Medical Errors and Adverse Events
Prevention and Reduction
Online Continuing Education Course

Course Description
7-contact-hour comprehensive overview on the prevention and reduction of medical errors and adverse events. This continuing education course defines the scope of the problem and includes many types of medical errors, including surgical, diagnostic, medication, devices and equipment, systems failures, healthcare-associated infections, and falls. Explore why medical errors are underreported and learn new strategies for how to decrease medical errors, optimize communication, and increase patient safety and quality of care.
Course Price: $42.00
Contact Hours: 7
Pharmacotherapeutic Hours: 0.5
Course updated on
May 2, 2024
"Well-organized course. Great information presented in a manner that you can relate to and therefore retain." - Patricia, RN in Arkansas
"One of the best online courses I have ever completed!" - Tami, OTA in Wisconsin
"Exceptional course! So helpful." - Sharon, RN in Georgia
"The course was well-written and easy to follow and the questions adequately assessed my understanding of the learning objectives." - Mary, OT in South Carolina
Medical Errors and Adverse Events
Prevention and Reduction
Copyright © 2024 Wild Iris Medical Education, Inc. All Rights Reserved.
LEARNING OUTCOME AND OBJECTIVES: Upon completion of this course, you will understand current, evidence-based interventions to prevent medical errors in the practice setting. Specific learning objectives to address potential knowledge gaps include:
- Define “medical errors” and associated terminology.
- Discuss the scope of medical errors in the U.S. healthcare environment.
- Describe the causes of medical errors.
- Review the most common medical errors and strategies to prevent them.
- Summarize the elements of effective clinical communication and documentation.
- Identify populations with special vulnerability to medical errors.
- Describe various initiatives of the patient safety movement in healthcare.
- Discuss healthcare accrediting agency standards and goals as they relate to preventing medical errors.
- Outline institutional strategies to identify and reduce the risk of medical errors.
TABLE OF CONTENTS
- Introduction
- Defining Medical Errors
- Scope of the Medical Error Problem
- Common Causes of Medical Errors
- Categories of Medical Errors and How to Prevent Them
- Developing Effective Documentation and Communication
- Error Risks Among Populations of Special Vulnerability
- Patient Safety Initiatives
- Accrediting Agencies’ Impact on Preventing Medical Errors
- Institutional Strategies for Addressing Errors
- Conclusion
- Resources
- References
INTRODUCTION
Healthcare providers know medical errors create a serious public health problem that poses a substantial threat to patient safety. Yet, despite providers’ best efforts, medical error rates remain high, with significant disability and death. Errors can occur at any point while an individual is in the healthcare system—in hospitals, clinics, surgery centers, dialysis centers, medical offices, dental offices, nursing homes, pharmacies, and even in patients’ homes—anywhere that patients receive healthcare services.
It is estimated that approximately 400,000 hospitalized patients experience some type of preventable harm each year, including surgical, diagnostic, medication, devices and equipment, system failures, infections, and falls. Most errors in outpatient healthcare are related to a missed or late diagnosis.
Analyzing why medical errors happen has traditionally been focused on the human factor, concentrating on individual responsibility for making an error, and the solutions have involved training or retraining, additional supervision, or even disciplinary action. Healthcare professionals experience profound psychological effects such as anger, guilt, inadequacy, depression, and suicide due to real or perceived errors. The loss of clinical confidence and fear of punishment can make healthcare professionals reluctant to report errors.
The alternative to this individual-centered approach is a system-centered approach, which assumes that humans are fallible and that systems must be designed so that humans are prevented from making errors. The trend is for patient safety experts to focus on improving the safety of healthcare systems to reduce the probability of errors and mitigate their effects rather than focus on an individual’s actions.
Errors represent an opportunity for constructive changes and improved education in healthcare delivery. Acknowledging that errors happen, learning from them, and working to prevent errors in the future are important goals and represent a major change in the culture of healthcare—a shift from blame and punishment to analysis of the root causes of errors and the creation of strategies to reduce the risk of errors. In other words, healthcare organizations must create a culture of safety that views medical errors as opportunities to improve the system. Every person on the healthcare team has a role in making healthcare safer for patients and workers (Rodziewicz et al., 2023).
DEFINING MEDICAL ERRORS
In 1999, the Institute of Medicine defined a medical error as “the failure of a planned action to be completed as intended (i.e., error of execution) or the use of a wrong plan to achieve an aim (i.e., error of planning)” (IOM, 1999). Errors can include problems in practice, products, procedures, and systems.
In 2008 the National Patient Safety Foundation defined medical errors as unintended healthcare outcomes caused by a deficit in the delivery of care to a patient, noting that there are two major types of medical errors:
- Errors of omission occur as a result of actions not taken. Examples: Failure to put on the brakes of a wheelchair before transferring a patient from bed to chair or not stabilizing a gurney prior to patient transfer.
- Errors of commission are the result of taking the wrong action. Examples: Administering a medication to a patient who has a known allergy to the drug or not labeling a laboratory specimen that is subsequently ascribed to the wrong patient.
Errors can be further described as adverse events. Important subcategories of adverse events include:
- Preventable events, in which harm may have been lessened or prevented had patient safety risk mitigation strategies been applied. Example: Performing surgery on the wrong body part.
- Negligent adverse events resulting from care that falls below the standards expected of clinicians in the community. Example: Not properly monitoring a patient under anesthesia.
- Unpreventable adverse events that result from complications that cannot be prevented given the current state of knowledge. Example: Appropriately prescribing, dispensing, and administering a drug to a patient not known to have an allergy who subsequently has an allergic reaction.
- Ameliorable events, which are not entirely preventable but may have resulted in less harm if the care had been provided differently. Example: A clinician failing to respond to a patient with medication-related symptoms.
(Boisvert & Pellet, 2022; Rodziewicz et al., 2023)
The most common adverse events reported in the literature are:
- Related to surgical specialties
- Medication- and fluid-related
- Healthcare-associated infections
(Skelly et al., 2022)
Other Terminology Associated with Medical Errors
In addition to adverse events, other terms used to describe medical errors include near misses, sentinel events, and serious reportable events (SREs).
NEAR MISSES
A near miss (also known as a close call) is an incident that might have resulted in harm but did not occur because of timely intervention by healthcare providers, the patient, or the patient’s family. Example: A nurse recognizes a potential drug overdose in a physician’s prescription and does not administer the drug but instead calls the error to the physician’s attention (Performance Health Partners, 2024).
SENTINEL EVENTS
Sentinel events are those medical errors resulting in death, permanent harm, or severe temporary harm and intervention required to sustain life. Such events are called sentinel because they signal the need for immediate investigation and response (VA, 2023).
(Kamakshya & De Jesus, 2023)
SERIOUS REPORTABLE EVENTS (SREs)
The National Quality Forum has compiled a list of serious reportable events (SREs), which are consequential, largely preventable, harmful adverse events (also referred to as never events, or events that should never happen). SREs are grouped into seven categories, as follows:
Surgical SREs
- Surgery/invasive procedure performed on the wrong site
- Surgery/invasive procedure performed on the wrong patient
- Wrong surgical/invasive procedure performed on a patient
- Unintended retention of a foreign object in a patient after surgery or other invasive procedure
- Intraoperative or immediately postoperative/postprocedure death in an American Society of Anesthesiologists class I patient (an otherwise healthy person with no medical problems beyond those that the proposed surgery is intended to address)
Product/Device SREs
- Patient death/serious injury associated with use of contaminated drugs, devices, or biologics provided by the healthcare setting
- Patient death/serious injury associated with use or function of a device in patient care where the device is used for functions other than as intended
- Patient death/serious injury associated with intravascular air embolism occurring while being cared for in a healthcare setting
Patient-Protective SREs
- Discharge or release of a patient/resident of any age who is unable to make decisions to other than an authorized person
- Patient death/serious injury associated with patient elopement (disappearance)
- Patient suicide, attempted suicide, or self-harm resulting in serious injury while being cared for in a healthcare setting
Care Management SREs
- Patient death/serious injury associated with a medication error involving:
- Wrong drug
- Wrong dose
- Wrong patient
- Wrong time
- Wrong rate
- Wrong preparation
- Wrong route
- Patient death/serious injury associated with unsafe administration of blood products
- Maternal death/serious injury associated with labor or delivery in a low-risk pregnancy while in a healthcare setting
- Death/serious injury of a neonate associated with labor/delivery in a low-risk pregnancy
- Artificial insemination with the wrong donor sperm/wrong egg
- Patient death/serious injury associated with a fall while cared for in healthcare settings
- Any stage 3, stage 4, or unstageable pressure injury acquired after admission/presentation to a healthcare facility
- Patient death/serious disability resulting from the irretrievable loss of an irreplaceable biologic specimen
- Patient death/serious injury resulting from failure to follow up or communicate laboratory, pathology, or radiology test results
Environmental SREs
- Patient/staff death/serious injury associated with electric shock in the course of a patient care process in a healthcare setting
- Any incident in which systems designated for oxygen or other gas to be delivered to a patient contain no gas or the wrong gas, or are contaminated by toxic substances
- Patient/staff death/serious injury associated with burns incurred from any source in the course of the patient care process in a healthcare setting
- Patient death/serious injury associated with the use of physical restraints/bedrails while cared for in a healthcare setting
Radiologic SREs
- Patient/staff death/serious injury associated with the introduction of a metallic object into an MRI area
Potential Criminal SREs
- Any instance of care ordered or provided by someone impersonating a physician, nurse, pharmacist, or other licensed healthcare provider
- Abduction of a patient/resident of any age
- Sexual abuse/assault on a patient or staff member within or on the grounds of a healthcare setting
- Death or serious injury of a patient or staff member resulting from a physical assault (i.e., battery) that occurs within or on the grounds of a healthcare setting
(NQF, 2024)
Latent and Active Errors
Active errors (human errors) are those that occur at the point of contact between a human and some aspect of a large system (e.g., a machine). They are generally readily apparent (e.g., pushing an incorrect button or ignoring a warning light) and almost always involve someone at the front line. Active errors or active failures are sometimes referred to as errors “at the sharp end,” referring figuratively to a scalpel. They are noticed first because they are committed by the person closest to the patient. Example: A surgeon amputates the wrong foot.
Latent errors refer to a less apparent failure in system or process design, faulty installation or maintenance of equipment, or ineffective organizational structure that contributes to the occurrence of errors, allowing them to cause harm to patients. Latent errors are those at the other end of the scalpel, the “blunt end,” referring to the many layers of the healthcare system that affect the person “holding the scalpel.” They are accidents waiting to happen. They are present but may go unnoticed for a long time with no ill effect. When a latent error occurs in combination with an active human error, some type of event manifests in the patient, causing an active error. Example: A hospital does not follow a consistent system for stocking central supply carts (Sameera et al., 2021).
CASE
St. Vincent Hospital
At St. Vincent Hospital all cylinders containing medical gases used in the operating room are stored in metal tubes in a tank room. All cylinders containing any concentration of carbon dioxide are color-coded gray and labeled “carbon dioxide.” Beneath that, a continuation of the label identifies any other gas with which it is combined, such as oxygen. When the cylinders are in their metal tubes, the capped connecting neck and top several inches of each cylinder, as well as several inches of the top of the label, are visible above the top of the tube. (Since the full label is not visible, this is an example of a latent error.)
On Tuesday a delivery of medical gas cylinders containing CO2 was accepted by a logistics technician from the cardiac catheterization lab. The delivery included at least one cylinder containing a CO2/O2 blend. As there was inadequate storage space for the entire delivery in the catheterization lab’s tank room, the technician asked his counterpart in the OR to store an extra tank of the gas blend. The OR logistics technician agreed but did not inform anyone in his or the OR’s chain of command.
On Thursday, during a routine laparoscopic cholecystectomy, the alarm for the pressure indicator in the gas delivery system sounded. The circulating nurse went to the tank room to obtain a cylinder replacement. She unknowingly selected the tank with the CO2/O2 blend and used it to replace the empty pure CO2 tank in the OR. (Selecting an incorrect medical gas cylinder is an example of an active error.)
The surgeon activated the electrosurgical cautery unit to stop oozing from the area of the liver from which the gallbladder had been bluntly dissected. There was a millisecond flash of flame (not an electrical arc, which can occur with the use of cautery) followed by a puff of smoke. The incident was confined to the contact area of the electrosurgical instrument, and careful examination indicated that there was no evidence of injury to the patient. (This is an example of a near miss; by chance, no adverse event occurred.)
Investigation of the incident used the “fire triangle” concept and revealed that the patient’s tissue was the fuel; the medical-grade CO2/O2 blend gas used to expand the patient’s abdomen was the oxidizing agent; and the instrument, the cord connecting it to the electrical generator, and the generator were the ignition source.
All elements of the system were eliminated as possible causes for the flash of flame except for one. The medical gas cylinder was found to contain not just CO2 but a CO2/O2 blend. The erroneous presence of this gas mixture was determined to be the single deviation from normal practice and the cause of the accident.
(Continued under “Root Cause Analysis” later in this course.)
SCOPE OF THE MEDICAL ERROR PROBLEM
The World Health Organization reports that 1 in every 10 patients around the world is harmed while receiving hospital care, and more than 3 million deaths occur annually due to unsafe care. In low-to-middle-income countries, as many as 4 in 100 people die from unsafe care (WHO, 2023).
In the United States, 1,441 sentinel events were voluntarily reported to the Joint Commission in 2022, a 19% increase compared to 2021 and a 78% increase from 2020. In terms of outcomes, 20% of reported sentinel events were associated with death, 44% with severe temporary harm, and 13% with unexpected extended stay or additional procedures or treatment resulting from the event. However, as reporting to the Joint Commission is voluntary, no conclusions can be drawn about the actual frequency of events over time (TJC, 2023).
Medication errors are the most common errors in both outpatient and inpatient settings. Each year in the United States, over 7 million people are affected and 7,000 to 9,000 people die due to a medication error. In addition, many other patients experience but often do not report an adverse reaction or other complications related to medication. The total cost of caring for patients with medication-associated errors exceeds $40 billion each year (Tariq et al., 2023).
MORTALITY STATISTICS CONTROVERSY
Currently there is debate about the actual medical error statistics in the United States, as it has been demonstrated that many research studies are based on flawed methods.
It has been estimated that medical errors are the “third leading cause of death” in the United States, with 250,000 to 400,000 deaths annually being due to medical errors, which translates to 62% of hospital deaths. Other studies indicate that at least 251,454 people in U.S. hospitals die due to mistakes in the delivery of care, which amounts to a third or more of all people who die in a hospital. Lower estimates, however, have found evidence of about 22,000 preventable deaths annually, mostly in people with less than three months to live.
A more recent study showed that approximately 400,000 hospitalized patients experienced some type of preventable harm each year. Depending upon the study, medical errors in hospitals and clinics resulted in approximately 100,000 people dying each year (Rodziewicz et al., 2023; Jarry, 2021; Jaklevic, 2023).
Looking at malpractice payout statistics in all settings provides a broad view of medical errors overall; however, these show only a fraction of the actual number of medical errors, as most patients who are harmed by error do not seek damages, and many who do are denied compensation. U.S. Department of Health and Human Services’ National Practitioner Data Bank Public Use Data reports that for the first nine months of 2023, the number of malpractice payments per practitioner type were:
- Registered nurses: 497
- Medical doctors: 11,891
- Licensed practical nurses: 70
- Technicians/assistants: 61
- Nursing paraprofessionals: 35
(NPDB, 2023)
Over the 10-year period from 2013 to 2022, the top 10 most common types of medical malpractice payouts were for:
- Failure to execute a medical procedure correctly
- Failure to make a diagnosis
- Poorly coordinated or monitored patient care
- Late recognition of a patient’s medical condition
- Failure to treat a diagnosed condition
- Failure to recognize complications
- Failure to monitor a patient’s condition
- Using an incorrect technique during a medical procedure
- Delay in treatment
- Allegation not otherwise classified
(NPBD, 2023)
Progress in Patient Safety
More than two decades have passed since the modern patient safety movement began, and errors remain a serious concern. However, the movement has made significant inroads into understanding why medical errors occur and effective strategies for their prevention.
| Year | Accomplishments |
|---|---|
| (AHRQ, 2019; Haskins, 2019; WHO, 2021; AHA, 2024) | |
| 1999 |
|
| 2000 |
|
| 2001 |
|
| 2002 |
|
| 2003 |
|
| 2004 |
|
| 2005 |
|
| 2006 |
|
| 2007 |
|
| 2008 |
|
| 2009 |
|
| 2010 |
|
| 2017 |
|
| 2019 |
|
| 2022 |
|
| 2024 |
|
COMMON CAUSES OF MEDICAL ERRORS
The majority of medical errors are not caused by individual recklessness or the actions of a particular group. More commonly, errors are caused by faulty systems, processes, and conditions that lead people to make mistakes or fail to prevent them. Individuals, of course, should still be held accountable when an error can be attributed to them; however, blaming an individual does little to make the system safer and prevent someone else from committing the same error. A blaming culture can also result in reluctance to disclose or report an error, which may contribute to increased harm to a patient and risk to other patients in the future.
Medical errors usually occur in stressful, fast-paced environments such as emergency departments, intensive care units, and operating rooms. Errors often occur when staffing is inadequate and necessary personnel are not available when needed (Carver et al., 2023).
Common causes for medical errors include:
- Communication issues (the most common cause)
- Miscommunications between patients and providers
- Absence of solid leadership
- Not knowing to whom a problem should be reported
- Failing to disclose issues
- Having a system with no problem-solving ability
- Inadequate staffing and/or poor supervision
- Changes in clinician’s ability to make good judgments and quick decisions:
- Failing to seek advice from peer
- Misapplying expertise
- Failing to formulate a plan
- Failing to consider the most obvious solution due to the assumption of complexity
- Deficiencies in education, training, orientation, and experience
- Inadequate methods of identifying patients
- Incomplete assessment on admission
- Failure to obtain consent
- Failure to provide patient education
- Inadequate policies and procedures to guide healthcare workers
- Lack of consistency in procedures
- Technical failures associated with medical equipment
- No audits in the system
- No one prepared to accept responsibility or to change the system
(Rodziewicz et al., 2023)
Classification of Errors
The classification of different types of errors involved in healthcare is based on human cognitive processes that involve planning, storage, and execution. One such classification system is the Skill, Rule, and Knowledge (SRK)–based approach. It refers to the degree of conscious control that an individual exerts over activities.
SKILL-BASED ERRORS
Errors that occur at the skill-based (automatic) mode involve execution/action failures (slips) and storage/memory failures (lapses). Skill-based errors are associated with familiar and frequently performed tasks that require little conscious attention. Slips are usually errors of inattention or misplaced attention in which the intention is correct but failure occurs while carrying out the activity. Memory lapses occur after formation of a plan and before execution during the time the plan is stored in the brain (Carr, 2022).
RULE-BASED ERRORS
Rule-based processing requires mental resources and conscious decision-making based on rules that may have been learned through education, formal training, and experience. Rule-based processing is used when a person becomes aware that there is a problem. The conditions of the problem are matched with the conditions of problems the individual has encountered in the past. The solution used for the similar situation in the past is then applied using the “if this happens, then do that” rule.
Rule-based mistakes can occur if the current problem is assessed incorrectly and therefore incorrectly matched to a previous problem, or when a usually good rule is applied at the wrong time. Such mistakes (planning failures) include using a good rule incorrectly or using a bad rule (Carr, 2022).
KNOWLEDGE-BASED ERRORS
In the knowledge-based (analytical) mode, the person is facing a unique and unfamiliar situation that must be carried out in an almost completely conscious manner taking considerable mental effort to assess the problem. Decisions are not automatic and skill based, and there are no rules for guidance. This would occur in a situation where a provider is performing a task that is new or when an experienced provider is faced with a completely novel situation and has no experience or rules to fall back on.
In such an instance the person is required to think of possible consequences and create a plan based on knowledge and experience. When the solution arrived at is incorrect, the error is called a planning failure or mistake. Knowledge-based mistakes arise from the considerable demands placed on the information-processing capabilities of the provider (Carr, 2022).
CATEGORIES OF MEDICAL ERRORS AND HOW TO PREVENT THEM
Errors can be placed into five general categories: surgical, diagnostic, medication, devices and equipment, and systems failures (including healthcare-associated infections, falls, and healthcare technology). Common areas in each of these categories are described below.
Surgical Errors
At least 4,000 surgical errors occur in the United States each year. Surgical errors include retained foreign bodies, mislabeled surgical specimens, wrong-site errors, wrong-procedure errors, and wrong-patient errors. Errors can occur at various stages in the surgical process, including during:
- Preoperative planning
- Patient positioning
- Anesthesia administration
- Intraoperative period
- Postoperative period
Most surgical errors, however, appear to be more common before and after the surgical procedure rather than errors made in the operating room. Some causes of surgical errors include:
- Lack of adequate surgeon training and education
- Absence of standardized rules and regulations
- Major gap in communication between surgeon, anesthesiologist, and other ancillary staff
- Gap in communication between the surgeon and the patient
- Use of unreliable systems or protocols
- Rushing to complete cases
- Human factors
(Santos & Jones, 2023; Rodziewicz et al., 2023)
UNINTENDED RETENTION OF FOREIGN OBJECTS (URFOs)
URFOs are defined as objects retained after skin closure following an invasive procedure and that have the potential to cause significant harm to the patient.
Instances of retained surgical items are known to occur approximately 40 times per week in the United States, and the most common are surgical sponges or laparotomy pads, which then become gossypibomas, or masses within the body comprised of a cotton matrix surrounded by a foreign body reaction. These gossypibomas account for 48% to 69% of retained foreign bodies (Steris Healthcare, 2024).
Clamps and retractors are the most common types of retained instruments. The second most common category are catheters and drains. Needles and blades are the third most common category, a majority of which are suture needles.
Contributing factors to URFOs include:
- Communication failures
- An emergent or urgent procedure
- Intra-abdominal surgery
- Patient with obesity
- Elements of teamwork, including leadership, human factors, and communication
- Unanticipated/unexpected change during the procedures (e.g., a change in approach/incision, type of procedure, complications developing during the procedure)
- Involvement of multiple surgical teams and staff turnovers during a procedures
- Equipment failure
- Distraction, multitasking, and time pressure
(Steris Healthcare, 2024; Sirihorachai et al., 2022)
Prevention strategies include:
- Systematic manual counting of materials during the procedures
- Standardized count reconciliation procedures
- Methodical wound exploration
- Radiologic confirmation
- Use of assistive devices, such as radio frequency identification (RFID) tracking devices
- Accurate and complete sharing about the surgical field and contents when personnel changes occur during a procedure
Good communication among the staff in the operating environment and transparent reporting are critical to reducing the incidence of URFOs (Seabra et al., 2023).
ANESTHESIA-RELATED ADVERSE EVENTS
Anesthesia-related adverse events are fairly uncommon, although not rare. The American Society of Anesthesiologists reports that approximately 1 in every 200,000 patients experiences an anesthesia-related complication leading to mortality.
Common anesthesia errors include, but are not limited to:
- Dosage errors (overdose and underdosing)
- Delayed delivery of anesthesia
- Improper intubation
- Improper monitoring
- Failure to respond to a patient’s vital signs
- Equipment malfunction
- Failure to complete a thorough patient history to identify allergies or drug interactions
- Poor communication
(Shaked, 2024)
Prevention strategies include:
- Barcode reader providing automatic auditory and visual verification of selected drugs
- Color-coded syringes
- Prefilled syringes
- Reorganization of workspace into a standardized model
- Labels with large, bold fonts; colorcoded labeling
- Improved labeling for infusion medications with clear fonts, color-coding, pharmacologic class, and specific drug name
- Anesthesia carts with a computer system for storage of drugs and automatic recording of information
(Maximous et al., 2021)
WRONG-SITE, WRONG-PROCEDURE, WRONG-PERSON ERRORS (WSPEs)
Data from the Joint Commission Sentinel Event Annual Review showed there were 85 WSPE sentinel events in the year 2022, and 65% of these errors were surgeries performed on the wrong site.
Contributing factors include:
- Scheduling, documenting, and inappropriate history and examination information
- Preoperative surgical site marking not done by the surgeon
- Information not tallied with the patient
- Inconsistent site marking and inadequate patient verification
- In the OR:
- Lack of efficient patient handoffs
- Inaccurate information
- Organizational culture
Interprofessional collaboration is crucial for preventing surgical errors, including safety checklists, briefing and debriefing, error reporting, and effective communication. Nurses are essential in monitoring patient vital signs, administering appropriate medications and fluids, and ensuring all necessary steps are taken before and after procedures (TJC, 2023).
Prevention strategies recommended by the Joint Commission’s Universal Protocol and National Patient Safety Goals include:
- Identifying instruments needed for the procedure and ensuring medical equipment is functional and alarm systems are in working order and audible
- Using a minimum of two people, conducting a preprocedure verification process to address missing information or discrepancies before starting the procedure
- Using a minimum of two patient identifiers to confirm patient identity before administering sedative medication, if possible
- Marking the operative site by the surgeon and confirming by the patient in the preoperative holding area
- Performing a standardized “time-out” or planned pause immediately before starting a procedure or making an incision, during which team members agree on the patient’s correct identity, correct site, and correct procedure to be done
(TJC, 2024b)
The WHO Surgical Safety Checklist was developed after extensive consultation aimed at decreasing errors and adverse events, and increasing teamwork and communication in surgery. This checklist has gone on to show significant reduction in both morbidity and mortality and is now used by a majority of surgical providers around the world (WHO, 2021).
ELEMENTS OF THE WHO SURGICAL SAFETY CHECKLIST
A surgical checklist is an algorithmic listing of actions to be taken in any given clinical situation intended to make everyone aware that others expect these things to be done.
“SIGN IN” checklist must be completed orally and in writing before induction of anesthesia (with at least a nurse and anesthetist).
- Has the patient confirmed their identify, site, procedure, and consent?
- Is the site marked?
- Is the anesthesia machine and medication check complete?
- Is the pulse oximeter on the patient and functioning?
- Does the patient have a:
- Known allergy?
- Difficult airway or aspiration risk?
- Risk of >500 mL blood loss (7 mL/kg in children)?
“TIME-OUT” checklist must be completed orally and in writing before skin incision (with nurse, anesthetist, and surgeon).
- Confirm all team members have introduced themselves by name and role.
- Confirm the patient’s name, procedure, and where the incision will be made.
- Has antibiotic prophylaxis been given within the last 60 minutes?
- For the anticipated critical event:
- To surgeon:
- What are the critical or nonroutine steps?
- How long will the case take?
- What is the anticipated blood loss?
- To anesthetist:
- Are there any patient-specific concerns?
- To nursing team:
- Has sterility (including indicator results) been confirmed?
- Are there equipment issues or any concerns?
- To surgeon:
- Is essential imaging displayed?
“SIGN OUT” checklist must be completed orally and in writing before the patient leaves the operating room (with nurse, anesthesia provider, and surgeon).
- Nurse verbally confirms:
- The name of the procedure
- Completion of instrument, sponge, and needle counts
- Specimen labeling (read aloud specimen labels, including patient name)
- Whether there are any equipment problems to be addressed
- To surgeon, anesthetist, and nurse:
- What are the key concerns for recovery and management of this patient?
(WHO, 2024)
CASE
Cheryl, a left-hand-dominant patient, was scheduled for a left carpal tunnel release to alleviate her left-hand pain. Immediately prior to her being transferred to the operating room, her surgeon verified the procedure and side with her and marked the surgical site with a purpose-made surgical site marker in accordance with facility policy.
After the time-out and induction of general anesthesia, the site was prepped and draped, the surgeon made a Z-shaped incision from the proximal phalanx of Cheryl’s left middle finger to the middle of her left palm and began to carefully dissect down through the soft tissue. The scrub, an experienced perioperative nurse, was perplexed by the placement of the incision, since the usual incision for a carpal tunnel release goes from the palm (in line with the ring finger) toward the wrist. The scrub did not say anything, since the surgeon was new to the facility, had just completed a fellowship in hand surgery, and had already performed several newly developed procedures with which the nursing personnel were not familiar.
After examining the tissue in Cheryl’s palm, the surgeon commented on the lack of thickening of the ligament in the palm and the inconsistency between his findings and her reported symptoms of ring finger pain and difficulty in doing keyboard work. At this point, both the circulating nurse and anesthesia provider stated that the proposed procedure was a carpal tunnel release. This was confirmed by the surgeon, anesthesia provider, circulating nurse, and scrub visualizing the surgical schedule and Cheryl’s chart (history and physical, surgical consent, and surgical safety checklist).
The surgeon closed the incision and made an appropriate incision for a carpal tunnel release, and the procedure was completed without further issue. After Cheryl was transported to the postanesthesia care unit (PACU), the surgeon spoke with her husband. He informed him of the incident and told him that a complete review of all that had transpired would be done that day. The surgeon later spoke to Cheryl and told her that he would give her a complete explanation the following day once all of the medications she had received were no longer affecting her understanding or memory.
The surgeon met with Cheryl and her husband and adult daughter the following day. He described the nature of the error (that a trigger finger release incision was made instead of the carpal tunnel release incision intended), how it had occurred, and what steps would be taken to improve that aspect of OR safety. The night of surgery, the family had briefly considered filing a lawsuit, but after meeting with the surgeon, they were satisfied with the full and honest disclosure of the incident and decided not to sue.
Diagnostic Errors
Diagnostic errors are common. The overall misdiagnosis rate is approximately 10% to 15%, resulting in injury or death of between 40,000 and 80,000 people each year. Diagnostic errors most commonly occur in primary care solo practice, where there is an inability to confer easily with colleagues.
Most diagnostic errors that occur in primary care settings include failure to order appropriate tests, faulty interpretation of data, failure to follow up, and failure to refer. A common cognitive error is closing the diagnostic process prematurely, which can result in a common, benign diagnosis for a patient with uncommon, serious disease. Delaying treatment after the diagnosis is made is the third most common error, resulting in increased costs for readmission and further treatment (Rodziewicz et al., 2023).
Making a diagnosis is a very complex process, with over 10,000 known diseases and 3,500 kinds of laboratory tests but only a small number of symptoms, so that any one symptom may have dozens or hundreds of possible explanations. Diagnostic testing may be helpful in clarifying the issue, but it is mostly a matter of observing the clinical course, which takes time. An error can occur at any step in the diagnostic process: getting a complete patient history, doing an appropriately thorough examination, obtaining the right tests, or interpreting tests correctly (SIDM, 2024b).
Cognitive psychology applied to healthcare has shown that clinicians frequently use heuristics (shortcuts or “rules of thumb”) to come up with a provisional diagnosis, especially when faced with a patient with common symptoms. Heuristics allow people to solve problems and make judgments quickly and efficiently. There are two types of heuristics:
- Availability heuristic: the process of making a decision based on previous experience in a similar situation
- Anchoring heuristic: using a certain piece of information as an “anchor point” for a diagnosis without considering other presenting signs and symptoms equally
(Aliouche, 2022)
Other factors involved in making inaccurate diagnoses may include:
- Incomplete communication during care transitions
- Lack of standardized measures for understanding performance in the diagnostic process, as providers rarely get feedback if a diagnosis was incorrect or changed
- Limited support to help with clinical reasoning
- Feeling rushed by limited appointment times
- Unclear responsibility for closing the loop on test results and referrals and how to communicate follow-up
- Limited amount of published evidence to identify what improves the diagnostic process
(SIDM, 2024)
HIGH-RISK DIAGNOSES
Prevention must include clinician awareness of the most commonly misdiagnosed conditions and taking extra precautions to seek and confirm the diagnosis. This includes considering the following common high-risk diagnoses:
- Acute renal failure
- Acute pyelonephritis
- Acute vascular occlusion
- Adverse effect of medication
- Aneurysms
- Angina
- Appendicitis
- Arrhythmias
- Asthma exacerbation
- Cellulitis
- Decompensated heart failure
- Hypertension
- Metabolic disorders like hypoglycemia, gout
- Metastatic cancer
- Osteomyelitis
- Pneumonia
- Primary malignancy
- Spinal cord compression
- Symptomatic anemia
- Urinary tract infection
(Rodziewicz et al., 2023)
Prevention strategies may include:
- Being aware of the most commonly misdiagnosed conditions and taking extra precautions to seek and confirm a diagnosis
- Fostering critical thinking by taking a diagnostic time-out and including the use of de-biasing questions
- Making diagnosis a “team sport” and seeking input from colleagues, including the interdisciplinary team
- Considering biases, both cognitive and implicit, and seeking data on disparities
- Improving content-specific knowledge through training programs and simulation
(Raffel & Ranji, 2023)
CASE
A serious outbreak of the Ebola virus was underway in Liberia in western Africa. A man traveled from Liberia back to his home in Texas, where he began to experience fever, nausea, and abdominal pains, prompting him to go to the emergency department (ED). There he reported to the nurse his recent travel to Liberia but denied contact with sick people. He was misdiagnosed and sent home. Days later he returned to the ED, tested positive for Ebola, and began receiving care, but died soon after.
Investigation of this misdiagnosis discovered that the patient’s travel history was obtained by the nurse and entered into his electronic medical record (EMR). The patient, however, had not mentioned the fact that he had had contact with an Ebola patient prior to leaving Liberia. Additionally, the examining physician did not see the travel portion of the patient’s history because it was in the nursing section of the EMR, which physicians can, but often don’t, routinely check. Every facility makes choices about what information shows up routinely in what part of the EMR, and this hospital chose not to include the travel history in the physician section of the EMR.
Nurses are not required to inform doctors about everything they do and document. However, important information is generally personally communicated to the physician. Although the importance of this patient’s travel history should have been recognized because of the amount of publicity surrounding the Ebola outbreak at that time, the nurse did not inform the physician personally.
The nurse asked the right questions about travel, but the patient failed to disclose important information for an unknown reason. The nurse correctly entered the travel history into the medical record but failed to verbally inform the physician, and the physician chose not to read the nurse’s notes. All of these actions illustrate the importance of communication in the prevention of medical errors such as this misdiagnosis and delayed treatment.
Medication Errors
Close to 6,800 prescription medications and countless over-the-counter drugs are available in the United States. Every year in the United States, 7,000 to 9,000 people die due to a medication error. In addition, hundreds of thousands experience but often do not report an adverse reaction or other complication related to a medication.
To further complicate a practitioner’s responsibility during patient care, there are thousands of health supplements, herbs, potions, and lotions used by the public regularly to treat their health problems, which can increase mistakes made when prescribing or dispensing medications.
Medication errors are most common during the ordering or prescribing stage. Errors typically include the healthcare provider writing the wrong medication, wrong route or dose, or wrong frequency. These ordering errors account for nearly 50% of medication errors. Nurses and pharmacists identify from 30% to 70% of medication-ordering errors.
Medication errors may be due to human errors but often result from a flawed system with inadequate backup to detect mistakes (Tariq et al., 2023).
CAUSES AND TYPES OF MEDICATION ERRORS
Causes of medication errors include:
- Expired product, usually related to improper storage
- Administering medication for a shorter or longer duration than prescribed
- Incorrect preparation before final administration
- Incorrect strength
- Incorrect rate, most commonly with IV push or infusions
- Incorrect timing, which may lead to under or overdosing
- Incorrect dose, including overdose, underdose, and extra dose
- Incorrect route, often resulting in significant morbidity and mortality
- Incorrect dosage form, such as immediate release instead of extended release
- Incorrect patient action correctible only with patient education
- Known allergen
- Known contraindication
The most common system failures include:
- Inaccurate order transcription
- Failure to disseminate drug knowledge
- Failing to obtain allergy history
- Incomplete order checking
- Mistakes in tracking of medication orders
- Poor professional communication
- Unavailable or inaccurate patient information
(Tariq et al., 2023)
Prescribing/Ordering
The most common errors in the ordering/prescribing step include:
- Ordering the incorrect drug
- Ordering the incorrect dose
- Ordering the wrong interval or drug schedule
- Ordering the wrong route of administration
- Ordering the wrong infusion rate
- Ordering the wrong dose form (tabs, liquid, immediate-release instead of extended-release)
- Distortions, including illegible handwriting, misunderstood symbols, use of abbreviations, or improper translation
- Distractions (attributed to nearly 75% of medication errors)
- Use of abbreviations
- Inappropriate use of decimal points
- Incomplete order
- Lack of awareness of new drugs with similar names requiring the diagnosis to be written when ordering or prescribing
- Ordering and not being alerted to allergies
- Lack of awareness of known contraindications
- Ordering and not being aware of preexisting medical conditions
- Ordering without reviewing and being aware of current medications being taken
(Tariq et al., 2023)
The National Medication Error Reporting program permits subscribing healthcare institutions to report and track medication errors and finds that medical abbreviation errors account for 4.7% of those errors reported to MedMarx. The most common medical abbreviation error involved the use of “QD” (one daily), accounting for 43.1% of all errors, followed by “U” for units, “cc” for “mL,” and decimal errors.
The most common drug abbreviation name that led to an error was the use of “MS” or “MSO4” for morphine sulfate. At least 81% of the errors were noted to occur at the time of ordering the medication, while errors at the transcribing and dispensing stage occurred at a lower frequency. Overall, the three most common errors due to medical abbreviations were errors in prescribing, improper dose/quantity, and incorrect preparation of the medication (Tariq & Sharma, 2023).
The Institute for Safe Medication Practices has developed a list of abbreviations that are routinely misinterpreted (see “Resources” at the end of this course).
Dispensing
Dispensing errors are usually judgmental or mechanical. Mechanical errors include mistakes in dispensing or preparing a prescription, such as dispensing an incorrect drug, dose, quantity, or strength. Judgement errors include:
- Failure to detect drug interactions
- Inadequate drug utilization review
- Inappropriate screening
- Failure to counsel the patient appropriately
- Giving improper directions
- Inappropriate monitoring
The most common causes for dispensing errors involve:
- Workload
- Similar drug names
- Interruptions
- Lack of support staff
- Insufficient time to counsel patients
Administration
Medication errors in the administration process have been estimated to be 8% to 25% in hospitals and long-term care facilities. Intravenous administration had a higher estimated error rate, ranging from 48% to 53%.
A substantial proportion of medication administration errors occur in hospitalized children, largely due to weight-based pediatric dosing.
Medication errors in the home are reported to occur at rates up to 33%. Wrong dose, missing doses, and wrong medication are the most common. Factors include low health literacy and poor provider-patient communication (MacDowell et al, 2021).
BAD HANDWRITING
Bad handwriting by physicians had become such a major problem that the Institute of Safe Medication Practices recommended the complete elimination of handwritten orders and prescriptions. This problem has been resolved using electronic records in which orders are entered by keystrokes. Poor handwriting has been found to be less common an issue than in the past (Tariq et al., 2023).
PREVENTION OF MEDICATION ERRORS
Prevention strategies when prescribing and ordering include:
- Always preparing one prescription for each medication
- Besides signing the prescription, always circling one’s name on the preprinted prescription pad
- Double-checking the dose and frequency
- Considering that each medication has the potential for adverse reactions
- Not using drug abbreviations when writing orders
- Always adding the patient’s age and weight to each prescription
- Checking the patient for liver and renal function before ordering any medication
- Spelling out the frequency and route of dosage; not using abbreviations
- Always specifying the duration of therapy; not indicating “give out X number of pills”
- Being aware of high-risk medications
- When writing a prescription, stating the condition being treated
- Using computerized provider order entry (CPOE)
- Reconciling medication at times of care transitions
- Double-checking orders by two healthcare professionals prior to dispensing or administering
(Tariq et al., 2023; Loria, 2023)
Prevention strategies when dispensing include:
- Taking time to speak to the patient and double-checking their understanding of the dose, drug allergies, and any other medications they may be taking
- Ensuring the entry of the prescription is correct and complete
- Being aware of look-alike, sound-alike drugs and utilizing tall-man lettering (TML), a technique that uses uppercase lettering to highlight the differences between similar drug names by capitalizing dissimilar letters (e.g., “CISplatin” vs. “CARBOplatin”)
- Using computer alerts or stocking a single strength of the medication in the pharmacy
- Organizing work space, work environment, and workflow
- Reducing distractions whenever possible
- Focusing on reducing stress and balancing heavy workloads
- Storing drugs properly
- Always providing thorough patient counseling
(Tariq et al., 2023; ISMP, 2023)
Prevention strategies when administering a medication include adhering to the “five rights” of medication administration:
- Right patient
- Right drug
- Right dose
- Right route
- Right time
Nurses are frequently the last person to check to see that a medication has been correctly prescribed and dispensed prior to administration. However, a growing number of studies have identified the inadequacy of these “rights” in reducing errors due to factors that place strains on nursing staff members, such as being understaffed and dealing with interruptions. Likewise, the value of nurses’ critical thinking, the role of patient advocacy, and clinical judgment are not accounted for by these five rights.
Therefore, an additional four “rights” have been proposed. These include:
- Right documentation: entering the administration of a medication in the patient’s medical record
- Right action/reason: confirming why patients are being given a medication, and that it is an appropriate treatment for their condition
- Right form: ensuring the correct form of administration within a given route (e.g., tablet, powder, liquid, suppository, etc.)
- Right response: monitoring the person’s response to the medication and ensuring it’s having the desired effect
Another strategy that has been shown in some studies to decrease administration errors up to 50% is the use of barcode medication administration (BCMA), which allows nurses to verify the five rights of medication administration by electronically scanning a patient’s wristband to confirm the information and crossmatch with the patient’s electronic medical chart. This has been shown to decrease administration errors by 23% to 50% (Hanson & Haddad, 2023).
Other prevention strategies for prevention in inpatient settings include:
- Smart infusion pumps for intravenous administration
- Single-use medication packages
- Package design features, such as tall-man lettering for look-alike drug names
- Minimizing interruptions
Few of these interventions are likely to be successful in isolation, and efforts to improve safe medication use must also focus on transitions to home, primary care, and patient caregiver understanding and administration of medication. These efforts include:
- Patient education
- Revised medication labels to improve patient comprehension of administration instructions
- Multicompartment medication devices for patients taking multiple medications in ambulatory or long-term care settings
- Being proficient in medication calculations
- Maintaining up-to-date pharmacologic knowledge
- Informing patients of a medication’s therapeutic effects
- Documenting accurately once a medication has been administered
(AHRQ, 2021)
PATIENT-CENTERED CARE AND MEDICATION COMPLIANCE
Research has found medication noncompliance in 50% of older adult patients. Patients who are noncompliant tend to have multiple chronic conditions, be forgetful, and experience adverse effects from medications. Patient noncompliance may result in medication errors that can lead to hospitalization or serious injury.
Patient-centered care requires that healthcare organizations and healthcare professionals actively understand what patients value, and recognize that patients need to be treated as full partners in their care (Liu et al., 2023).
ERRORS IN MEDICATION MONITORING
Monitoring and assessment are essential to the process of administration of medications. Errors can occur regarding the assessment of vital signs, lab values, ability to swallow, and patient’s self-report. Monitoring involves observing the patient to determine if the medication is working, is being used appropriately, and is not harming the patient. Types of errors in monitoring that can occur include:
- Failure to monitor effectiveness of therapeutic action of a medication
- Lack of awareness of side effects of a medication
- Failure to monitor, assess, and report laboratory tests
- Failure to monitor, assess, and report vital signs
- Failure to educate patients about potential side effects
- Failure to comply with a pain management program
- Communication failures during handoff procedures to accepting nurse
(Tariq et al., 2023, MacDowell et al., 2021)
ERRORS AND HIGH-ALERT MEDICATIONS
The Institute for Safe Medication Practices (ISMP) defines a high-alert medication as a drug that has a heightened risk of causing significant patient harm when used in error. Although errors may or may not be more common with such medications, the consequences of errors are much more devastating. High-alert medications are at the top of the list of drugs involved in moderate-to-severe patient outcomes when an error occurs.
The ISMP lists high-risk medications according to what is commonly used in acute care settings, community settings, and long-term settings. These lists are updated every few years based on error reports submitted to ISMP, reports of harmful errors in the literature, and input from practitioners and safety experts.
High-alert medications specific to acute care settings include:
- U-500 insulin
- Potassium or chloride for injection concentrate
- Epidural and intrathecal medications
- Sodium chloride for injection, greater than 0.9%
- Chemotherapeutic agents, parenteral and oral
- Insulin, subcutaneous and IV
- Neuromuscular blocking agents
- Antithrombotic agents
- Potassium phosphates injection
- Methotrexate, oral, nononcologic use
- Epoprostenol (e.g., Flolan) IV
- Opioids, all routes
- Parenteral nutrition preparations
- Cardioplegic solutions
- Anesthetic agents, general, inhaled and IV
- Sterile water for injection, inhalation, and irrigation, excluding pour bottles 100 mL or more
- Nitroprusside sodium for injection
- Adrenergic agonists, IV
- Antiarrhythmics, IV
- Inotropic medications, IV
- Moderate sedation agents, IV
- Epinephrine, IM and subcutaneous
- Magnesium sulfate injection
- Promethazine injection
- Opium tincture
- Vasopressin, IV and intraosseous
- Dextrose, hypertonic, 20% or greater
- Moderate and minimal sedation agents, oral for children
- Dialysis solutions, peritoneal and hemodialysis
- Liposomal forms of drugs and conventional counterparts
- Adrenergic antagonists, IV
- Sulfonylurea hypoglycemics, oral
- Oxytocin, IV
(ISMP, 2024)
Preventing Errors with High-Alert Medications
ISMP makes the following recommendations for reducing errors with high-alert medications in the acute care settings, community/ambulatory settings, and long-term care settings, which may include:
- Standardizing the ordering, storage, preparation, and administration of these medications
- Improving access to information about these drugs
- Using auxiliary labels and automated alerts
- Employing redundancies—duplicate devices used for backup purposes to prevent or recover from the failure of a specific part of the process (e.g., asking another nurse to perform an independent check)
- Providing mandatory patient education
(ISMP, 2024)
FDA Warnings for High-Risk Medications
A box warning (formerly known as Black Box Warning) is the highest safety-related warning assigned to a medication by the U.S. Food and Drug Administration (FDA). Box warnings are not meant to be absolute contraindications for drugs; rather, they are to bring to the attention of clinicians and pharmacists the potential severe side effects or other major risks of a drug.
Over 400 different medications currently have box warnings.
Before adding a box warning, however, the FDA must have evidence that the drug poses a significant risk. This comes from observations and studies conducted after the drug has been on the market. This also means that new drugs that have just been put on the market rarely will have these warnings (Delong & Preuss, 2023).
PREVENTING ADVERSE EVENTS DUE TO PATIENT-CONTROLLED ANALGESIA (PCA)
Checklists for safe use of PCA pumps are available. The Physician-Patient Alliance for Health and Safety checklist recommends certain steps be taken when initiating, refilling, or reprogramming PCA pumps, and PCA checks to be done at shift change and hourly.
PCA pump initiation, refilling, or programming a change requires:
- Assessment of the patient for increased risk of respiratory distress due to:
- Obesity
- Low body weight
- Current medication that can potentiate sedative effects
- Preexisting conditions such as asthma, COPD, and sleep apnea
- Advanced age
- Preprocedural cognitive assessment to determine the capability of the patient to participate in pain management (may not be suitable for pediatric patients)
- Provision of information to the patient on proper use of the PCA and purpose of monitoring
- Two healthcare providers independently verify the patient’s:
- Identification
- Allergies, if any
- Drug selection and concentration as prescribed
- Dose adjustment, if any
- PCA pump settings
- Line is attached to the patient and tubing is inserted into the pump
- Electronic monitoring:
- Pulse oximetry
- Capnography
Change of shift and every hour requires:
- Assessing patient for level of pain, alertness, and adequacy of ventilation
- Verifying PCA pump settings
- Verifying electronic monitoring of pulse oximetry and capnography
- Documenting patient assessment and condition, PCA dosing, and monitoring
(Wong, 2022)
CASE
A nurse working on the obstetrics unit of a local hospital was halfway through the second of two eight-hour shifts, and she asked to go home because she was tired. The hospital denied her request, stating staffing would be inadequate (fatigue and RN staffing). The nurse was assigned a young female in active labor. The patient stated that she had spoken to her doctor beforehand and had agreed to an epidural for delivery.
In order to save time (workload and time pressures), the nurse took a bag of epidural anesthesia from a storage locker without a doctor’s order, brought it to the patient’s room, and laid it on the work counter (deliberate violation of medication administration guidelines, policies, and procedures). The IV bag had a bright-red label that read “for epidural use only.”
In the meantime, an IV antibiotic was ordered and delivered to the patient’s room. The nurse picked up what she believed was the IV antibiotic (similar packaging or product) and hung it (deliberate violation of medication administration guidelines, policies, and procedures). Shortly thereafter, the patient had a seizure and died. Her infant was delivered live by cesarean section.
The investigation of the incident revealed that the nurse:
- Was fatigued and under time pressure
- Failed to follow hospital procedures requiring a doctor’s order before removing drugs from the storage locker
- Failed to recognize the bright-red intrathecal warning label on the IV bag
- Failed to follow the hospital’s policy and procedure to scan medication labels before drugs were administered
- Failed to follow the “rights” of medication administration as described in the hospital’s policy and procedure manual
Investigation further revealed that shortcuts were common practice on the unit.
Initially, the nurse was charged with a felony, which was later reduced to civil charges, and her license was suspended.
Tubing Misconnections
The FDA reports that medical device misconnections can occur when one type of medical device is attached in error to another type of medical device that performs a different function. Tubing misconnections can occur for several reasons. The most common reason is that many types of tubing lines for different medical devices incorporate common Luer lock connectors, which consist of a male taper with an associated threaded “skirt” and a female taper having flanges to engage the threads (FDA, 2023a).
EXAMPLES OF TUBING MISCONNECTIONS
- Enteral feeding tube connected to an IV
- Enteral feeding tube connected to ventilator in-line suction catheter
- Blood pressure cuff tubing connected to an IV port
- IV tubing connected to tracheostomy cuff
- IV tubing connected to nebulizer
- Oxygen tubing connected to a needleless IV port
- IV tubing connected to nasal cannula
- Syringe connected to tracheostomy cuff
- Epidural solution connected to a peripheral or central IV catheter
- Epidural line connected to an IV infusion
- Bladder irrigation solution utilizing primary IV tubing connected to a peripheral or central IV catheter
- Foley catheter connected to NG tube
- IV infusion connected to an indwelling urinary catheter
- IV infusion connected to an enteral feeding tube
- Primary IV tube connected to a blood product meant for transfusion
(FDA, 2023a)
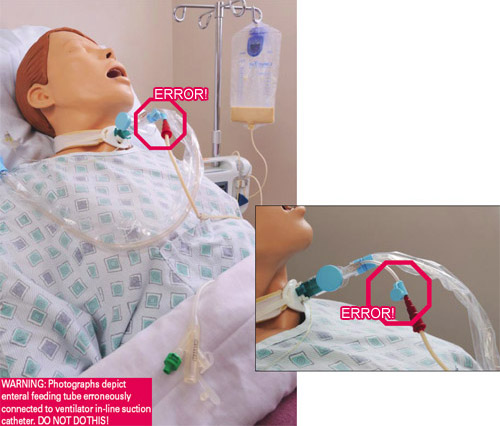
Patient’s feeding tube is incorrectly connected to the instillation port on the ventilator in-line suction catheter, delivering tube feeding into the patient’s lungs, causing death. (Source: FDA, 2023a.)
PREVENTING TUBING MISCONNECTIONS
Attempts to prevent device misconnections have included color-coding, labels, tags, and training. However, these methods alone have not effectively solved the problem, because they have not been consistently applied, nor do these methods physically prevent the misconnections.
In order to reduce the chances of tubing misconnections, non-Luer lock connections have been introduced. These include the NR-Fit connector for neuraxial and regional anesthesia catheters and the Enfit connectors for feeding tubes.
These connectors are designed to be incompatible with Luer adaptors, which are commonly used in IV applications. The connectors look and secure very similarly to a Luer threaded lock system, although the design is larger and, therefore, incompatible with connectors for unrelated delivery systems such as tracheostomy tubes, IV lines, and catheters (Rodziewicz et al., 2023).
Until new connectors are universally adopted, the following interventions offer healthcare providers with strategies such as the use of “ACT” to prevent device misconnections (see table).
| Label | Step | Actions |
|---|---|---|
| (FDA, 2023a) | ||
| A | Assess equipment |
|
| C | Communicate |
|
| T | Trace |
|
Errors Related to Medical Devices and Equipment
There are about 5,000 types of medical devices used by healthcare providers throughout the world, making it inevitable there will be device-related errors. Such errors may involve:
- Inadequate maintenance
- Inadequate plan for implementing technology into practice
- Poor technology design that does not consider human factors and ergonomic principles
- Poor technology interface with the environment and patient
- Differences in function between devices from different manufacturers
- Inadequate testing
- Lack of standardization
(Rodziewicz et al., 2023)
The FDA categorizes medical devices into three classes (I, II, and III) based on potential risks to the patient.
- Class I devices do not come into contact with a patient’s internal organs, the central nervous system, or the cardiovascular system. Examples include:
- Bedpan
- Tongue depressor
- Oxygen mask
- Bandage
- Surgical mask
- Manual stethoscope
- Hospital bed
- Class II devices are more likely to come into sustained contact with a patient, such as those that come into contact with a patient’s cardiovascular system or internal organs, and diagnostic tools. Examples include:
- Catheter
- Blood pressure cuff
- Syringe
- Blood transfusion kit
- Surgical gloves
- Absorbable sutures
- Powered wheelchair
- Class III devices usually sustain or support life, are implanted, or present a potential unreasonable risk of illness or injury. Examples include:
- Breast implant
- Implantable pacemaker
- Implantable prosthetic
- Defibrillator
- High-frequency ventilator
- Fetal blood sampling monitor
- Cochlear implant
As experience and knowledge about a device increase, the original classification of a device can be changed through reclassification. To reclassify a device, the FDA must:
- Publish a proposed order in the Federal Register that includes a summary of valid scientific evidence that supports the reclassification
- Convene a device classification panel meeting
- Consider comments from the relevant public docket
(FDA, 2021)
Design flaws, misuse, and malfunction of medical devices and equipment are all common causes of medical errors. Subtle differences in a familiar pattern using a device can affect the speed and accuracy of data entry, and the lack of standardization invites user mistakes. Poor medical device design and lack of usability testing have also been repeatedly discussed as being key factors in many device-related incidents.
An increasing number of medical devices are also implanted in patients. These include complex programmable cardiac pacemakers, defibrillators, deep-brain stimulation neurotransmitters, and laser surgical devices. Any malfunction of such devices can be serious and even life-threatening. Each year the FDA receives several hundred thousand reports of suspected device-associated deaths, serious injuries, and malfunctions.
Mandatory reporting of such events must be done by manufacturers, importers, and device user facilities. Healthcare professionals, patients, caregivers, and users are also encouraged to voluntarily report adverse events to MedWatch, the FDA’s Safety Information and Adverse Event Reporting Program.
User facilities are hospitals, ambulatory surgical facilities, nursing homes, outpatient diagnostic facilities, or an outpatient treatment facility that is not a physician’s office. Such facilities must report a medical device-related serious injury to the manufacturer, or to the FDA if a medical device manufacturer is unknown. A user facility is not required to report a device malfunction but can voluntarily advise the FDA of such product problems (FDA, 2023b).
CASE
Jory, a 17-year-old boy, fractured his arm in several places following a tackle and fall while playing football. He was taken to the nearby hospital, where he underwent surgical repair. Postoperatively he was placed on morphine delivered via a pump. His heart rate, respirations, and blood oxygen levels were being monitored. Through the evening hours, Jory was alert, oriented, and had stable vital signs. When the night shift took over, it was ordered that the morphine should be shut off and that he should be placed on routine vital sign checks and oral pain medication.
During the night, the nurse entered his room to assess his vital signs and found that he was nonresponsive and barely breathing. It was discovered that the morphine pump, a newly acquired piece of equipment, had not been shut off but had accidently been turned to the “high” setting. Jory was lucky; he survived the overdose.
The following investigation found that the new device was designed differently than the old one, with an additional step required in the shutoff process, and the nurse had not received training in the use of the new pump.
PREVENTING ERRORS RELATED TO MEDICAL DEVICES AND EQUIPMENT
Workplaces, instruments, devices, and equipment should be designed and developed to consider human factors. A healthcare professional can maximize safety through participating in the selection process, utilizing proactive risk-assessment methods, and confirming that equipment is maintained.
Prevention strategies for health professionals include:
- Standardizing equipment, such as infusion pumps and monitors, in similar care environments
- Being involved in setting and evaluating institutional, organizational, and public policy related to technology
- Ensuring that the technology used meets quality and safety standards
Prevention strategies for institutions include:
- Making decisions concerning technology with the input of critical stakeholders (those with a financial interest, medical leaders, clinicians, patients, and vendors)
- Adopting policies and processes related to maintenance, training, monitoring, and reporting adverse events related to technology
(Rodziewicz et al., 2022)
Healthcare-Associated Infections (HAIs)
HAIs are infections that occur while receiving healthcare in a hospital or other healthcare facility and that first appear 48 hours or more after admission or within 30 days after having received healthcare. HAIs are considered system failures and are often preventable. As many as 1 in 31 hospitalized patients and 1 in 43 nursing home residents contract at least one HAI in association with their healthcare (CDC, 2022).
HAIs AND HAND HYGIENE
One of the most important reasons in healthcare settings for the spread of bacteria resulting in HAIs, some of which are antibiotic resistant and can prove life-threatening, is the failure of physicians, nurses, and other caregivers to practice basic hand hygiene. Studies show that some healthcare providers practice hand hygiene on fewer than half the occasions they should. Providers might need to clean hands as many as 100 times per 12-hour shift, depending on the number of patients and intensity of care (CDC, 2023a).
CATHETER-ASSOCIATED URINARY TRACT INFECTIONS (CAUTIs)
CAUTIs occur at a rate of approximately 3% to 10% per day of catheterization, making duration of catheterization an important risk factor. Other risk factors include:
- Female sex
- Older age
- Diabetes mellitus diagnosis
- Bacterial colonization of the drainage bag
- Errors in catheter care
The most common causative pathogens are:
- E. coli, 24%
- Candida subspecies, 24%
- Enterococcus subspecies, 14%
- P. aeruginosa, 10%
- Klebsiella subspecies, 10%
Complications of CAUTIs include sepsis, bacteremia, and involvement of the upper urinary tract. Almost 20% of bacteremias arise from the urinary tract, and the mortality rate associated with this condition is about 10% (Fekete, 2023).
Recommended prevention strategies include:
- Inserting catheters only for appropriate indications and leaving in place only as long as needed
- Considering other methods for bladder management such as intermittent catheterization or external male or female collection devices, when appropriate
- Using appropriate technique for catheter insertion
- Practicing hand hygiene immediately before insertion of the catheter and before and after any manipulation of the catheter site or apparatus
- Inserting catheters following aseptic technique and using sterile equipment
- Using a catheter with the smallest feasible diameter to minimize urethral damage
- Properly securing indwelling catheters after insertion to prevent movement and urethral traction
- Maintaining a sterile, continuously closed drainage system
- Replacing the catheter and collecting system using aseptic technique when breaks in aseptic technique, disconnection, or leakage occur
- When collecting a urine specimen, collecting a small sample by aspirating urine from the needleless sampling port with a sterile syringe after cleaning the port with disinfectant
- Maintaining unobstructed urine flow
- Using portable ultrasound devices for assessing urine volume to reduce unnecessary catheterizations
- Ensuring the collecting bag remains below the level of the bladder
- Not placing the bag on the floor
- Preventing kinking of the catheter and collecting tube
- Emptying the collection bag regularly using a separate collecting container for each patient, and avoiding touching the drainage spigot to the container
- Cleaning the meatal area with an antiseptic solution
(Patel et al., 2023)
CASE
Brenda is a nursing assistant instructor at the local technical college. Today she has taken a group of students to their clinical site, the Marshall Green Nursing Home, which has had a higher than usual number of urinary tract infections over the last several months. One of her students, Annie, is assigned to an older adult male who has an indwelling urinary catheter in place. The care plan indicates he should use a bedside drainage bag during the night and a leg bag during the day. The nursing assistant assigned to the patient tells Brenda his leg bag is in the bedside stand wrapped in a towel.
When Annie locates the bag, it is in a washbasin wrapped in a towel. She finds there is no cap on the end of the tubing that is to be inserted into the catheter, and she shows this to Brenda. Annie has been taught that the end of the tubing must be protected by capping it with a sterile cap in order to maintain a closed system and to prevent bacteria from contaminating the system. Brenda approaches the nursing assistant and tells her about the lack of the cap and the risk for infection. The nursing assistant replies, “We never put a cap on the end of it.”
Brenda tells Annie to obtain a new leg drainage bag, instructing her to ensure that she cleans the end of the bedside drainage bag connection and caps it with the cap removed from the new leg-bag tubing before storing it in the bedside cabinet. She then brings the contaminated leg bag to the supervising nurse, who says she will report it and speak to the nursing assistant about it. With the help of Brenda, Annie completes an incident report.
SURGICAL SITE INFECTIONS (SSIs)
SSIs remain a substantial cause of morbidity, prolonged hospitalization, and mortality. SSIs affect approximately 0.5% to 3% of patients undergoing surgery, accounting for 20% of all HAIs. SSIs are associated with up to an 11-fold increase in risk of mortality, with 75% of SSI-associated deaths directly attributable to the SSI (Seidelman et al., 2023).
The CDC recommends the following prevention measures for SSIs:
- Administering preoperative antimicrobial agents only when indicated by published clinical practice guidelines, and timing the administration so that a bactericidal concentration is established when incision is made
- Administering appropriate parenteral prophylactic antimicrobial agents before skin incision in all cesarean section procedures
- In clean and clean-contaminated procedures, not administering additional prophylactic antimicrobial agent doses after the surgical incision is closed in the OR, even in the presence of a drain
- Not applying antimicrobial agents (i.e., ointments, solutions, or powders) to the surgical incision with the aim of preventing SSI
- Considering the use of triclosan-coated sutures for prevention of SSI
- Implementing perioperative glycemic control and using blood glucose target levels lower than 200 mg/dL in patients with and without diabetes
- Maintaining perioperative normothermia
- Advising patients to shower or bathe the entire body with either antimicrobial or nonantimicrobial soap or an antiseptic agent on at least the night before the day of the procedure
- Performing intraoperative skin preparation with an alcohol-based antiseptic agent unless this is contraindicated
- Not applying microbial sealant immediately after intraoperative skin preparation, as it is not necessary for prevention of SSI
- Avoiding the use of plastic adhesive drapes with or without antimicrobial properties
- Considering intraoperative irrigation of deep or subcutaneous tissues with aqueous iodophor solution
- Not withholding transfusion of necessary blood products from surgical patients undergoing prosthetic joint arthroplasty as a means of preventing SSI
- In clean or clean-contaminated prosthetic joint arthroplasties, not administering additional antimicrobial prophylaxis doses after the surgical incision is closed in the OR, even in the presence of a drain
(Singhal, 2023)
CENTRAL LINE–ASSOCIATED BLOODSTREAM INFECTIONS (CLABSIs)
CLABSIs are laboratory-confirmed bloodstream infections that are not secondary to an infection at another body site and that are due to the presence of an intravascular catheter that terminates at or close to the heart, or in one of the great vessels, that is used for infusion, withdrawal of blood, or hemodynamic monitoring. An estimated 30,000 central line–associated infections still occur in intensive care units and wards of U.S. acute care facilities each year (NHSN, 2024).
| (Buetti et al., 2022; Kolikof et al., 2023; Srinivasan et al., 2023) | |
| Prior to insertion |
|
| During site selection |
|
| During insertion |
|
| After insertion |
|
PERIPHERAL INTRAVENOUS CATHETER (PIVC)-ASSOCIATED BLOODSTREAM INFECTIONS
PIVC-associated bloodstream infections are estimated to occur in 70% of people with PIVCs, necessitating premature PIVC removal, suspended administration of ongoing therapies, and catheter replacement (Catarino et al., 2022).
Peripheral intravenous catheters are the most commonly used invasive medical device in healthcare. They should be used for short-term IV therapies (up to 7 days), should not be used if oral treatment is available, and dwell time should be short (less than 4 days).
| (Jacob & Gaynes, 2020; Zingg et al., 2023) | |
| During site selection |
|
| During catheter selection |
|
| During insertion |
|
| During catheter and site care |
|
| Replacing administration sets |
|
CLOSTRIDIOIDES DIFFICILE INFECTIONS (CDIs)
Clostridioides (formerly known as Clostridium) difficile (C. diff) infections (CDIs) cause life-threatening diarrhea. They are usually a side effect of taking antibiotics. Those most at risk are patients, especially older adults, who take antibiotics and also receive medical care and people staying in hospitals and nursing homes for a long period of time. In the United States, half a million CDIs cause 15,000 deaths each year (Cleveland Clinic, 2024).
Prevention strategies for CDIs include:
- Isolating and initiating Contact Precautions for suspected or confirmed CDI
- Maintaining Contact Precautions for at least 48 hours after diarrhea has resolved, or longer, up to the duration of hospitalization
- Adhering to recommended hand hygiene practices
- Using dedicated patient-care equipment (e.g., blood pressure cuffs, stethoscopes)
- Implementing daily patient bathing or showering with soap and water
- When transferring patients, notifying receiving wards or facilities about the patient’s CDI status
- Performing daily cleaning of CDI patient rooms using C. difficile sporicidal agent at least once a day, including toilets
- Cleaning and disinfecting all shared equipment prior to use with another patient (e.g., wheelchair)
- Performing terminal cleaning after CDI patient transfer/discharge using a C. difficile sporicidal agent (EPA List K agent)
- Cleaning additional areas that are contaminated during transient visits by patients with suspected or confirmed CDI (e.g., radiology, emergency rooms, physical therapy) with C. difficile sporicidal agent (EPA List K agent)
- Restricting use of antibiotics with the highest risk for CDI (e.g., fluoroquinolones, carbapenems, and third- and fourth-generation cephalosporins)
- Limiting the use of nonantibiotic patient medications (e.g., proton pump inhibitors, H2-receptor blockers) that are hypothesized to increase risk for CDI
- Evaluating and testing asymptomatic patients at high risk for CDI; isolating those testing positive, but not treating in the absence of symptoms
- Considering additional disinfection of CDI patient room with no-touch technologies (e.g., UV light)
- Dedicating healthcare personnel to care of patients with CDI only to minimize risk of transmission to others
- Expanding the use of environmental disinfection strategies (e.g., sporicidal agents [EPA List K agent]) for daily and terminal cleaning in all rooms on affected units
(CDC, 2021)
HOSPITAL-ACQUIRED PNEUMONIA (HAP)
HAP occurs 48 hours or more after hospital admission at a rate of 5 to 10 per 1,000 hospital admissions. Ventilator-associated pneumonia (VAP) is a subset of HAP occurring in intensive care units that presents more than 48 to 72 hours after tracheal intubation and is thought to affect 10% to 20% of patients receiving mechanical ventilation for more than 48 hours (Shebl & Gulick, 2023).
Prevention strategies for ventilator-associated pneumonias include:
- Avoiding intubation and preventing reintubation
- Using routine infection control practices and hand hygiene
- Minimizing sedation
- Maintaining and improving physical conditioning
- Elevating the head of the bed to 30 to 45 degrees
- Providing oral care with toothbrushing but without chlorhexidine
- Providing early enteral vs. parenteral nutrition
- Changing the ventilator circuit only if visibly soiled or malfunctioning (or per manufacturer’s instructions)
- Using selective oral or digestive decontamination in ICUs with low prevalence of antibiotic resistant organisms
- Utilizing endotracheal tube with subglottic secretion drainage ports for those patients expected to require greater than 48–72 hours of mechanical ventilation.
- Considering early tracheostomy
- Considering postpyloric rather than gastric feeding for patients with gastric intolerance or who are at high risk for aspiration
(SHEA, 2022)
MULTIDRUG-RESISTANT ORGANISM (MDRO) INFECTIONS
MDROs are pathogens resistant to multiple antibiotics or antifungals. MDROs can be difficult to treat and can therefore cause serious illness or event death. Some MDROs are found and transmitted almost exclusively within healthcare settings. Several key pathogens include:
- Carbapenem-resistant Enterobacterales (CRE)
- Carbapenem-resistant Acinetobacter
- Multidrug-resistant Pseudomonas aeruginosa
- Candida auris
In the United States more than 2.8 million antimicrobial-resistant infections occur each year and more than 35,000 people die as a result (WIDHS, 2022; CDC, 2022).
Prevention measures for multidrug-resistant organism infections include:
- Following Standard Precautions during all patient encounters in all healthcare settings, regardless of suspected or confirmed infection or colonization status
- Following hand hygiene procedures
- Carefully cleaning rooms and medical equipment used for patients with an MDRO with an appropriate disinfectant
- Removing temporary medical devices as soon as possible
- Using antibiotics only when necessary
- Using enhanced barrier precaution during high-contact patient care activities
- Using Contact Precautions for all patients with MDRO infection or those identified as MDRO colonized
- Decolonizing patients with MDROs to eliminate MDRO carriage using chlorhexidine gargling, bathing, and showering, along with nasal mupirocin
(Binghui & Weijiang, 2024)
Falls
Falls are the most common type of accidents in people 65 years of age and older, with over 30% of such individuals falling every year. In approximately one half of these cases, the falls are recurrent. These percentages increase to around 40% in individuals 85 years and older.
Approximately 10% of falls result in serious injuries, including fracture of the hip, other fractures, traumatic brain injury, or subdural hematoma. They are the major cause of hospitalization related to injury in those 65 years and older and are associated with increased mortality.
Falls in institutional settings occur more frequently and are associated with greater morbidity than falls that occur in the community. Approximately 50% of individuals in the long-term care setting fall yearly (Appeadu & Bordoni, 2023; Kiel, 2023).
FALL RISKS
Fall risk can be categorized as either intrinsic or extrinsic.
Intrinsic Factors
Intrinsic factors include issues that are unique to the individual and concern medical, psychological, and physical issues. They include:
- Advanced age
- Previous falls
- Gender (women fall more often than men)
- Race (Whites fall most often)
- Taking more than four medications
- Lower extremity weakness
- Impairment in gait and mobility
- Immobility/deconditioning
- Being sedentary
- Poor nutrition
- Postfall anxiety syndrome (fear of falling following a recent fall)
- Inner ear disorders
- Cardiovascular or cerebrovascular disorders
- Cognitive disorders
- Poor nutrition
- Poor vision
- Substance use
- Postural hypotension
- Chronic conditions such as arthritis, stroke, incontinence, diabetes, Parkinson’s disease, diabetes
- Foot problems leading to balance issues
(Appeadu & Bordoni, 2023)
Extrinsic Factors
Extrinsic factors generally can be changed and address environmental risks that patients encounter. They include:
- Clutter
- Inadequate lighting, glare
- Uneven or wet floors, raised thresholds, missing floor tiles
- Unstable or lightweight furniture
- Insecure toilet seat or handrail
- Hard-to-reach personal items
- Unstable wheels
- Low toilet seat
- Throw rugs
- Lack of grab bars inside and outside tub and shower and next to toilet
- Lack of railings on both sides of stairs
(CDC, 2023b)
PREVENTING FALLS
Preventing falls involves assessing patients for risk for falls, developing a personalized plan of care, and utilizing consistent preventive interventions. Fall prevention interventions are to be considered in both hospitalized and ambulatory settings.
Hospitalized Patients
Risk factors for falls and injury in hospitalized patients include:
- Age or frailty
- Osteoporosis or a recent fracture
- Bleeding disorders/taking anticoagulants
- Recent surgery
A fall risk assessment is done on admission, and reassessment is done whenever there is a change in a patient’s condition or when a patient is being transferred to another unit. A reliable, standardized, and validated assessment scale should be used that includes a history of falls, mobility problems, use of assistive devices, medications, and mental status.
There are many fall assessment tools. The following tools have been extensively studied and recommended:
- Morse Fall Scale
- STRATIFY Scale
- Schmid Fall Risk Assessment Tool
MORSE FALL SCALE (MFS)
The MFS is used widely in both hospital and long-term care inpatient settings. The MFS requires systematic, reliable assessment of a patient’s fall risk factors upon admission, after a fall, upon change in status, and at discharge or transfer to a new setting (see table below).
| Risk Factor | Score |
|---|---|
| History of falling, immediate or within 3 months |
|
| Secondary diagnosis |
|
| Ambulatory aid |
|
| IV/heparin lock |
|
| Gait/transferring |
|
| Mental status |
|
| MFS Score | Risk Level | Action |
|---|---|---|
| 0 | None | Basic nursing care |
| <25 | Low | Standard fall prevention interventions |
| 25–45 | Moderate | Standard fall prevention interventions |
| >45 | High | High-risk fall prevention interventions |
(AHRQ, 2023)
After assessment of fall risk, collaboration with the patient and family takes place in order to develop a personalized plan that addresses each identified risk factor. Tailored prevention interventions are described in the table below.
| (Turner et al., 2022) | |
| Mobility and identification practices |
|
| Bed modification practices |
|
| Patient monitoring practices |
|
| Education practices |
|
| Patient safety practices |
|
| Restructuring strategies |
|
Community-Dwelling Patients
The Centers for Disease Control and Prevention’s STEADI initiative (Stop Elderly Accidents, Deaths, and Injuries) is a coordinated approach for the implementation of practice guidelines for fall prevention in community-dwelling adults. The STEADI initiative provides clinical practice guidelines for fall prevention that consists of the three core elements of screen, assess, and intervene. The STEADI Algorithm for Fall Risk Screening, Assessment, and Intervention outlines how to implement these three elements, as follows:
- Screen for fall risk annually, or any time the patient presents with an acute fall.
- For patients found not at risk, prevent future risk by recommending effective prevention strategies:
- Educate patient about fall prevention.
- Assess vitamin D intake and recommend supplement if deficient.
- Refer to community exercise or fall prevention program.
- Reassess yearly or any time the patient presents with an acute fall.
- For patients found not at risk, prevent future risk by recommending effective prevention strategies:
- Assess patients who are found to be at risk:
- Assess the patient’s modifiable risk factors and fall history and evaluate gait, strength, and balance. Common assessments include:
- Timed Up and Go (TUG)
- 30-second Chair Stand
- 4-stage Balance Test
- Identify medications that increase fall risk (e.g., using Beers criteria) (see “Resources” at the end of the course).
- Ask about potential home hazards.
- Measure orthostatic blood pressure.
- Assess visual acuity (Snellen eye test).
- Assess feet and footwear.
- Assess vitamin D intake.
- Identify comorbidities (e.g., depression, osteoporosis).
- Assess the patient’s modifiable risk factors and fall history and evaluate gait, strength, and balance. Common assessments include:
- Intervene to reduce identified risk factors.
- Discuss patient and provider health goals and develop an individualized patient care plan.
- Poor gait, strength, and/or balance observed:
- Refer for physical therapy evaluation.
- Refer to evidence-based exercise or fall prevention program.
- Medication(s) likely to increase fall risk:
- Optimize medications by stopping, switching, or reducing dosages of medication.
- Home hazards as described:
- Refer to occupational therapist to evaluate home safety.
- Poor gait, strength, and/or balance observed:
- Orthostatic hypotension observed:
- Stop, switch, or reduce dose of medications that increase fall risk.
- Educate about importance of exercises (e.g., foot pumps).
- Establish appropriate blood pressure goal.
- Encourage adequate hydration.
- Consider compression stockings.
- Visual impairment observed:
- Refer to ophthalmologist/optometrist.
- Stop, switch, or reduce dosage of medication affecting vision (e.g., anticholinergics).
- Consider benefits of cataract surgery.
- Provide education on depth perception and single-vision multifocal lenses.
- Feet/footwear issues identified:
- Provide education on shoe fit, traction, insoles, and heel height.
- Refer to a podiatrist.
- Vitamin D deficiency observed or likely:
- Recommend daily vitamin D supplement.
- Comorbidities documented:
- Optimize treatment of conditions identified.
- Be mindful of medications that increase fall risk.
- Follow up with the patient in 30 to 90 days to discuss ways to improve patient receptiveness to care plan and to address barrier(s).
(CDC, 2023c)
- Discuss patient and provider health goals and develop an individualized patient care plan.
PHYSICAL THERAPY FOR FALL PREVENTION
For patients who are found to be at fall risk, a physical therapist will perform a thorough evaluation, including:
- Review of medical history
- Review of medications
- Checking heart rate and blood pressure measurements at rest and while changing positions
- Simple vision test
- Assessment of balance, strength, range of motion, ambulation, and gait pattern
- Home safety assessment
- Simple test of cognitive abilities
- Assess feet and footwear
Based upon the findings, the physical therapist designs a treatment plan tailored to the patient’s needs, which may include:
- Balance training
- Prescribed exercise program that includes walking and other functional mobility activities
- Dual-task training program
- Strength training
- Pain management
- Education on nutrition, sleep, choosing appropriate footwear
- Fear management
- Referral to community programs
- Home safety guidance
(APTA, 2023)
OCCUPATIONAL THERAPY FOR FALL PREVENTION
Occupational therapists’ role in any setting involves striking a balance between optimizing patient safety and positive risk-taking. They assist in helping patients who have a fear of falling to overcome the fear and regain mobility, educate them on actual risks, and provide opportunities to learn by doing (Keenan, 2021).
Occupational therapists consider how the person functions in their day-to-day environment. They assess for hazards and patient limitations that contribute to falls and offer safety education to patients and/or caregivers during activities of daily living. Occupational therapists can also earn specialty certification in fall prevention.
Fall risk factors addressed by occupational therapy include:
- Lower-extremity weakness
- Impaired balance
- Cognitive impairment
- Urinary incontinence
- Sensory impairment
- Fear of falling
- Side effects of medication
- Throw rugs and loose carpeting
- Lighting and glare
- Pets
- Clutter
- Uneven sidewalks and thresholds
- Unstable or nonexistent handrails
(AOTA, 2021)
Health Information Technology (IT) Problems
Healthcare facilities across the United States have made great efforts in moving from paper to electronic systems and processes since the Health Information Technology for Economic and Clinical Health (HITECH) Act was passed in 2009. Currently, there are no regulatory agreed-upon design standards that successfully limit potential errors.
As these systems become more and more integrated into the delivery of healthcare and the monitoring of safety and quality, how providers interact with these systems as users has become increasingly significant. While health IT systems have great potential to improve patient safety and help ensure high-quality healthcare, they can also present unintended risks.
Challenges include:
- Electronic health records are often designed to be additive, and it may impossible to correct errors leading to incorrect information being forwarded to other clinicians.
- Copying and pasting can represent a significant source of error.
- Computerized prescription order entry is beneficial for prescribers, pharmacists, and nurses. However, only about one third of hospitals have these systems in place, and fewer use barcode medicine administration.
Strategies to decrease errors in information technology include:
- Automating dispensing devices
- Barcoding
- Computerizing medical administration records
- Computerizing order entry and decision support
- Intercepting error messages at the time medications are ordered
- Prompt warnings for drug interaction, allergy, or overdose
- Providing drug-specific information
- Filling prescriptions using robotics
- Proving up-to-date information on new drugs
(Rodziewicz et al., 2023)
DEVELOPING EFFECTIVE DOCUMENTATION AND COMMUNICATION
It is clear that good communication lies at the heart of good practice and thus promotes patient safety. Many errors have been demonstrated to arise from the lack of adequate or accurate communication. There is a great deal of support for the development of effective documentation and communication in the provision of safe patient care.
Documenting to Prevent Errors
Documentation is a form of communication. It must be credible, timely, and accurately reflect the patient’s condition as well as the actions taken in response to that condition. Healthcare professionals must learn and follow their facility’s policies and procedures about documenting care.
COMMON DOCUMENTATION MISTAKES
Common documentation mistakes in patient records that can lead to errors in treatment include:
- Failing to record pertinent health or drug information
- Failing to record relevant details of a patient’s treatment, especially when treating multiple patients and across shifts
- Failing to record that medications have been administered, including dose, route, and time
- Recording in the wrong patient’s record (e.g., patients with similar names, with similar conditions, in physical proximity, or having the same attending physician)
- Failing to document discontinuation of medication
- Failing to record drug reactions or changes in a patient’s condition
- Transcribing orders improperly or transcribing improper orders
(NSO, 2023)
To help prevent medical errors, the following documentation (charting) do’s and don’t’s are recommended:
| Do | Don’t |
|---|---|
| (NSO, 2023) | |
|
|
Communication Tools to Prevent Errors
Research indicates that poor communication is a root cause of the great majority of all sentinel events.
RISK FACTORS FOR POOR COMMUNICATION
Verbal communication is a common source of medical error. Risk factors for such errors include:
- Disruptive behavior, rudeness, or verbal abuse
- Environmental noise issues
- Cultural differences between patients and providers
- Hierarchy issues
- Providers acting as autonomous agents
- Personality differences
- Language barriers
- Failure to work as a team
- Multiple conversations occurring at the same time
- Education and literacy
(Rodziewicz et al., 2023)
TOOLS FOR EFFECTIVE COMMUNICATION
Communication among healthcare providers using a standard framework and proven tools for reporting and sharing information can enable more effective communication. Examples of such tools include:
- SBAR (see below)
- BATHE protocol (Background, Affect, Trouble, Handling, and Empathy) is an interviewing process utilized in outpatient settings to connect with patients, screen for mental health problems, and empower patients to handle identified issues more constructively.
- Ticket-to-Ride for handoffs is a short, in-house document ensuring that transporters and providers unfamiliar with the patient will have important information readily available if problems arise or the patient is away from the unit longer than expected.
- Hourly rounding to each patient’s room or bedside is an intervention that helps to proactively anticipate and address each patient’s needs.
- Patient teach-back is a technique for healthcare providers to ensure that medical information has been explained clearly so that patients and families understand the information given to them.
- I-PASS is a clinical handoff verbal and written protocol for patient in-house transfer that includes Patient summary, Action to-do list, Situation awareness and contingency plan, and Synthesis or Summary of the information by the receiver.
- Technologic communication tools:
- Bedside tablets for patients instead of call lights
- HIPAA-compliant text messaging platforms for communicating among members of the care team
(HIPAA Journal, 2021)
SBAR
SBAR is one of the most commonly used communication tools for structured communication to ensure that information is transferred accurately between two clinicians, such as during a shift transfer. SBAR stands for Situation (S), Background (B), Assessment (A), and Recommendation (R). It uses prompt questions in four areas to guide a conversation to ensure efficient transfer of concise information (IHI, 2021).
| (IHI, 2021) | ||
| S | Situation | What is happening right now? |
|---|---|---|
| B | Background | What are the circumstances that led up to this situation? |
| A | Assessment | What do I think the problem is with this patient? |
| R | Recommendation | What should be done to correct the situation? |
“Speak Up” for Patients
Well-informed patients are better able to avoid serious medical errors. Clinicians should follow protocols that guide care, health education, and communication to help in both their own and their patients’ decision-making about appropriate healthcare.
The Joint Commission encourages patient participation through their Speak Up initiative, which encourages hospitals to inform patients about the importance of their contributions to the care they receive, making them active participants in avoiding medical errors (Rodziewicz et al., 2023).
| (TJC, 2021a) | |
| S | Speak up if you have questions or concerns. |
|---|---|
| P | Pay attention to badges worn by healthcare staff and remind staff to wash their hands. |
| E | Educate yourself about your illness, medical tests, and treatment plan. |
| A | Ask a trusted family member or friend to be your advocate. |
| K | Know what medicines you take and why you take them. |
| U | Use a hospital, clinic, surgery center, or other facility that meets standards of care. |
| P | Participate in all decisions about your treatment; you are the center of the healthcare team. |
ERROR RISKS AMONG POPULATIONS OF SPECIAL VULNERABILITY
The safety of all patients is of paramount concern for all healthcare providers. However, some patients—for example, the very young, the very old, and the very sick—are particularly vulnerable to the effects of medical errors, often due to their inability to participate actively as a member of the healthcare team due to communication issues. In addition, their physical status (including but not limited to body weight and body mass composition, nutritional status, and metabolism) may also cause them to react differently to interventions, putting them at special risk. Healthcare providers must recognize the special needs of these patients and act accordingly.
Older Adults
There are multiple issues of concern when providing healthcare to adults ages 65 and over. Failure to recognize the unique problems of this age group can result in adverse events.
POLYPHARMACY
Polypharmacy (the use of multiple medications) is strongly associated with an increased risk of falls, emergency care, hospitalization, and mortality in older adults. Studies have shown that more than one third of independent-living adults and half of those residing in long-term care facilities are taking medications that the Centers for Medicare and Medicaid Services (CMS) has labeled “unnecessary.”
Taking multiple medications is within guidelines for treatment of hypertension, diabetes, and heart failure, but additional comorbidities requiring simultaneous medications may result in a prescribing cascade, which occurs with medications being added to treat or prevent side effects of other medications.
Prescribers treating the older adult must consider aging physiology, functional status, cognitive issues, nutritional status, and social-support systems as well as mental, somatic, and psychological health in order to avoid negative outcomes.
With aging come changes in physiology including a decline in drug clearance and metabolism, which contributes to drug accumulation. Adjusting doses of medications in those with declining clearance is crucial.
The following steps help to identify drug therapy problems in cases of polypharmacy:
- Reconciling medications (to identify discontinued medications, missing medications, correct usage)
- Assessing for adherence
- Identifying drug-drug interactions using interaction databases
- Screening for drug-disease interaction
- Assessing for overtreatment such as duplicated medications resulting in toxicity
- Identifying high-risk drugs using Beers Criteria or STOPP/START
- Assessing for undertreated indications or missed therapy
- Monitoring medications for efficacy and safety
- Evaluating supplements, herbal supplements, multiple vitamins
(Hoel et al., 2021)
COGNITIVE IMPAIRMENT
Confusion and/or delirium in the older adult, especially someone with preexisting cognitive impairment, can be due to certain aspects of hospitalization, such as changes in environment, pain, and interruption in sleep patterns, as well as by several classes of medications. Delirium can also be the result of polypharmacy. The most common medications to cause delirium are opiates, benzodiazepines, and anticholinergics. An anticholinergic effect can also worsen a developing dementia. Confusion can also worsen when sensory input is affected, such as when the patient does not have access to eyeglasses or hearing aids.
Effective measures include orientation protocols, environmental modification, nonpharmacologic sleep aids, early and frequent mobilization, minimizing use of physical restraints, use of vision and hearing aids, adequate pain relief, and reduction in polypharmacy (Mattison, 2024).
FUNCTIONAL DECLINE
Functional decline may be the result of lack of mobility resulting in physical deconditioning and muscle weakness. Immobility can increase the risk for adverse events such as falls, delirium, skin breakdown, and venous thromboembolic disease.
The older adult experiences functional decline when unable to engage in activities of daily living. When an older adult is hospitalized, they spend most of their hospitalization in bed, and functional decline can occur as early as the second day of hospitalization.
Although a few conditions require absolute bed rest, most medical conditions do not. Improved mobility during hospitalization has been linked to decreased risk of death. For example, patients should be assisted out of bed to a chair for meals (which can also decrease the risk of aspiration) and be encouraged to walk several times a day (Mattison, 2024).
FALL RISK
Risk for falls is increased in the older adult and may be due to the effects of acute illness compounded by an unfamiliar environment and side effects of treatments. Tethering medical devices (e.g., urinary catheters, IV lines, cardiac monitor leads, restraints) makes it more difficult to mobilize patients safely and is associated with increased rates of falls.
Strategies to help prevent falls may include weighing the risks and benefits of medications with significant psychotropic and anticholinergic effects, monitoring patients whose prescribed drugs may increase fall risk, supervising high-risk patients when ambulating, and encouraging time out of bed walking or sitting in a chair to prevent orthostatic hypotension associated with prolonged immobility. Intravenous lines and urinary catheters should be discontinued as early as possible. Restraints, either physical or pharmacologic, should be avoided (Mattison, 2024).
MALNUTRITION
Poor nutrition for older patients may result from several factors. which can include:
- Impaired cognition or delirium
- Poor appetite, nausea, or constipation (due to underlying illness or as side effects of medications)
- Restriction of movement
- No access to dentures/poor dentition
- Missed or interrupted meals
- Lack of assistance with feeding
- Difficulty in self-feeding
- Severely restricted diet order (e.g., nothing by mouth [NPO])
Simple interventions such as getting the person out of bed at mealtime and providing assistance with eating can be of benefit. Inpatient assessment by a nutritionist can identify deficiencies and, combined with nutritional follow-up after discharge, may decrease mortality.
Careful consideration before placement of a feeding tube should be done, particularly in older patients with multiple morbidities. Whenever possible, oral feedings are preferred to the use of feeding tubes (Mattison, 2024).
DEHYDRATION
Older adults, especially those living with multiple chronic diseases, are more vulnerable to dehydration. Dehydration is associated with adverse health outcomes and acts as an independent factor of hospital length of stay, readmission, intensive care, in-hospital mortality, and poor prognosis. Dehydration has been shown to affect cognitive performance and increase risk of metabolic and renal diseases.
Rehydration therapy is particularly difficult in light of unawareness within that population and because no recommended volume of daily water intake has yet been determined in older patients with certain diseases, especially those with heart failure, kidney disease, or other causes of fluid overload. It is important to individualize fluid intake recommendations for older adults (Li et al., 2023).
Infants and Children
The pediatric and neonatal patient population is three times more likely to be affected by medication errors than adults and at greater risk of harm than adults when they occur. The most common error is in dosage preparation as a result of failure to consider the weight of a child. Significantly high error rates have been reported during prescribing, dosage preparation, and administration.
Implementation of drug therapy in children and infants can be complex due to treatment of rare and life-threatening conditions requiring continuous adjustment of dosing and drug therapy; narrow therapeutic range of some drugs; and individualized dosing based on factors such as age, weight, renal function, and maturation of enzyme systems.
Children are more prone to experiencing medication errors and the resulting harm because of the following:
- Most medications used in the care of children are formulated and packaged primarily for adults, requiring preparation of doses in different volumes or concentrations.
- The need to alter the original medication dosage requires a series of pediatric-specific calculations and tasks, each increasing the possibility of error.
- Most healthcare settings are primarily built around the needs of adults, and emergency departments may be particularly risk prone for children.
- Children are usually less able physiologically to tolerate a medication error due to still-developing renal, immune, and hepatic functions.
- Many children, especially very young children, cannot communicate effectively to providers regarding any adverse effects that medications may be causing.
Pediatric-specific strategies for reducing medication errors include:
- Effectively standardizing and identifying medication as well as the processes for drug administration
- Ensuring full pharmacy oversight in verifying dosage preparation, dispensing, and administration of both neonatal and pediatric medications
- Orienting all pharmacy staff and practitioners involved in ordering, preparing, and administering medication to pediatric patients in all settings
- Monitoring children who are under sedation during office-based procedures, particularly pulse oximetry, using age- and size-appropriate monitoring equipment and following uniform procedures
- Weighing all pediatric patients in kilograms at the time of admission (including outpatient and ambulatory clinics) or within four hours of admission in an emergency situation
- Using barcoding technology with pediatric capability
- Ensuring clinical pharmacists check the dosing calculation, screen for drug-drug interactions, and counsel caregivers on proper administration and medication-storage safety
- Using accurate weight scales that only measure in metric units (kilograms or grams), standardized equipment throughout a system, drug dose ranging limits, and “smart” infusion pumps for hospitals and standardized order sets.
- Avoiding high-risk drug dispensing or administration if the pediatric patient has not been weighed, unless it is an emergency
- Requiring both inpatient and outpatient prescribers to include the calculated dose and the dosing determination, such as the dose per weight (e.g., milligrams per kilogram) or body surface area, to facilitate an independent double-check of the calculation by a pharmacist, nurse, or both
- Using commercially available pediatric-specific formulations and concentrations whenever possible
- Communicating verbally and in writing information about the child’s medication to the child, caregivers, and parents/guardians, including information about potential side effects
(TJC, 2021b)
Intensive Care Patients
Intensive care settings are one of the most complex environments in healthcare. Preventable harms contribute significantly to ICU morbidity, mortality, and costs. Medical errors and deaths due to preventable harms are more common in the ICU due to higher patient acuity and complexity of care. Medication errors are the most common type of error and account for 78% of serious errors in ICU.
It has been found to be helpful to provide mandatory teaching and training sessions for all ICU staff to ensure all caregivers are up to date in the field of critical care, with patient safety being of paramount importance.
Implementing bundles of care in the ICU has been found to reduce risk of nosocomial infection, and a bedside checklist is a cost-effective and simple method to prevent errors of omission. A bundle of care is a collection of interventions that may be applied to the management of a particular condition.
The use of checklists is an effective way to improve patient safety in intensive care units and appears in many clinical guidelines. Clinical and process-of-care outcomes associated with checklists are predominantly positive (Patil et al., 2023; Erikson et al., 2023).
Patients with Limited English Proficiency
Persons with limited English proficiency (LEP) have a limited ability to read, speak, write, or understand English. According to U.S. census data, there at least 25 million people in the United States who can be classified as LEP (Claros, 2021).
Individuals with LEP have problems with language competence that negatively affect communication and can greatly define the ease with which they navigate all areas of the healthcare system. An analysis of adverse incidents in the hospital setting found that 49.1% of LEP patients experienced physical harm, compared to 29.5% of English-speaking patients (Claros, 2021).
Patients with linguistic differences may have problems advocating for themselves and may not be able to describe or explain their chief complaints or express their level of pain. They are at higher risk for complications because of poor comprehension of medication errors, inaccurate assessment, increased psychological stress, and poor compliance with treatment and follow-up. In addition, the use of family or friends as interpreters increases chances of error (Claros, 2021).
Both the Joint Commission and the Affordable Care Act mandate adequate medical interpreter and translation services for patients with LEP (Language Scientific, 2020; TJC, 2021c).
Patients with Low Health Literacy
Health literacy is defined as the degree to which individuals have the capacity to obtain, process, and use information and services to inform health-related decisions and actions for themselves and others. This includes the ability to understand basic health information and services needed to make appropriate health decisions. Low health literacy may have a negative effect on a person’s adherence to a treatment regimen, which may decrease its benefits.
Low health literacy is associated with patients who are older, have limited education, have lower income, have chronic conditions, and are non-native English speakers. People can also face health literacy issues when:
- They aren’t familiar with medical terms or how their bodies work
- They have to interpret statistics and evaluate risks and benefits that affect their health and safety
- They are diagnosed with a serious illness and are frightened and confused
- They have health conditions that require complicated self-care
(CDC, 2023d)
Since limited health literacy is common and may be difficult to recognize, it is recommended that clinicians assume all patients and caregivers may have difficulty comprehending health information and that they communicate in ways that anyone can understand. This includes:
- Answering questions using nonmedical language
- Confirming comprehension for all patients
- Encouraging patients to take part in decisions and letting them make healthcare decisions that fit their values, goals, preferences, and circumstances
(ODPHP, 2021)
PATIENT SAFETY INITIATIVES
When the book To Err Is Human made headlines across the country in 1999, it captured the attention of the public and launched the modern patient safety movement. Federal funding for patient safety initiatives increased, accreditation and reporting standards tightened, and research on effectiveness of patient safety measures expanded. Over the ensuing years, the patient safety movement has grown to involve many agencies and organizations in both the public and private sectors, and many important milestones have been achieved along the way.
Annually, the Patient Safety Movement Foundation meets to nominate and elect new patient safety challenges to be addressed for the following year in attempt to reach their primary goal of zero preventable deaths by 2030. In 2020, organizations were asked to commit to implementing and sustaining a foundation for safety and reliability that includes three critical components:
- A person-centered culture of safety
- A holistic, continuous improvement framework
- An effective model for sustainment
(PSMF, 2021)
AGENCIES AND ORGANIZATIONS IN THE PATIENT SAFETY MOVEMENT
AAAHC (Accreditation Association for Ambulatory Health Care): Develops standards to advance and promote patient safety, quality care, and value for ambulatory healthcare settings, including ambulatory surgery centers, community health centers, medical and dental group practices, medical home practices, and managed care organizations, as well as Indian Health Service and student health centers
ABMS (American Board of Medical Specialties): Recognizes medical specialists and establishes standards for physician certification
ACGME (Accreditation Council for Graduate Medical Education): Responsible for accrediting the majority of medical residency and internship programs
AHRQ (Agency for Healthcare Research and Quality): Produces evidence to make healthcare safer, of higher quality, more accessible, more equitable, and more affordable, working with the U.S. Department of Health and Human Services
ANA (American Nurses Association): Represents the interests of registered nurses to advance the profession to improve healthcare
CDC (Centers for Disease Control and Prevention): Promotes health and disease prevention and preparedness
ECRI (Emergency Care Research Institute): Independent nonprofit organization that aims to improve the safety, quality, and cost-effectiveness of care across all healthcare settings worldwide
ECRI (Economic Cycle Research Institute): Federally designated evidence-based practice center, recognized as a trusted source of guidance and consulting on and monitoring of new and emerging medical technologies, procedures, genetic tests, and clinical guidelines
HCUP (Healthcare Cost and Utilization Project): Maintains hospital care data, enabling research on a range of health policy issues
HRET (Health Research and Education Trust): Research and education affiliate of the American Hospital Association that promotes research and education efforts
IHI (Institute for Healthcare Improvement): Redesigns healthcare into a system without errors, waste, delay, or unsustainable costs; in 2017, joined together with the National Patient Safety Foundation
IOM (Institute of Medicine): Asks and answers the nation’s most pressing questions about health and healthcare
ISMP (Institute for Safe Medication Practices): Watchdog organization devoted to medication error prevention and safe medication use
NIH (National Institutes of Health): Conducts medical research
NQF (National Quality Forum): Leads national collaboration to improve health and healthcare quality through measurement
TJC (The Joint Commission): Accredits and certifies healthcare organizations and programs in the United States
WHO (World Health Organization, World Alliance for Patient Safety): Serves as the directing and coordinating authority for health within the United Nations system
Federal and State Efforts
The National Action Plan centers on four foundational and interdependent areas, prioritized as essential to create total system safety:
- Culture, Leadership, and Governance
- Ensure safety is a demonstrated core value
- Assess capabilities and commit resources to advance safety
- Widely share information about safety to promote transparency
- Implement competency-based governance and leadership
- Patient and Family Engagement
- Establish competencies for all healthcare professionals for the engagement of patients, families, and care partners
- Engage patients, families, and care partners in the coproduction of care
- Ensure equitable engagement for all patients, families, and care partners
- Promote a culture of trust and respect for patients, families, and care partners
- Workplace Safety
- Implement a systems approach to workforce safety
- Assume accountabilities for physical and psychological safety and a healthy work environment
- Develop and execute priority programs that equitably foster workforce safety
- Learning System
- Facilitate both intra- and interorganizational learning
- Accelerate the development of the best possible safety learning networks
- Initiate and develop systems to facilitate interprofessional education and training on safety
- Develop shared goals for safety across the continuum of care
- Expedite industry-wide coordination, collaboration, and cooperation on safety
(IHI, 2022)
The Centers for Medicare and Medicaid Services (CMS) announced in 2007 that Medicare would no longer pay for additional costs associated with many preventable errors, including those considered “never events.” Since then, many states and private insurers have adopted similar policies. Since February 2009, CMS has not paid for any costs associated with wrong-site surgeries. Never events are also being publicly reported, with the goal of increasing accountability and improving the quality of care.
Since the National Quality Forum disseminated its original never events list in 2002, 11 states have mandated reporting of these incidents whenever they occur, and an additional 16 states mandate reporting of serious adverse events. Healthcare facilities are accountable for correcting systematic problems that contribute to the events, with some states mandating performance of a root cause analysis and reporting its results (Shebell & Shebell, 2023).
PREVENTABLE COMPLICATIONS (NEVER EVENTS) NOT COVERED BY MEDICARE AND MEDICAID
The following preventable complications are not reimbursed by Medicare and Medicaid if acquired during an inpatient stay:
- Foreign object retained after surgery
- Air embolism
- Blood incompatibility reaction
- Stage 3 and 4 pressure injuries/ulcers
- Falls and trauma:
- Fractures and dislocation
- Intracranial injuries
- Burns
- Crushing injuries
- Other injuries
- Manifestations of poor glycemic control:
- Diabetic ketoacidosis
- Nonketotic hyperosmolar coma
- Hypoglycemic coma
- Secondary diabetes with ketoacidosis
- Secondary diabetes with hyperosmolarity
- Catheter-associated urinary tract infection
- Vascular catheter-associated infection
- Surgical site infection following:
- Mediastinitis following coronary artery bypass graft
- Bariatric surgery for obesity
- Laparoscopic gastric bypass
- Gastroenterostomy
- Laparoscopic gastric restrictive surgery
- Surgical site infection following certain orthopedic procedures:
- Spine
- Neck
- Shoulder
- Elbow
- Surgical site infection following cardiac implantable electronic device
- Deep vein thrombosis/pulmonary embolism following total knee or hip replacement
- Iatrogenic pneumothorax with venous catheterization
Medicare and Medicaid also will not reimburse for wrong-site, wrong-procedure, and wrong-patient surgery (CMS, 2023a).
Evidence-Based Practice (EBP)
EBP is the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of the individual patient. It means integrating individual clinical expertise with the best available external clinical evidence from systematic research.
EBP is vital for improvement in the quality of treatment and for assuring patient safety. EBP attempts to standardize practices in order to make outcomes more predictable. EBP involves collecting, evaluating, and implementing practices that can improve patient care safety and outcomes. EBP is beneficial in decreasing healthcare costs and reducing medical complications. It is the integration of clinical expertise, patients’ values and preferences, and best research evidence into the decision-making process for providing patient care (Duke University Medical Center, 2023).
Components of evidence-based practice. (Source: J. Swan.)
FIVE STEPS TO IMPLEMENT EVIDENCE-BASED PRACTICE
Evidence-based practice begins and ends with the patient.
- Assess the patient and your own knowledge gaps.
- Ask a well-built clinical question derived from the patient’s case.
- Acquire evidence by selecting an appropriate resource and by conducting a search.
- Appraise evidence for validity and applicability.
- Apply what has been learned, talk with the patient, and integrate the evidence with your clinical expertise and patient preferences.
Following this process, it is important to evaluate performance with the patient (Duke University Medical Center, 2023).
CASE
Jai, a pharmacist working in skilled nursing facilities, was involved in assessing and updating a facility’s manual of medication policies and procedures. While reviewing the section on digoxin monitoring, he found that an apical pulse should be taken daily before administering digoxin and that the drug should not be given if the pulse is below 60 beats per minute.
While looking over medication administration records, he found that residents with hypertension receiving antihypertensives had their blood pressure taken once a week and other residents had vital signs done once a month. Apical pulses for residents receiving digoxin were obtained daily.
As he thought about this, he realized that in all the time he had been working as a pharmacist in healthcare facilities, he could only recall digoxin being withheld once or twice because of a pulse below 60. He began to question the necessity for performing apical pulses and asked, “Why are medication nurses in skilled nursing facilities checking apical pulses daily?”
With that question in mind, Jai began to acquire relevant resources by talking with medication nurses, directors of nursing, and other pharmacists about their experiences with digoxin monitoring. All of the nurses he questioned had been in nursing for 10 or more years in skilled nursing facilities, and none could remember holding digoxin more than once or twice for a pulse below 60 on a single day that returned to normal on the next day. This number was compared to the hundreds of doses they had administered over their careers.
Jai then began to search databases for the best evidence for digoxin monitoring. He found that the initiation of digoxin occurred in hospital settings, and that it was critical to take apical pulses to determine the correct dosage. Once the patient was properly dosed and discharged, this monitoring was no longer required. Indeed, the research showed that patients discharged to “home” are not instructed to monitor their apical pulse every day, and there were no negative outcomes reported.
Following his critical appraisal of the resources, Jai determined that persons who reside in nursing homes have been discharged to their “home” and that medication nurses were performing a time-consuming, unnecessary procedure.
Jai brought his findings to the director of nursing and the medical director, and together they enacted (applied) a new policy that stated the apical pulse rate of residents receiving digoxin is to be obtained once a week. If the apical pulse is less than 60, digoxin should be given as ordered, and the apical pulse is to be monitored daily for three days while continuing to give the medication. If it continues to be below 60 after three days, the medication should be withheld and the attending physician notified.
The change in policy was explained to patients and their families so there would be no perception of the staff “cutting corners” once the new practice started. Aside from a few joking comments by patients about missing the “hand holding,” there was no push back.
The policy was assessed after it was in place for nine months. During that time there was not a single dose of digoxin held. It was determined that this change resulted in one less procedure to be performed by the medication nurse, leaving more time to provide other care for the patients (Vogenberg, 2004).
Quality Assurance and Performance Improvement (QAPI)
Quality assurance (QA) is the process of meeting quality standards and assuring that care reaches an acceptable level, and performance improvement (PI) is the continuous analysis of performance and the development of systematic efforts to improve it. Beginning in 2011, the Centers for Medicare and Medicaid Services began mobilizing some of the best practices in nursing homes. QA and PI were combined, and a prototype QAPI program was begun in a small number of facilities, which provided the agency with best practices for helping nursing homes upgrade their current quality programs.
QAPI is made up of five elements:
- Design and Scope
- Must be comprehensive and ongoing
- Should address all systems of care and management practices
- Aims for safety and high quality with all clinical interventions
- Emphasizes autonomy and choice in daily life for residents
- Utilizes the best available evidence to define and measure goals
- Governance and Leadership
- Develops a culture that seeks input from facility staff, residents, and families
- Assures adequate resources exist
- Designates person(s) to be accountable
- Ensures staff time, equipment, and technical training as needed
- Ensures that policies are developed to sustain QAPI
- Ensures a culture of safety
- Feedback, Data Systems, and Monitoring
- Uses performance indicators to monitor a wide range of care processes and outcomes
- Reviews findings against established benchmarks or targets
- Includes tracking, investigating, and monitoring of adverse events
- Develops action plan to prevent adverse event recurrences
- Performance Improvement Projects
- Gathers information systematically to clarify issues or problems
- Intervenes to make improvements
- Systematic Analysis and Systemic Action
- Uses a systematic approach to determine when in-depth analysis is needed
- Uses a thorough and highly organized structured approach to examine the way care and services are organized or delivered
- Develops policies and procedures and demonstrates proficiency in the use of root cause analysis
- Takes systemwide actions to prevent future events
- Focuses on continual learning and continuous improvement
(CMS, 2023b)
ACCREDITING AGENCIES’ IMPACT ON PREVENTING MEDICAL ERRORS
Accreditation is a voluntary process in which trained external peer reviewers evaluate a healthcare organization’s compliance and compare it with preestablished performance standards. Accreditation focuses on continuous improvement strategies and achievement of optimal quality standards intended to ensure patient safety.
The Joint Commission
The Joint Commission (TJC) is an independent, not-for-profit agency whose mission is to continuously improve the safety and quality of care provided to the public. TJC accredits and certifies more than 22,000 healthcare organizations and programs in the United States, including hospitals and healthcare organizations that provide ambulatory and office-based surgery, behavioral health, home healthcare, laboratory, and nursing care center services.
Accreditation by TJC is not mandatory. Healthcare organizations, programs, and services voluntarily pursue accreditation and certification. TJC surveyors visit accredited healthcare organizations a minimum of once every 36 months (two years for laboratories) to evaluate standards compliance. This visit is referred to as a survey. All regular Joint Commission surveys are unannounced.
During a survey, the surveyors randomly select patients, and using their medical records, the surveyors evaluate standards compliance. As they review each patient’s experience, they talk to doctors, nurses, and other staff who interacted with the patients. They also observe doctors, nurses, and other caregivers providing care and often speak to the patients themselves.
TJC Quality Reports give the public information on the safety and quality of care for all TJC-accredited/certified healthcare organizations. Reports include:
- Accreditation decision and date
- Programs and services accredited by the Joint Commission and other bodies
- National Patient Safety Goal performance
- Hospital National Quality Improvement Goal performance
- Special quality awards
(TJC, 2024c)
SENTINEL EVENT POLICY
TJC adopted a formal Sentinel Event Policy in 1996 to help hospitals that experience serious adverse events improve safety and learn from those sentinel events. The policy explains how TJC partners with healthcare organizations that have experienced a serious patient safety event to protect the patient, improve systems, and prevent further harm.
Each accredited organization is strongly encouraged, but not required, to report sentinel events to TJC. Benefits of reporting include:
- TJC can provide support and expertise during the review of a sentinel event.
- The organization can collaborate with a patient safety expert in TJC’s Sentinel Event Unit of the Office of Quality and Patient Safety.
- Reporting raises the level of transparency in the organization and promotes a culture of safety.
- Reporting conveys the healthcare organization’s message to the public that it is doing everything possible, proactively, to prevent similar patient safety events in the future.
(TJC, 2024a)
NATIONAL SAFETY GOALS
Every year TJC gathers information about patient safety issues from widely recognized experts and stakeholders. This information is the basis for their National Patient Safety Goals, which are tailored for each specific healthcare setting. The information also informs their sentinel event alerts, standards and survey processes, performance measures, educational materials, and Joint Commission Center for Transforming Healthcare projects.
The National Safety Goals address multiple healthcare sites:
- Ambulatory healthcare
- Assisted living community
- Behavioral healthcare and human services
- Critical access hospital
- Home care
- Hospital
- Laboratory
- Nursing care center
- Office-based surgery
Specific goals for each may include any of the following that have been identified as pertinent for the setting:
- Identify patients correctly
- Improve staff communication
- Use medicines safely
- Improve healthcare equity
- Reduce the risk for suicide
- Use alarms safely
- Prevent infection
- Identify patient safety risks
- Prevent mistakes in surgery
- Prevent patients from falling
- Prevent bed sores (pressure injuries)
(TJC, 2024d)
“DO NOT USE” ABBREVIATION LIST
Misreading medical abbreviations can be a cause of serious medication errors, and TJC has created a “do not use” list of abbreviations that endanger patients’ safety and that it requires its members to follow (see table below).
| Do Not Use | Potential Problem | Instead Use |
|---|---|---|
| (TJC, 2024e) | ||
| U, u | Mistaken for “0” (zero), the number “4” (four), or “cc” | Unit |
| IU | Mistaken for IV (intravenous) or the number 10 (ten) | International unit |
| Q.D., QD, q.d., qd | Mistaken for each other | Daily |
| Q.O.D., QOD, q.o.d, qod | Period after the “Q” mistaken for “I” and the “O” mistaken for “I” | Every other day |
| Lack of leading zero | Decimal point is missed | 0.X mg |
| MS | Can mean morphine sulfate or magnesium sulfate | Morphine sulfate or Magnesium sulfate |
| MSO4 and MgSO4 | Confused for one another | Morphine sulfate or Magnesium sulfate |
| Trailing zero * | Decimal point is missed | X mg |
| * Exception: A “trailing zero” may be used only where required to demonstrate the level of precision of the value being reported, such as for laboratory results, imaging studies that report size of lesions, or catheter/tube sizes. It may not be used in medication orders or other medication-related documentation. | ||
The Institute for Safe Medication Practices has also compiled an extensive list of abbreviations, symbols, and dose designations that are frequently misinterpreted and involved in harmful medication errors, which can be accessed online (see “Resources” at the end of this course).
ROOT CAUSE ANALYSIS (RCA)
RCA has been adopted widely as a method for investigating serious adverse events. The Joint Commission has mandated use of RCA to analyze sentinel events since 1997.
RCA cause analysis identifies underlying problems that increase the likelihood of errors while avoiding focusing on mistakes made by individuals. The approach identifies both active errors and latent errors and is one of the most widely used retrospective methods for detecting safety hazards. The ultimate goal of RCA is to prevent future harm by eliminating the latent errors that often underlie adverse events.
RCAs follow a prespecified protocol, beginning with data collection and reconstruction of the events through record review and participant interviews. A multidisciplinary team then analyzes the sequence of events leading to the error, with goals of identifying how the event occurred (by identifying active errors) and why the event occurred (by systematic identification and analysis of latent errors). The steps in this process include:
- Verifying the incident and define the problem
- Commissioning the RCA investigation
- Mapping a timeline (event and causal factor chart
- Identifying critical events
- Analyzing the critical events (cause and effect chart)
- Identifying root causes
- Supporting each root cause with evidence
- Identifying and selecting the best ways of addressing the problem
- Developing recommendations
- Writing and presenting the report
(VDH, 2021)
ROOT CAUSE ANALYSIS AND ACTION PLAN TEMPLATE
The Joint Commission has developed a template to be used while conducting a root cause analysis that recommends the following 24 questions be asked and answered and an action plan developed for any finding that can be considered a risk-reduction strategy.
- What was the planned flow of the procedure?
- What steps in the procedure did not occur as planned?
- What human factors were pertinent to the outcome?
- How did performance of equipment affect outcome?
- What controllable environmental factors directly affected the outcome?
- What external controllable factors affected the outcome?
- Were there any other factors that directly affected the outcome?
- In what other areas of the organization could this happen?
- Was the staff properly qualified and currently competent at the time of the event?
- How did real staffing compare with ideal levels?
- What is the plan for dealing with unforeseen staffing problems?
- Were such problems a factor in this event?
- Did staff perform to expectations during the event?
- Was all the necessary information available when needed? Was it accurate, complete, and explicit?
- Was communication among participants sufficient for this situation?
- Was this the appropriate physical environment for the situation?
- What systems are in place to recognize environmental risks?
- What planned and tested emergency and failure-mode responses are in place?
- How does the culture support risk reduction?
- What barriers exist to the communication of potential risk factors?
- What methods are utilized to communicate the high priority of prevention of adverse outcomes?
- What orientation and in-service training revisions are necessary to reduce risk of events in the future?
- Was available technology used as intended?
- What technology or redesign of technology might reduce risk in the future?
(TJC, n.d.)
CASE
St. Vincent Hospital
(continued from above under “Active and Latent Errors”)
Following identification of the cause of the accident in St. Vincent Hospital’s operating room, a root cause analysis was begun that day. The root cause was determined to be the use of an inappropriate gas mixture to expand the abdomen during laparoscopic surgery.
Contributing factors included:
- All extra cylinders containing medical gases used in the OR are stored in metal tubes in a tank room, but only the top several inches of each cylinder and a portion of each tank’s label is visible above the top of the storage tubes. The tube height is to provide adequate support for the cylinders, so shortening the tubes to allow visualization of the entire label is not an appropriate option.
- All tanks containing any percentage of CO2 are color-coded the same (gray). This is an industry standard over which individual facilities have no control.
- When an OR logistics technician allowed a logistics technician from the catheterization lab to store an extra CO2/O2 tank in the OR tank room, no one in the OR, anesthesia, or logistics chain of command was informed. This is an example of well-intentioned interunit cooperation gone awry due to lack of appropriate communication.
- The circulating nurse mistakenly replaced an empty CO2 tank with a blended CO2/O2 tank, not noticing the difference because they were both gray, with similar labeling, and because there was no history of anything but pure CO2 being stored in the OR tank room or used in the OR.
- There was no pin indexing at the connection point between the cylinder and the gas delivery system that differentiates between pure CO2 and CO2/O2 blends. Any cylinder containing any percentage of CO2 fits to any yoke designed to accept CO2 in any concentration.
Corrective actions included policy changes and an intensive education initiative for all involved personnel:
- Only medical gases intended for use in the OR are to be stored in the OR tank room.
- Should a deviation from this policy be indicated for safety reasons and no other alternatives exist:
- Tanks containing gases not used in the OR are to be stored in the OR tank room only until safe storage elsewhere is available.
- If no alternative storage is available, storage in the OR tank room may be approved only by the senior professional and technical personnel in the OR and the anesthesia service. If the decision is made during “off” hours by a shift charge person, that person is responsible for notifying the appropriate senior personnel by the next shift or delegating and documenting that this notification is to be made by a specific, named person.
- Any such tanks are to be indicated by orange fluorescent tags reading “Not for use in the OR” and placed in the most remote storage tubes in the tank room.
- Information about the temporary storage is to be conveyed at each OR and anesthesia shift report and in the OR shift change log until the tank is removed.
- Medical gases are elevated to the status of medications and the triple-check policy used for medications will be implemented for medical gases.
- The OR manager will personally brief each shift for the next two days to minimize rumors.
- All members of the involved surgical and logistics teams will be debriefed by their supervisors.
- A description of the incident and follow-up will be published in the quality assurance journal for the healthcare system.
CASE
Céline is an 82-year-old patient who has suffered a stroke and been transferred to a local nursing home where inadequate staffing has been a recurrent problem. Céline has right-sided paralysis and requires total care. Her care plan includes repositioning every two hours. Today the nurse does the required biweekly skin assessment and finds a small open crater with visible subcutaneous tissue on the heel of her right foot (i.e., a stage 3 pressure injury).
The nurse documents and reports this long-term care sentinel event per facility policy, and a root cause analysis is begun by a multidisciplinary team that will identify how and why the event occurred. By asking questions as outlined in the facility’s root cause analysis template, the first step is to identify and define the problem:
- Stage 3 pressure injury (damage to tissue leading to death of tissue) has developed on the heel of the patient’s right foot.
- Tissue damage has negatively impacted the goal of patient safety.
The second step is to identify the cause:
- Death of tissue caused by mechanical damage
- Mechanical damage caused by pressure
- Pressure injury due to patient remaining in same position
- Patient remaining in same position due to failure to reposition every 2 hours
- Failure to reposition every 2 hours due to inadequate level of staffing
The third step in the process is to select the best solution to reduce the risk of pressure injuries in the future.
- Reposition patients at risk every 2 hours and document the action.
- Utilize pressure-relieving devices such as beds, mattresses, or overlays.
- Review and revise staffing formulas; improve staffing to meet the U.S. Department of Health and Human Services recommendations of 1 hour per resident per day for total licensed staff, 27 minutes per day for RNs, and 2 hours per day for nursing assistants.
Following completion of the root cause analysis, the facility determines to institute the action plan:
- Alternating pressure pads are applied to the beds of all residents at high risk for pressure injuries.
- The use of heel/elbow protectors becomes standard for all patients with immobility issues.
- Documentation on the implementation and effectiveness of a turning schedule is instituted for each resident with immobility.
- Staffing issues remained unresolved due to budget restraints, but ongoing exploration of means to improve the staffing level is being carried out.
Accreditation Association for Ambulatory Health Care (AAAHC)
The AAAHC was founded in 1979 and is the leader in ambulatory healthcare accreditation, with more than 6,600 organizations accredited. AAAHC accredits a wide range of outpatient settings, which include:
- Ambulatory healthcare clinics
- Ambulatory surgery centers
- Birthing centers
- College and university health centers
- Community health centers
- Dental group practices
- Diagnostic imaging centers
- Endoscopy centers
- Federally qualified community health centers
- Health plans
- Independent physician associations
- Indian Health Service centers
- Lithotripsy centers
- Medical home organizations
- Military healthcare facilities
- Multispecialty group practices
- Occupational health centers
- Office-based anesthesia organizations
- Office-based surgery centers and practices
- Oral and maxillofacial surgeons’ offices
- Pain management centers
- Podiatry practices
- Radiation oncology centers
- Single specialty group practices
- Surgery recovery centers
- Urgent or immediate care centers
- Women’s health centers
AAAHC advocates for the provision of high-quality healthcare through the development and adoption of nationally recognized standards, providing a voluntary survey experience founded on a peer-based, educational approach to in-site review every three years.
The AAAHC Certificate of Accreditation demonstrates an organization’s commitment to providing safe, high-quality services to its patients every day of the 1,095-day accreditation cycle. Accreditation is recognized by third-party payors, medical professional associations, liability insurance companies, state and federal agencies, and the public (AAAHC, 2024).
INSTITUTIONAL STRATEGIES FOR ADDRESSING ERRORS
Essential strategies healthcare facilities must consider in their efforts to reduce medical errors include:
- Changes in organizational culture
- Involvement of leadership
- Education of providers
- Development of patient safety committees
- Adoption of safe protocols and procedures
- Use of technology
Patient Safety Culture
Patient safety culture describes the extent to which an organization’s culture supports and promotes patient safety and refers to the values, beliefs, and norms shared by practitioners and other staff throughout the organization that influence their behaviors and actions. Key features of a safety culture include:
- Strong support from organizational leadership
- Acknowledgement of the high-risk nature of an organization’s activities
- Determination to achieve consistently safe operations
- Responsibility by everyone for safety, implementing and reporting unsafe conditions
- A blame-free environment for individual reporting of errors or near misses without fear of reprimand or punishment
- Encouragement of collaboration about decision-making across all staff levels and disciplines to seek solutions to worker and patient safety problems
- Organizational commitment of resources to address safety concerns
(CDC, 2023e)
JUST CULTURE MODEL
In a just culture, adverse events are recognized as valuable opportunities to understand contributing factors and to learn from them. The principles of a just culture require a shift away from how healthcare organizations typically responded to adverse events in the past by immediately assigning blame and punishing the individual involved. This approach resulted in fear of consequences and, in turn, failure to disclose errors.
A just culture acknowledges that errors occur not only as a result of an individual’s behavioral choices but also as a result of system failures. When an adverse event occurs in a just culture, it is necessary to understand the behavioral choices of the individual as well as system factors. This approach to remedying the problem results in promoting of learning, managing behavioral choices, and designing safe systems to prevent recurrence.
When using the just culture model, people recognize that when they report errors, fair treatment will be received (Murray et al., 2023).
Addressing Staffing Concerns
Since 2020, nearly 1 in 5 healthcare workers have quit their jobs, and research indicates that up to 47% of healthcare workers plan to leave their positions by 2025. When an experienced healthcare professional departs the field, their knowledge goes with them. Collective knowledge loss can be damaging to both providers and patients by lowering the average experience level of hospital employees. When inexperienced personnel are trained by less-experienced staff, the knowledge deficit deepens.
With fewer healthcare personnel, the risk for medical error increases. Overworked and understaffed medical teams may make more avoidable mistakes when cognitive failure is high. Cognitive failure can be a result of stress, lack of expertise, and a heavy patient load, among other factors. When cognitive failure is high, healthcare workers may be more likely to find shortcuts to safety procedures, insufficiently monitor patients following a procedure, or suffer injuries themselves from physical hazards.
Studies have demonstrated the association between staffing ratios and patient safety, documenting an increased risk of adverse events, morbidity, and mortality as staffing ratios decrease.
Nurses play a critically important role in ensuring patient safety while providing care directly to them. Nurses are a constant presence at the bedside and regularly interact with physicians, pharmacists, families, and all other members of the healthcare team. They are crucial to timely coordination and communication of a patient’s condition to the team.
In regards to safety, a nurse’s role includes monitoring patients for clinical deterioration, detecting errors and near misses, and understanding care processes and weaknesses inherent in some systems. Nurses communicate changes in a patient’s condition and perform countless other tasks to ensure patients receive high-quality care.
Determination of adequate nurse staffing is a complex process that changes on a shift-by-shift basis, requiring close coordination between management and nursing, and is based on:
- Patient acuity and turnover
- Availability of support staff and skill mix
- Settings of care
The high-risk nature of the work, stress caused by increased workload and interruptions, and the risk of burnout due to involvement in errors or exposure to disruptive behavior likely combined with unsafe conditions precipitated by low nurse-to-patient ratios result in an increased risk of adverse events (Phillips et al., 2021).
Organizations need to be creative in meeting the needs of nurses with an environment that motivates and empowers their autonomy in staffing ratio decisions that consider high volume and acuity levels that will lead to less burnout and desire to exit the workforce (Haddad et al., 2023).
It has been found that differences in both physical and occupational therapist staffing levels produce differences with respect to quality. Those facilities with the highest PT and OT staffing levels were, on average, associated with more favorable performance scores, compared to those facilities with lower staffing levels. Studies have found that PT and OT staffing is significant in regard to fall prevention. Both disciplines perform environmental modifications, manage pain to reduce negative behavioral symptoms, and provide networks of support to educate patients on functioning capabilities which has been shown to reduce the incidence of falls (Livingstone et al., 2018).
Leadership
Leadership is essential for the achievement of goals related to quality care and patient safety. Safety leadership in healthcare must be encouraged at all levels in an organization, from the bedside to the executive office. Inefficient, invisible leadership is a significant cause of adverse patient outcomes.
Effective leadership is necessary for communication, teamwork, situation awareness, workload management, error management, decision-making, and human performance, all of which determine quality of care. Open communication between professionals, regardless of their occupation, is also critical for the safety of patient care (Murray & Cope, 2021).
Leaders in healthcare have many responsibilities, including:
- Instilling a culture of safety
- Assessing, reducing, mitigating, and managing safety risk in a caring environment
- Maintaining a safe patient allocation based on the acuity and skill mix of the clinicians
- Evaluating team performances
- Sharing and educating patient safety measures
- Managing risky behaviors among staff
Healthcare leaders have a direct impact on the climate based on their commitment to a culture of safety that includes:
- Communication
- Fostering teamwork
- Productivity
- Scheduling
- Recognition of clinicians’ achievements that support patient safety
- Promoting a learning culture
Orientation, regular in-service training programs, and unit-specific competency training should be offered to empower clinicians to assess their own learning needs and practice safely (Haskins & Roets, 2022).
CONCLUSION
Everyone has a stake in the safety of the healthcare system—healthcare workers as well as the general public. In the past, patient safety was not a traditional part of the education of most healthcare workers, but today this is no longer true. All healthcare workers are being actively educated about their roles in the prevention of avoidable negative outcomes for those they care for. It is essential that all clinicians understand the journey every patient makes through the system, recognizing how the system can fail, and take action to prevent those failures.
To counter errors and safeguard patients, changes must continue to be made in how the workforce is deployed; in how work processes are designed; and in the leadership, management, and culture of healthcare organizations. Because communication issues are so commonly involved in medical errors, it is crucial that physicians, nurses, therapists, and other healthcare personnel work together as a team, respecting each other’s contributions to the well-being of the patients in their care. Collaborative teamwork is essential for optimizing quality and safety in healthcare.
RESOURCES
AGS Beers Criteria (American Geriatrics Society)
Error-prone abbreviations, symbols, and dose designations (Institute for Safe Medication Practices)
Institute for Healthcare Improvement
List of high-alert medications (Institute for Safe Medication Practices)
National Coordinating Council for Medication Error Reporting and Prevention
REFERENCES
NOTE: Complete URLs for references retrieved from online sources are provided in the PDF of this course.
Accreditation Association for Ambulatory Health Care (AAAHC). (2024). Raise your standard of care. https://www.aaahc.org
Agency for Healthcare Research and Quality (AHRQ). (2023). Preventing falls in hospitals. https://www.ahrq.gov
Agency for Healthcare Research and Quality (AHRQ). (2021). Medication administration errors. http://psnet.ahrq.gov
Agency for Healthcare Research and Quality (AHRQ). (2019). Adverse events, near misses, and errors. https://psnet.ahrq.gov
Aliouche H. (2022). Heuristic decision making in medicine. https://www.news-medical.net
American Hospital Association (AHA). (2024). American Hospital Association patient initiative goals. https://www.aha.org
American Occupation Therapy Association (AOTA). (2021). Occupational therapy and prevention of falls. https://www.aota.org
American Physical Therapy Association (APTA). (2023). Physical therapy guide to falls. https://www.choosept.com
Appeadu M & Bordoni B. (2023). Falls and falls prevention in older adults. https://www.ncbi.nlm.nih.gov
Binghui J & Weijiang Y. (2024). Prevention and control of hospital-acquired infections with multidrug-resistant organism: A review. Medicine, 103(4), e37018.
Boisvert S & Pellett J. (2022). Patient safety in dentistry: Managing adverse events in the practice setting. https://www.thedoctors.com
Buetti N, Marschal J, Drees M, et al. (2022). Strategies to prevent central line-associated bloodstream infection in acute-care hospitals: 2022 update. Infect Control Hosp Epidemiol, 43(5), 553–69. https://doi.org/10.1017/ice.2022.87
Caetano B. (2024). FDA Medical device classification: Classes, examples. https://www.simplerqms.com
Carr B. (2022). Skill, rule, and knowledge models. https://www.taproot.com
Carver N, Gupta V, & Hipskind. (2023). Three ways to prevent medical errors. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
Catarino F, Lourenco C, Correia C, et al. (2022). Nursing care in peripheral intravenous catheters (PIV): Protocol of a best practice implementation project. Nurs Rep, 12(3), 515–9.
Centers for Disease Control and Prevention (CDC). (2023a). Show me the science. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2023b). Facts about falls. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2023c). STEADI—older adult fall prevention. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2023d). Understanding health literacy. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2023e). Safety culture in healthcare settings. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2022). Multi-drug-resistant organisms (MDROs). https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021). Strategies to prevent Clostridioides difficile infection in acute care facilities. https://www.cdc.gov
Centers for Medicare and Medicaid Services (CMS). (2023a). Hospital-acquired conditions. https://www.cms.gov
Centers for Medicare and Medicaid Services (CMS). (2023b). QAPI description and background. https://www.cms.gov
Claros E. (2021). Impact of language barriers on patient safety. http://rn-journal.com
Cleveland Clinic. (2024). C. diff (Clostridioides difficile) infection. https://my.clevelandclinic.org
Delong C & Preuss C. (2023). Box warning. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
Duke University Medical Center. (2023). Evidence-based practice. https://guides.mclibrary.duke.edu
Erikson E, Edelman D, Brewster F, et al. (2023). The use of checklists in the intensive care unit: A scoping review. Critical Care, 27(1), 468.
Fekete T. (2023). Catheter-associated urinary tract infection in adults. UpToDate. https://www.uptodate.com
Haddad L, Annamarju P, & Toneye-Butler T. (2023). Nursing shortage. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
Hanson A & Haddad L. (2023). Nursing rights of medication administration. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
Haskins J. (2019). 20 years of patient safety. Association of American Medical Colleges (AAMC). https://www.aamc.org
Haskins HEM & Roets L. (2022). Nurse leadership: Sustaining a culture of safety. Health SA Gesondheid, 27, 2009. https://doi.org/10.4102/hsag.v27i0.2009
HIPAA Journal. (2021). Communication tools in nursing. https://www.hipaajournal.com
Hoel R, Giddings Connolly R, & Takahashi P. (2021). Polypharmacy management in older adults. Mayo Clin Proc, 96(1), 242–56.
Institute for Healthcare Improvement (IHI). (2022). National action plan to advance patient safety. https://www.ihi.org
Institute for Healthcare Improvement (IHI). (2021). SBAR tool: Situation-background-assessment-recommendation. http://www.ihi.org
Institute for Safe Medication Practices (ISMP). (2024). High-alert medications in acute care settings. https://www.ismp.org
Institute for Safe Medication Practices (ISMP). (2023). Look-alike drug names with recommended tall man (mixed case) letters. https://www.ismp.org
Institute of Medicine (IOM). (1999). To err is human: Building a safer health system. National Academies Press.
Jacob J & Gaynes R. (2020). Intravascular catheter-related infection: Prevention. UpToDate. https://www.uptodate.com
Jaklevic M. (2023). “Medical errors are the third leading cause of death” and other statistics you should question. https://healthjournalism.org
Jarry J. (2021). Medical error is not the third leading cause of death. https://www.mcgill.ca
Kamakshya P & De Jesus O. (2023). Sentinel event. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
Keenan S. (2021). How do occupational therapists contribute to patient safety? https://www.pslhub.org
Kiel D. (2023). Falls in older persons: Risk factors and patient evaluation. UpToDate. https://www.uptodate.com
Kolikof K, Peterson L, & Baker A. (2023). Central venous catheter. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
Language Scientific. (2020). A quick primer on Affordable Care Act language. https://languagescientific.com
Li S, Xiao X, & Zhang X. (2023). Hydration status in older adults: Current knowledge and future challenges. Nutrients, 15(11), 2609. https://doi.org/10.3390/nu15112609
Liu J, Yu Y, Yan S, et al. (2023). Risk factors for self-reported medication adherence in community-dwelling older patients with multimorbidity and polypharmacy: A multicenter cross-sectional study. BMC Geriatrics, 23, 75.
Livingstone I, Hefele J, Nadash P, Barch D, & Leland N. (2018). Examining the effects of professional non-nursing staffing in quality of care in nursing homes. Innovation in Aging, 2(supp l), 536. https://doi.org/10.1093/geroni/igy023.1979
Loria K. (2023). Technology trends to prevent prescription errors. https://www.drugtopics.com
MacDowell P, Cabri A, & Davis M. (2021). Medication administration errors. https://psnet.ahrq.gov
Mattison M. (2024). Hospital management of older adults. UpToDate. https://www.uptodate.com
Maximous R, Wong J, Chung F, & Abrishami A. (2021). Interventions to reduce medication errors in anesthesia: A systematic review. Can J Anesth/J Can Anesth, 68(6), 880–93. https://doi.org/10.1007/s12630-021-01959-7
Murray M & Cope V. (2021). Leadership: Patient safety depends on it! Collegian, 28(6), 604–9.
Murray S, Clifford J, Larson S, et al. (2023). Implementing just culture to improve patient safety. Military Medicine, 188(7–8), 1596–9.
National Healthcare Safety Network (NHSN). (2024). Bloodstream infection event (central line-associated bloodstream infection and non-central line associated bloodstream infection). https://www.cdc.gov
National Practitioner Data Bank (NPDB). (2023). Public use data file. https://www.npdb.hrsa.gov
National Quality Forum (NQF). (2024). List of SREs. https://www.qualityforum.org
Nurses Service Organization (NSO). (2023). Do’s and don’t’s of documentation. https://www.nso.com
Office of Disease Prevention and Health Promotion (ODPHP). (2021). Health literacy in Healthy People 2030. https://health.gov
Patel P, Advani S, Kofman A, et al. (2023). Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2022 update. Infection Control & Hospital Epidemiology, 44, 1209–31.
Patient Safety Movement Foundation (PSMF). (2021). Commitment FAQs. https://patientsafetymovement.org
Patil SJ, Ambulkar R, Kulkarni AP. (2023) Patient safety in intensive care unit: What can we do better? Indian J Crit Care Med, 27(3) 163–5.
Performance Health Partners. (2024). How to avoid near miss events in healthcare. https://www.performancehealthus.com
Phillips J, Malliaris A, & Bakerjian D. (2021). Nursing and patient safety. https://psnet.ahrq.gov
Raffel K & Ranji S. (2023). Diagnostic errors. UpToDate. https://www.uptodate.com
Rodziewicz T, Houseman B, & Hipskind J. (2023). Medical error reduction and prevention. Publishing. https://www.ncbi.nlm.nih.gov
Sameera V, Bindra A, & Rath G. (2021). Human errors and their prevention in healthcare. J. Anaesthesiol Clin Pharmacol, 37(3), 328–35.
Santos G & Jones M. (2023). Prevention of surgical errors. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
Seabra A, De Souza A, Artioli R, Tabaytayan R, et al. (2023). Unintentionally retained foreign objects (URFOs). J Patient Saf Risk Manag, 6, 25160435231185437.
Seidelman J, Mantyh C, & Anderson D. (2023). Surgical site infection prevention. JAMA, 329(3), 244–52.
Shaked P. (2024). Most common anesthesia errors and complications. https://prosperlaw.com
Shebell & Shebell. (2023). Never events: Preventable medical errors that should never happen. https://www.shebell.com
Shebl E & Gulick P. (2023). Nosocomial pneumonia. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
Singhal H. (2023). What are the CDC guidelines for prevention of surgical site infection (SSI)? Medscape. https://www.medscape.com
Sirihorachai R, Saylor K, & Manojlovich M. (2022). Interventions for the prevention of retained surgical items: A systematic review. World J Surg, 46(2), 370–81.
Skelly C, Cassagnol M, & Munakomi S. (2022). Adverse events. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
Society for Healthcare Epidemiology of America (SHEA). (2022). New guidance released for preventing hospital-acquired pneumonia. https://shea-online.org
Society to Improve Diagnosis in Medicine (SIDM). (2024a). Factors in diagnostic error. https://www.improvediagnosis.org
Society to Improve Diagnosis in Medicine (SIDM). (2024b). Improving diagnosis. https://www.improvediagnosis.org
Srinivasan A, Naidu V, & Dhivya P. (2023). Arterial line placement using modified Seldinger technique: A novel approach. Indian J Crit Care Med, 27(7), 515–6. https://www.ncbi.nlm.nih.gov
Steris Healthcare. (2024). What are retained surgical items? https://www.steris.com
Tariq R & Sharma S. (2023). Inappropriate medical abbreviations. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
Tariq R, Vashisht R, Sinha A, & Scherbak Y. (2023). Medication dispensing errors and prevention. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
The Joint Commission (TJC). (2024a). Sentinel event policy and procedures. https://www.jointcommission.org
The Joint Commission (TJC). (2024b). The universal protocol. https://www.jointcommission.org
The Joint Commission (TJC). (2024c). Who we are. https://www.jointcommission.org
The Joint Commission (TJC). (2024d). National patient safety goals. https://www.jointcommission.org
The Joint Commission (TJC). (2024e). Do not use list fact sheet. https://www.jointcommission.org
The Joint Commission (TJC). (2023). Sentinel event data 2022 annual review. https://www.jointcommission.org
The Joint Commission (TJC). (2021a). Speak up campaigns. https://www.jointcommission.org
The Joint Commission (TJC). (2021b). Preventing pediatric medication errors. https://www.jointcommission.org
The Joint Commission (TJC). (2021c). Quick Safety 13: Overcoming the challenges of providing care to limited English proficient patients. https://www.jointcommission.org
The Joint Commission (TJC). (n.d.). Framework for root cause analysis and corrective actions. https://www.jointcommission.org
Turner K, Staggs V, Potter C, et al. (2022). Fall prevention practices and implementation strategies: Examining consistency across hospital units. J Patient Saf, 18(1), e236–42. https://www.ncbi.nlm.nih.gov
U.S. Department of Veterans Affairs (VA). (2023). Glossary of patient safety terms. https://www.patientsafety.va.gov
U.S. Food and Drug Administration (FDA). (2023a). Examples of medical device misconnections. https://www.fda.gov
U.S. Food and Drug Administration (FDA). (2023b). Medical device reporting (MDR): How to report medical device problems. https://www.fda.gov
U.S. Food and Drug Administration (FDA). (2021). Reclassification. https://www.fda.gov
Victoria Department of Health (VDH). (2021). Clinical incident investigations—Root cause analysis. https://www.health.vic.gov
Vogenberg AJ. (2004). A fresh look at digoxin monitoring. Pharmacy Times. http://www.pharmacytimes.com
Wisconsin Department of Health Services (WIDHS). (2022). Multi-drug-resistant organisms (MDROs). https://www.dhs.wisconsin.gov
Wong M. (2022). PCA safety checklist. https://ppahs.org
World Health Organization (WHO). (2024). WHO surgical safety checklist. https://www.who.int/teams/integrated-health-services/patient-safety/research/safe-surgery/tool-and-resources
World Health Organization (WHO). (2023). Patient safety. https://www.who.int/news-room/fact-sheets/detail/patient-safety
World Health Organization (WHO). (2021). Global patient safety action plan 2021–2030. https://www.who.int/publications/i/item/9789240032705
Zingg W, Barton A, Bitmead J, et al. (2023). Best practice in the use of peripheral venous catheters: A scoping review and expert consensus. Infect Prev Pract, 5(2), 100271.





Customer Rating
4.9 / 538 ratings