There appears to be a minimal to moderate amount of drainage and a strong malodor. The skin surface extending beyond the ulcer is red and excoriated. The patient states that this area is painful, and the hospice nurse explains that this is due to the tape used to keep the dressings in place.
Shondra recommends irrigating the wound with tap water or allowing water to flow over the wound bed while the patient is taking a shower in order to reduce the bacterial load in the ulcer. She also recommends that the hospice nurse ask Ms. Williams’s healthcare provider for an order for metronidazole tablets, since crushed metronidazole when applied to the wound bed has been shown to be successful in odor control. Other recommended options are the application of charcoal dressings or honey dressings.
Shondra further suggests applying thick abdominal pads (ABD pads) to control wound drainage. Adhesive tape should be discontinued and the dressings held in place with a stockinette tube vest that fits comfortably over the patient’s chest area.
Ms. Williams voices her agreement with the care plan. Shondra checks back two weeks later with the hospice nurse, Dante. The patient’s primary care provider has approved the use of metronidazole, and the patient has noticed a marked decrease in the degree of odor. The use of the ABD pads and the stockinette vest have made the dressings more comfortable, and Ms. Williams is now able to spend more quality time with her family and friends.
ADVANCED WOUND CARE MODALITIES
Advanced therapies (also known as adjunctive therapies) are used when standard wound care is not sufficient to bring about wound healing. Advanced therapies include negative-pressure wound therapy, hyperbaric oxygen therapy, and bioengineered skin products (Baranoski & Ayello, 2020).
Negative-Pressure Wound Therapy (NPWT)
Negative-pressure dressings (also called vacuum-assisted closure devices or sub–atmospheric-pressure dressings) are labor-saving devices that remove excess fluid from wounds while improving healing time (Shah et al., 2018). NPWT can be used in both acute and chronic wound care. Prior to applying NPWT, the wound bed must be free from necrotic tissue and infection under control (being actively treated).
Typically, NPWT is used for full-thickness wounds that require granulation tissue growth and contraction. It is the recommended treatment for stage 3 and stage 4 pressure injuries (Baranoski & Ayello, 2020).
NPWT has become the primary treatment used by military surgeons and trauma surgeons treating complex wounds such as penetrating trauma, fasciotomy incisions, and open fractures. For patients who require split-thickness grafts, NPWT has taken the place of established bolster dressings as the optimum means for preserving close interaction between the graft and the underlying wound bed (WOCN, 2022).
There are several types of NPWT systems, and all systems are based on either foam or gauze dressings. Whichever type is used, the dressing is fitted into the wound and covered by a special plastic film. A suction tube is applied through a hole made in the film and connected to the sponge or gauze via a disc. When the NPWT unit is turned on, a vacuum is applied to the tube, and it continuously sucks fluid from the wound. The vacuum also pulls the plastic film tightly over the top of the wound, sealing the wound from the environment, protecting the wound from outside contaminants, and keeping the wound warm.
For complex wounds, it may be necessary to continue use of negative pressure for several weeks. NPWT dressings are usually changed every 48 to 72 hours and sometimes more frequently for heavily infected wounds or heavily exudating wounds, depending on physician orders.
In addition to removing excess wound fluid, reducing the growth medium for bacteria, and removing wound inhibitory factors, a negative-pressure dressing encourages contraction of and granulation around the wound, thereby reducing edema, supporting development of local circulation, and providing more oxygen and nutrients to the cells.
The pull of negative pressure on cells has been demonstrated to stimulate new cell growth. Studies show NPWT to be very effective early in treatment of deep pressure injuries by reducing the depth of the ulcer via granulation and also as an aid to healing of chronic pressure injuries. Research indicates that granulation tissue develops more quickly with intermittent rather than continuous therapy; however, continuous therapy is needed when there are high volumes of drainage.
Studies also show that NPWT significantly increases healing rates and decreases healing time in diabetic foot ulcers and decreases the frequency of major amputations. In one study of diabetic patients who had undergone a partial foot amputation, 56% of those treated with NPWT achieved complete wound healing compared to 39% in the control group (Zhang et al., 2025).
NPWT can be used along with instillation of solutions into the wound bed. Several different solutions can be used, including normal saline and hypochlorous acid, with the intent to clean and irrigate the wound and to diminish bacterial colonization on the wound bed. This therapy is only available for hospitalized patients at this time (Baranoski & Ayello, 2020).
NPWT can be safely used in children older than one year. In the pediatric population, the pressure setting is modified and the therapy closely monitored by the wound clinician (WOCN, 2022).
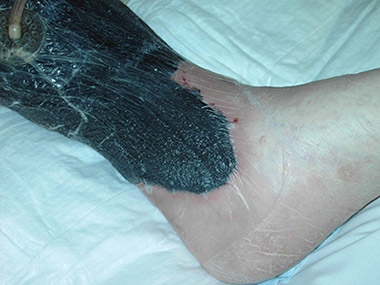
Negative-pressure wound therapy, with foam dressing, plastic film, and suction tube. (Source: Shortcut27, CC BY-SA 3.0.)
SPECIAL CONSIDERATIONS
When using NPWT, some special considerations must be kept in mind:
- Painful dressing changes can be prevented by placing a contact layer next to the wound bed beneath foam dressings (with prior physician/surgeon approval). If foam is adherent to the wound bed, it can be soaked with normal saline for a few minutes prior to removal, allowing it to be removed more easily. As well as decreasing pain, both these measures reduce the likelihood of causing wound bleeding.
- If tunneling is present, the wound is packed loosely with white foam (if a foam system is being used). This hydrophilic foam does not fall apart easily, reducing the possibility of unintentionally leaving a piece of foam in the area of tunneling.
- Special care is taken to protect the periwound area. This can be done using spray-on skin sealants or “picture-framing” the wound edges with strips of hydrocolloid dressings.
- Most NPWT devices have two settings: continuous suction or intermittent suction. Continuous suction is recommended at the beginning of therapy, and it is also the most appropriate setting for heavily draining wounds. Intermittent suction is usually applied when the amount of drainage has decreased and the goal is to enhance the growth of granulation tissue. However, some patients complain of wound discomfort with intermittent therapy.
- NPWT is continued as long as there is evidence of wound healing (i.e., the growth of new, healthy granulation tissue and decreasing wound size). Once a healing wound has filled in with granulation tissue close to surface level, NPWT is discontinued.
Hyperbaric Oxygen Therapy (HBOT)
Adequate levels of oxygen are essential for all phases of wound healing. Hyperbaric oxygen therapy is a process whereby the patient inhales 100% oxygen while inside a pressurized hyperbaric chamber. The delivery of oxygen under pressure increases the amount of dissolved oxygen in the plasma, and this in turn allows for increased amounts of oxygen distribution to the tissues. The goal is to stimulate healing by exposing the wound to high levels of oxygen. Increased oxygen levels also increase leukocyte activity with the destruction of aerobic gram-positive and gram-negative organisms.
The treatment can be delivered in single-unit chambers that accommodate one patient or in multi-unit chambers in which several patients are treated simultaneously. In most instances, HBOT is provided on an outpatient basis. HBOT has an excellent safety record in the United States (Baranoski & Ayello, 2020).
HBOT benefits patients with wounds that are severely infected (e.g., the presence of refractory osteomyelitis) or in cases in which wound healing is impeded by poor circulation. It is used to treat bone infection (osteomyelitis) because the increased delivery of oxygen to the bone has been shown to improve the functioning of white blood cells.
HBOT has also proved to be a positive factor in the healing of diabetic foot ulcers. HBOT is a proven therapy used to decrease the number of major amputations in person with diabetes and concurrent chronic foot ulcers. In one study where persons with diabetes and diabetic foot ulcers received HBOT, there was a notably quicker time to wound healing (Baranoski & Ayello, 2020; WOCN 2022).
It is also successfully used to advance the healing of surgical flaps and grafts. However, HBOT is not beneficial to a wound that is covered with necrotic tissue (e.g., dry gangrene).
The first step is a patient evaluation to determine whether the treatment is appropriate. For any patient who has a recent history of malignancy or is being treated for malignancy, a consultation with the treating oncologist is necessary before deciding about HBOT.
Contraindications to HBOT include:
- Untreated pneumothorax
- Ear disorders
- Medications such as doxorubicin (Adriamycin) and cisplatinum
- Claustrophobia
- Seizure disorder (to be determined on an individualized basis, since a side effect of HBOT is oxygen toxicity, which can induce seizure activity)
(WOCN, 2022)
Bioengineered Skin Products
This is a fast-evolving area in wound care. Such products can be quite expensive, but they offer several advantages, the most important being speedy wound closure. They can also obviate the need for a graft with skin harvested from the patient (usually removed from the thigh area), which produces a second, and many times painful, wound site.
Patients must be carefully selected for this therapy, and their overall health status and ability to heal must be taken into consideration. The wound must be free from infection and the wound bed well vascularized, free of necrotic tissue, and adequately prepared prior to application of the specific skin substitute product. Most facilities require that authorization from the payment source be obtained prior to the treatment.
A key consideration for the wound care team is choosing the product that is most appropriate for the particular wound; this requires expertise, research, and collaboration with other wound care specialists.
Bioengineered skin substitutes can be classified as either cellular or acellular. Cellular products contain living cells. Acellular products contain collagen, usually derived from a porcine or bovine source; they do not contain living cells (Zhang et al., 2023).
Adjunct Therapies for Wound Care
The following therapies are not used alone but in conjunction with other treatments to heal wounds:
ELECTRICAL STIMULATION THERAPY (E-STIM)
Electrical stimulation therapy is the use of an electrical current to transfer energy to a wound. E-stim is performed by physical therapists and occupational therapists who have training and experience in the use of this therapy.
Effects attributed to e-stim include increased blood flow to the area, which increases oxygen and nutrient transport to the wound; reduced edema and pain; and increased fibroblast and collagen development. It may be useful for pressure injuries, venous ulcers, surgical wounds, donor sites, burn wounds, and others. Studies have shown positive benefits in the treatment of diabetic foot ulcers. E-stim has also been shown to increase the blood supply to ulcer sites and decrease the healing time. This is an area of ongoing research. Studies show that electrical stimulation also has bacteriostatic and bactericidal effects on organisms that are present in chronic wounds.
E-stim for wound care is not to be used in the presence of a cardiac pacemaker, malignancy, osteomyelitis, and when electrodes would be placed near the heart, larynx, carotid sinus, eyes, head, and some other areas. E-stim should not be used when there is a possibility of basal cell or squamous cell carcinoma in the wound or the surrounding tissues. E-stim is contraindicated for individuals with infections (Baranoski & Ayello, 2020; WOCN, 2022).
ULTRASOUND
Noncontact low-frequency ultrasound has been shown to reduce wound size and “bio-burn.” In this modality, a trained, experienced clinician employs the ultrasound machine to vaporize normal saline into microdroplets that are then propelled into the wound bed.
Ultrasound is used to increase the elasticity of collagen, decrease muscle and joint stiffness, decrease pain and muscle spasms, decrease edema, increase oxygen transport, and accelerate wound healing. Ultrasound stimulates circulation to the treated area, which aids in cell metabolism. It also provides for an increase in macrophage activity and leads to increased protein production by fibroblasts.
Ultrasound is used on chronic wounds, pressure injuries, venous ulcers, and trauma wounds. It is not indicated in cases of infection, osteomyelitis, profuse bleeding, severe arterial insufficiency, or necrotic wounds.
Ultrasound therapy cannot be used over the following areas of the body:
- Eyes
- Heart
- Carotid sinuses
- Uterus during pregnancy
- Exposed central nervous system (e.g., a laminectomy site)
(Baranoski & Ayello, 2020)
Several studies on the use of ultrasound have been conducted, but more evidence on the efficiency of ultrasound treatment is recommended (Bryant & Nix, 2024).
IASTM
Instrument-assisted soft tissue mobilization (IASTM) is a therapeutic approach used by physical therapists to treat fascial dysfunction and pain. The trained therapist uses specialized handheld instruments to apply force to small specific areas. The result is soft tissue mobilization. The intent of treatment is to initiate an inflammatory healing reaction, with subsequent production of collagen and healing. Benefits of IASTM include increase in strength and diminished pain. It is not applied directly to a wound. There are several contraindications to the use of IASM, including acute injury or infection, local tissue inflammation or tumors, severe pain reported by the patient, and bruising at the location of treatment (Cheatham et al., 2019).
PATIENT TEACHING
It is important that patients be told what to expect during and after wound healing. Some helpful information to give a patient is described below:
- The wound area may tingle, feel strange, or itch. By pressing on the skin or by lightly rubbing it, the feelings can usually be lessened. These feelings may show up for many months, but they should be gone within a year.
- Deeper wounds may have injured some sensory nerves, so there may be numbness or lessened sensation distal to the wound. This problem usually improves on its own within a year.
- All full-thickness wounds, no matter how artfully repaired, leave a scar. Typical scars become darker and redder before they eventually fade. It can be a year or more before they reach their final appearance. Scar mobilization techniques taught by a physical therapist may be helpful.
- Currently, there is limited evidence that creams or lotions can limit scar formation, but Mederma cream can help to improve the overall appearance of the skin.
- If the patient is worried about something seen or felt in the wound or if the patient develops any of the signs and symptoms of an infection, it is important to contact the appropriate healthcare professional.
- If tissue glue has been used, it may cause a mild local inflammatory reaction.
(WOCN, 2022; Baranoski & Ayello, 2020)
A Patient-Centered Approach
At their first encounter, the clinician begins to develop a therapeutic relationship with the patient, emphasizing that the patient is the most important team member. The clinician explains the steps the patient can take to actively participate in their own care. This is especially important for those with chronic wounds, in which control of blood sugar levels, weight loss, and compliance with compression therapy may play key roles in wound healing.
All information is phrased in plain language that the patient will understand. However, caution is required; what is plain language to one individual may be confusing to another. The age, background, and ethnicity of the patient must be taken into consideration. For instance, instead of referring to “the medial aspect of your leg,” a more easily understood description might be “the inside of your leg.”
Clinicians view wounds from a professional perspective, but that is not how patients see wounds. Wounds are open areas on one’s own body that can, and do, make life miserable. The psychological distress that a wound causes has the potential to impede healing. Patient fears include:
- “Will the wound leave a scar?”
- “Will it come back again?”
- “Can I still work?”
- “How will my partner, family, etc., react to my wound?”
Pain and depression are also significant factors for patients with chronic wounds. For instance, individuals with diabetic foot ulcers often experience intense burning or shooting neuropathic pain that can last for several hours and interfere with sleep. Depression is a common finding among patients with diabetes who have open wounds. It is related to pain, the array of complications they face, and the real possibility of amputation of part of a foot or leg and the resultant deformity.
Wounds with a malodor are particularly debilitating for a patient. They can be a source of embarrassment, shame, and social isolation. Wound care clinicians must practice emphatic listening and give straightforward, honest answers (WOCN 2022; Baranoski & Ayello, 2020).
ANSWERING PATIENT QUESTIONS
Q: How long should a wound hurt?
A: This depends on the size and type of wound, but a wound that is healing should hurt less and less each day. If a wound starts hurting more, see your primary care provider immediately.
Q: How long will my wound take to heal?
A: Realistically, I can’t tell you exactly how long it will take since we each heal at a different rate, some faster than others.
Discharge Planning
When a patient is transferring to a new level of care or out of a facility, effective communication helps prevent a breakdown in care. All members of the wound care team should be involved in the discharge, with the case manager or social worker normally coordinating the process.
Patient concerns and anxiety about the impending transfer or discharge must be addressed. The patient is told what to expect at the next location of care. For example, a patient being transferred to a nursing home or rehabilitation facility is reassured that staff there will be able to continue the wound care and that a complete record of all treatments and interventions will be provided to the new facility.
Patients being discharged to home are also made aware of the availability of home health care and provided with a list of home health agencies in the area. Once an agency has been chosen, it is incumbent on the wound care team to communicate with the agency and arrange for the following:
- Patient’s projected date of discharge from the facility or clinic
- Date and time when the initial home health intake will occur
- Secure transfer of patient records and treatment plan to the agency
- If possible, a brief telephone conference with the home care wound clinician to discuss the plan of care and address any issues and concerns
(WOCN, 2022; Baranoski & Ayello, 2020)
Home Care Instructions
Home care plays an important role in wound care, especially for chronic wounds, and the patient and caregivers must feel comfortable providing this care. It is important that the clinician observe the patient or caregiver doing hands-on care and reinforce or correct their technique as appropriate.
A written set of instructions explaining how to care for the healing wound is given to the patient. Included is a list of signs and symptoms of an infected wound and instructions regarding showering, bathing, and swimming. Healthcare facilities generally develop protocol-based, preprinted instruction sheets for each specific type of wound (WOCN, 2022; Baranoski & Ayello, 2020).
CHECKING FOR SIGNS OF INFECTION
Patients are instructed to watch for signs and symptoms of an infected wound. These include:
- Pus or yellow, greenish, or thick whitish fluid in the wound
- Increased redness in the wound
- Redness radiating out into the skin around the wound
- Red lines progressing up an extremity
- Increasing pain or tenderness
- Swelling
- Wound getting warmer than normal skin
- Fever
(WOCN, 2022)
ELEVATING THE WOUND
Elevating the injured area will minimize swelling, reduce any throbbing pain, and speed up healing. If the wound is on an arm or a leg, patients are instructed to keep the injured area elevated during the first two days. For injuries to the hand or forearm, the patient can consider wearing a sling.
PROTECTING THE WOUND
Patients are instructed that:
- When healing normally, most wounds that have been directly closed will become impermeable to bacteria and water within two days.
- The edges of a directly closed healing wound are held together only weakly for the first five days; therefore, it is important to be especially careful with the wound for the first week. The new scar will then strengthen rapidly over the next month.
- A wound that has been splinted should remain immobilized until the sutures or staples have been removed.
- There is no general reason that most healing wounds need to be kept dry. Beginning on day three, or as ordered by the healthcare provider, patients with sutured or stapled wounds can shower then pat dry the wound and cover with a dressing if there is drainage, or as instructed.
CLEANSING THE WOUND
Patients with wounds will probably be sent home with a protective dressing. Minor wounds and many sutured or stapled wounds will no longer require these coverings after two days. The coverings can then be discarded and the wound left uncovered. However, if there is drainage, the wound is best left covered.
In most cases, patients can be advised that when treating the wound, cleanliness is needed but sterility is not.
A sutured or stapled wound without a dressing can be cleansed gently daily with soap and water beginning two days after the suturing or stapling. A major goal of these washings is to remove the crusting that develops from the wound exudate. Such a wound can remain uncovered starting on day three.
Almost any wound can benefit from being gently cleansed with soap and water in a shower. Taking a shower also cleans the skin around the wound much better than normal saline solution, and clean periwound skin also decreases risk of infection. About the only wounds that should not be in a shower are deep abdominal wounds or foot wounds (because foot wounds may end up soaking in water, which is not desirable).
When the wound is inside the mouth, the patient should rinse the injured area at least three times daily with warm normal saline solution (9% saline).
ANSWERING PATIENT QUESTIONS
Q: Is it true that I can use regular tap water to clean a wound?
A: Yes, tap water can be used to clean wounds.
Q: What should I do if I get my dressing wet during showering?
A: A dressing that gets wet should be changed. Leaving it in place can make the wound too wet and also damage the skin surrounding the wound.
PATIENT INSTRUCTIONS FOR CHANGING A WOUND DRESSING AT HOME
- Wash and dry your hands.
- Assemble and open the fresh dressing materials, being careful to touch only the wrappers or edges of the new dressings.
- Gently remove the used dressings and set them away from the new dressings.
- Wash the wound with soap and tap water unless otherwise instructed.
- Pat the wound dry with a clean cloth or gauze.
- Follow written wound care instructions regarding any ointments or types of dressings.
- Put on a fresh dressing.
- Discard the used dressing.
- Wash and dry your hands again.
AVOIDING ALCOHOL CONSUMPTION
As alcohol may interfere with or interact with some medications, such as antibiotics and analgesics, and may increase bleeding, patients should be informed on an individual basis as to alcohol consumption.
TELEHEALTH AND WOUND CARE
Teleheath (also known as telemedicine) can be a useful tool in the management and treatment of wounds. Telemedicine can improve wound care and healing outcomes for patients at home, those who attend remote clinics, and in circumstances where travelling is too difficult (Baranoski & Ayello, 2020; Bryant & Nix, 2024).
Studies have found better healing rates and fewer amputations when using telehealth with expert consultation continued throughout the progression of the patient’s treatment as compared to usual wound care. Study data has also indicated that the costs for telehealth consultations were lower than the cost of in-person consultations and that overall healthcare costs were also decreased through the use of telemedicine for wound care (AHRQ, 2019; Bryant & Nix, 2024).
Currently, there is not a great deal of evidence about the efficacy of telehealth in the overall delivery of wound care. What research does exist indicates that wound care via telehealth is not inferior to in-person care. The growth of telehealth during the COVID-19 pandemic highlights the need for telehealth models for wound care (Oropallo, 2022).
CONCLUSION
Regardless of their practice setting—acute care, outpatient, rehabilitation, nursing home, or home care—clinicians encounter patients who require wound care. Clinicians must provide holistic care, while using excellent assessment skills, thorough knowledge of state-of-the-art therapies, and respect for patient and caregiver preferences.
To meet these challenges successfully, the wound care clinician must have knowledge of evidence-based wound care and the skills needed to provide appropriate therapies. A commitment to continuous professional development and lifelong learning is essential for every clinician involved in wound care practice.
RESOURCES
American Professional Wound Care Association
National Alliance of Wound Care and Ostomy
National Pressure Injury Advisory Panel
Organization of Wound Care Nurses
Wound care (eMedicineHealth)
REFERENCES
Agency for Healthcare Research and Quality (AHRQ). (2019). Telehealth for acute and chronic care consultations (Publication No.19-EHCO12-EF). https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/cer-216-telehealth-final-report.pdf
American Physical Therapy Association (APTA). (n.d.). Become a board-certified wound management specialist. https://specialization.apta.org/become-a-specialist/wound-management
American Physical Therapy Association (APTA). (2020). APTA analysis: The value of physical therapy in wound care. https://www.apta.org/article/2020/10/30/analysis-value-physical-therapy-wound-Care
American Podiatric Medical Association (APMA). (2025). Diabetic wound care. https://www.apma.org/patients-and-the-public/conditions-affecting-the-foot-and-ankle/diabetic-wound-care/
American Professional Wound Care Association (APWCA). (n.d.). ABWH certification exams. https://www.apwca.org/ABWH-Certification-Exams
Amini D. (2018). American Occupational Therapy Association position paper: Role of occupational therapy in wound management. American Journal of Occupational Therapy, 72(suppl. 2), 7212420057. https://doi.org/10.5014/ajot.2018.72S212
Amirrah IN, Lokanathan Y, Zulkiflee I, Wee MFMR, Motta A, & Fauzi MB. (2022). A comprehensive review on collagen type 1 development of biomaterials for tissue engineering: from biosynthesis to bioscaffold. Biomedicines, 10(9), 2307. https://pmc.ncbi.nlm.nih.gov/articles/PMC9496548/
Armstrong DG & Lavery LA (Eds.). (2024). Clinical care of diabetic foot (4th ed.). American Diabetes Association.
Association for Professionals in Infection Control and Epidemiology (APIC). (2022). PPE do’s and don’ts. Infection Prevention and You. https://infectionpreventionandyou.org/infographic/PPE-dos-and-donts
Attaran RR & Carr JG. (2023). Chronic venous disease of the lower extremities: A state-of-the art review. Journal of the Society for Cardiovascular Angiography & Interventions, 2(1), 100538. https://www.sciencedirect.com/science/article/pii/S2772930322005543
Baranoski S & Ayello EA. (2020). Wound care essentials: Practice principles (5th ed). Wolters Kluwer.
Beitz JM & Kennedy-Evans KL. (2023). Good things don’t always come in small packages. Journal of Wound, Ostomy & Continence Nursing, 50(5), 365–74.
BioStem Technologies. (2024). Understanding hypergranulation: Causes, effects, and treatment. https://biostemtechnologies.com/understanding-hypergranulation-causes-effects-and-treatment/
Bryant RA & Nix DP. (2024). Acute and chronic wounds, current management concepts (6th ed.). Elsevier.
Cederholm T & Bosaeus I. (2024). Malnutrition in adults. New England Journal of Medicine, 391(2), 155–65. https://www.nejm.org/doi/full/10.1056/NEJMra2212159
Centers for Disease Control and Prevention (CDC). (2023). Current HAI progress report. https://www.cdc.gov/healthcare-associated-infections/php/data/progress-report.html
Chen Q & Zhou K. (2022). Acetic acid use in chronic wound healing. Journal of Wound Ostomy and Continence Nursing, 49(3), 286–9. https://doi.org/10.1097/WON.0000000000000863
Cheatham SW, Baker R, & Kreiswirth E. (2019). Instrument assisted soft-tissue mobilization: A commentary on clinical practice guidelines for rehabilitation professionals. Int J Sports Phys Ther, 14(4), 670–82. https://pmc.ncbi.nlm.nih.gov/articles/PMC6670063/
Chowdhry SA, Nieves-Malloure Y, Camardo M, Robertson JM, & Keys J. (2021). Use of oxidised regenerated cellulose/collagen dressings versus standard care over multiple wound types: A systemic review and meta-analysis. Intl Wound Journal, 19(2). https://doi.org/10.1111/iwj.13625
Conyers Y & Swoboda L. (2023). Getting ready for foot care certification. Journal of Wound, Ostomy, and Continence Nursing, 50(3), 250–2.
Cox J, Edsberg LE, Koloms K, & VanGilder CA. (2022). Pressure injuries in critical care patients in US hospitals. Journal of Wound, Ostomy, and Continence Nursing, 49(1), 21–8.
Department of Public Health (DPH) City of Philadelphia. (2024). Recommendations for caring for individuals with xylazine-associated wounds. https://hip.phila.gov/document/4148/Recommendations_for_Caring_for_People_with_Xylazine-Associated_Wounds_1.12.pdf/
DeYulis M & Hinson JW. (2023). Joint immobilization. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK557703/
Dunlop E, Fitzpatrick S, & Nagarsheth K. (2025). Case report: Treatment of chronic venous ulceration. Journal of Vascular Nursing, 43(1), 23–6. https://www.sciencedirect.com/science/article/abs/pii/S1062030324000876
European Pressure Injury Advisory Panel, National Pressure Injury Advisory Panel, and the Pan Pacific Pressure Injury Advisory Panel (EPIAP/NPIAP/PPPIA). (2019). International guideline. https://www.internationalguideline.com/
Eustice C. (2022). Symptoms of rheumatoid arthritis. Verywell Health. https://www.verywellhealth.com/rheumatoid-arthritis-signs-and-symptoms-190325
Evans K & Oropallo A. (2025). Overview of treatment of chronic wounds. UpToDate. https://www.uptodate.com/contents/overview-of-treatment-of-chronic-wounds
Friedlander T, Masterson R, & Mahesh K. (2023). Contractures. https://now.aapmr.org/contractures/
Gregory T. (2025). Seniors and chronic wounds: A growing concern in the United States. All About Seniors. https://www.allaboutseniors.org/seniors-and-chronic-wounds-a-growing-concern-in-the-united-states
Guarino MF, Bacci S, González LAP, Martínez MB, Matilla AC, & Bule MLH. (2023). The role of physical therapy in wound healing and assisted scarring. International Journal of Molecular Sciences, 24(8), 7487. https://pmc.ncbi.nlm.nih.gov/articles/PMC10144139/
Harding K, Carville K, Chadwick P, Moore Z, Nicodème M, Percival S, Romanelli M, et al. (2019). WUWHS consensus document: Wound exudate, effective assessment and management. Wounds International. https://www.woundsinternational.com/resources/details/wuwhs-consensus-document-wound-exudate-effective-assessment-and-management
Holman M. (2023). Using tap water compared with normal saline for cleansing wounds in adults: A literature review of the evidence. Wound Care, 32(8), 507–12. https://pubmed.ncbi.nlm.nih.gov/37572340/
International Skin Tears Advisory Panel (ISTAP). (2025). Best practice recommendations for the prevention and management of skin tears in aged skin (2nd ed.). Wounds International. https://woundsinternational.com/consensus-documents/best-practice-recommendations-for-the-prevention-and-management-of-skin-tears-in-aged-skin-2nd-edition/
Johns Hopkins Medicine. (2025). What is a scar? https://www.hopkinsmedicine.org/health/conditions-and-diseases/scars
Jones B. (2024). What is the hypodermis? The subcutaneous layer of skin. Verywell Health. https://www.verywellhealth.com/the-hypodermis-is-the-lowermost-layer-of-skin-2710144
Ju M, Kim Y, & Seo KW. (2023). Role of nutrition in wound healing and nutritional recommendations for promotion of wound healing: A narrative review. Annals of Clinical Nutrition and Metabolism, 15(3), 67–71. https://www.e-acnm.org/journal/view.php?number=287
Mackenzie J. (2020). The importance of interdisciplinary teamwork in wound rounds and assessment. Vohra Wound Physicians. https://cert.vohrawoundcare.com/the-importance-of-interdisciplinary-teamwork-in-wound-rounds-and-assessment/
Majmundar VD & Baxi K. (2022). Livedoid vasculopathy. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK559037/
Mayo Clinic. (2025). Rabies. https://www.mayoclinic.org/diseases-conditions/rabies/diagnosis-treatment/drc-20351826
Melter C & Mamou M. (2023). Pressure injury prevention and treatment: Assessment, wound care, and healing. Wild Iris Medical Education. https://wildirismedicaleducation.com/courses/pressure-ulcer-assessment-treatment-ceu
Mitchell A & Llumigusin D. (2021). The assessment and management of hypergranulation. British Journal of Nursing, 30(5). https://www.britishjournalofnursing.com/content/back-to-basics/the-assessment-and-management-of-hypergranulation
Mony MP, Harmon KA, Hess R, Dorafshar AH, & Shafikhani SH. (2023). An updated review of hypertrophic scarring. Cells, 12(5), 678. https://pmc.ncbi.nlm.nih.gov/articles/PMC10000648/
Morgan N. (2019). Sample procedure for nonsterile dressing changes. Wound Care Advisor. https://woundcareadvisor.com/smaple-procedure-nonsterile-dressing-change/
Münter K-C, Lázaro-Martínez JL, Kanya S, Sawade L, Schwenke C, Pegalajar-Jurado A, Swanson T, & Leaper D. (2024). Clinical efficacy and safety of a silver ion-releasing foam dressing on hard-to-heal wounds: a meta-analysis. Journal of Wound Care, 33(10), 726–36. https://pubmed.ncbi.nlm.nih.gov/39388210/
National Alliance of Wound Care and Ostomy (NAWCO). (2020). Wound care certification. https://www.nawccb.org/wound-care-certification
National Institute on Drug Abuse. (2024). Xylazine. How do you treat xylazine-related wounds? https://nida.nih.gov/research-topics/xylazine#treat-xylazine-related-wounds
National Pressure Injury Advisory Panel (NPIAP). (2025). Pressure ulcers/injuries: Definition and etiology. https://internationalguideline.com/etiology
Oropallo A. (2022). COVID-19: Issues related to wound care and telehealth management. UpToDate. https://www.uptodate.com/contents/covid-19-issues-related-to-wound-care-and-telehealth-management/print
Panahi A, Couch KS, White PB, & Chao JW. (2022). Acute skin failure associated with severe COVID-19. Plastic and Reconstructive Surgery, 151(1), 185e–6e. https://doi.org/10.1097/PRS.0000000000009748
Penn Medicine. (2025). Xylazine wounds. https://penncamp.org/clinical/xylazine-wounds/
Pontieri-Lewis V, Emmons, R, Scardillo J, Berke C, Alexander D, Byrant D, Yates S, et al. (2021). COVID-19 skin manifestations: A guide for WOC nursing practice. Journal of Wound, Ostomy & Continence Nursing, 48(5), 410–4. https://doi.org/10.1097/WON.0000000000000809
Ratner D. (2020). Suturing techniques periprocedural care. Medscape. https://emedicine.medscape.com/article/1824895-periprocedure#b6
Rosenthal BF. (2020). Recognizing the impact of nutrition in healing wounds. Podiatry Today, 33(10). https://www.hmpgloballearningnetwork.com/site/podiatry/recognizing-impact-nutrition-healing-wounds
Sen CK. (2025). Human wound and its burden: Updated 2025 compendium of estimates. Mary Ann Liebert, Inc. https://pubmed.ncbi.nlm.nih.gov/40660772/
Shah JB, Sheffield PJ, & Fife CE. (2018). Textbook of chronic wound care: An evidence-based approach for diagnosis and treatment. Best Publishing Company.
Spector JJ. (2025). Breaking down the stages of biofilm formation. Wound Source. https://www.woundsource.com/blog/breaking-down-stages-biofilm-formation
Staebel K. (2023). Wound care: Five evidence-based practices. American Nurse Journal, 18(2). https://www.myamericannurse.com/wound-care-five-evidence-based-practices/
Tambella AM, Galosi M, Angorini A, Dini F, Piccionello AP, Di Bella C, Serino F, Sassaroli S, & Troisi A. (2025). Advances in noncontact measurement of wound area using an application for smart mobile devices. Advances in Skin & Wound Care, 38(7), 360–6. https://journals.lww.com/10.1097/ASW.0000000000000296
Tchanque-Fossuo CN, Millsop JW, Johnson MA, Dahle SE, & Isseroff RR. (2018). Ulcerated basal cell carcinomas masquerading as venous leg ulcers. Advances in Skin and Wound Care, 31(3), 130–4. https://doi.org/10.1097/01.ASW.0000530068.44631.dc
Toussaint F, Erdmann M, Berking C, Erfurt-Berge C. (2021). Malignant tumors presenting as chronic leg wounds or foot ulcers. Journal of Clinical Medicine, 10(11), 2251. https://doi.org/10.3390/jcm10112251
University Hospitals Sussex. (2024). Identifying a wound infection. https://www.uhsussex.nhs.uk/wp-content/uploads/2024/01/2336-identifying-a-wound-infection-2024.pdf
UT Health East Texas. (2022). Find complete healing for chronic wounds. https://uthealtheasttexas.com/news/find-complete-healing-chronic-wounds
Vasculitis Foundation. (2022). What is vasculitis? https://www.vasculitisfoundation.org/vasculitis/
Warp PV, Hauschild M, Serota DP, et al. (2024). A confirmed case of xylazine-induced skin ulcers in a person who injects drugs in Miami, Florida, USA. Harm Reduction Journal, 21, 64. https://doi.org/10.1186/s12954-024-00978-z
WebMD. (2025). Rabies in dogs. https://www.webmd.com/pets/dogs/rabies-dogs
WebMD. (2024). Foods high in zinc. https://www.webmd.com/diet/foods-high-in-zinc
Wound, Ostomy, and Continence Nursing Certification Board (WOCNCB). (n.d.). Certification and Recertification. https://www.wocncb.org/
Wound, Ostomy, and Continence Nurses Society (WOCN). (2022). Wound management core curriculum. Wolters Kluwer.
Wound Source. (2025). Hydrofera Blue. https://www.woundsource.com/product/hydrofera-blue-classic
Wound Source. (2022). Defining chronic wounds. https://www.woundsource.com/blog/defining-chronic-wounds
Zhang Y, Huang B, Wang T, Dong H, Huang X, & Li X. (2025). A systematic review and meta-analysis of treatment modalities and their impact on the healing progression of diabetic foot ulcers. International Journal of Burns and Trauma, 15(2), 41–52. https://doi.org/10.62347/WVEM7973
Zhang M, Xing J, Zhong Y, Zhang T, Liu X, & Xing D. (2023). Advanced function, design and application of skin substitutes for skin regeneration. Materials Today Bio, 24, 100918. https://www.sciencedirect.com/science/article/pii/S2590006423003782






Customer Rating
5.0 / 505 ratings