Infection Control Training for New York State Healthcare Professionals
Online Continuing Education Course

Course Description
NEW YORK STATE MANDATED INFECTION CONTROL COURSE. NYSED- and NYSDOH-approved 4-contact-hour training covers infection prevention, infection control practices and procedures, barriers, PPE, safe environment principles, and preventing transmission of infectious disease to and from healthcare workers. Must be taken every four years. Bestseller course!
Course Price: $40.00
Contact Hours: 4
Course updated on
December 5, 2023
"I have been a practicing RN in NYS for over 45 years. This material provided an excellent review of the basic practices and principles required for a practicing nurse. Well done!" - Adrienne, RN in New York
"The overall structure of your online CEU program is very versatile, allowing me to download all material and study it at my convenience. Being able to take the test between my professional, academic, and family responsibilities sold me at once on your product. Thank you!" - Joa, Physician in New York
"I had a great experience with this course. It reminded me of the things I had forgotten over time." - Francisca, LPN in New York
"It was an excellent training course!" - Rosemarie, RN in New York
Infection Control Training for New York State Healthcare Professionals
Copyright © 2023 Wild Iris Medical Education, Inc. All Rights Reserved.
LEARNING OUTCOME AND OBJECTIVES: Upon completion of this course, you will have increased your knowledge of current, evidence-based information on preventing and controlling the spread of infection. Specific learning objectives to address potential knowledge gaps include:
- Summarize the principles and practices of infection prevention and control.
- Describe the modes and mechanisms of the transmission of pathogenic organisms, including the chain of infection.
- Discuss engineering and work practice controls designed to reduce patient and healthcare worker exposure to infectious materials.
- Identify barriers and personal protective equipment for protection from exposure to potentially infectious material.
- Describe the principles and practices for cleaning, disinfection, and sterilization in the healthcare environment.
- Identify occupational health strategies for preventing the transmission of bloodborne and other pathogens to and from healthcare workers.
- Recognize suspected sepsis and methods to prevent it.
TABLE OF CONTENTS
- The Need for Infection Prevention and Control Practices
- Infection Control Principles and Practices
- Methods for Preventing the Spread of Pathogens in Healthcare Settings
- Engineering and Work Practice Controls
- Barriers and Personal Protective Equipment
- A Safe Environment: Cleaning, Disinfecting, and Sterilizing
- Infectious Diseases and Occupational Health Strategies
- Sepsis
- Conclusion
- Resources
- References
THE NEED FOR INFECTION PREVENTION AND CONTROL PRACTICES
Infection control was born in the mid-1800s when Ignaz Semmelweis, a Hungarian obstetrician, demonstrated that handwashing could prevent infection. Semmelweis was director of two obstetrical clinics, one staffed by medical students, the other by midwives. Disturbed by the fact that the maternal mortality rate from puerperal (postpartum) fever in the clinic staffed with medical students was almost six times greater than in the clinic staffed by midwives, he set about analyzing the difference and found that medical students often performed dissection prior to assisting with deliveries without washing their hands.
Semmelweis came to the conclusion that the medical students performing dissection (which midwives did not do) were carrying some invisible poisonous material on their hands to the women they were assisting in the delivery room, and he instituted a policy requiring medical students to wash their hands in a solution of chlorinated lime prior to assisting in any obstetrical procedure. As a result of this practice, the mortality rate dropped from 18.27% to 1.27% in the medical students’ clinic, and in a period of two months, the death rate dropped to zero (Zoltán, 2023).
Later in that same century, Florence Nightingale described the relationship between the diseases that were killing her patients during the Crimean War and the conditions in which they were cared for. Nightingale instituted ways to improve overall hygiene through clean clothing and dressings, bathing, and supplying adequate nutrition. These measures helped prevent contamination and led to reductions in infections and only a 2% mortality rate. Her greatest influence has been on hospital infection control, and many modern healthcare practices (e.g., isolation, ventilation, routine cleaning, and medical and human waste disposal) are attributed to her (Selanders, 2023).
Today, we know about pathogenic microorganisms and how they are transmitted, and we have a great deal of knowledge of the principles of infection control. Despite these advances, preventable infections continue to occur. Every year the five most common healthcare-associated infections (HAIs) in U.S. hospitals cost $9.8 billion, with surgical site infections the leader. Healthcare costs from HAIs occur in every medical department, including the intensive care unit (Monegro et al., 2023). Healthcare providers clean their hands less than they should, despite it being known that hand hygiene is the most effective way to prevent HAIs (CDC, 2023a).
This discussion indicates that infection control is not just a matter of knowing what is effective but that there is a strong behavioral element involved in the process of carrying out infection control practices. Both factors must be addressed if the absence of HAIs is the goal. To accomplish this, each healthcare worker should have the necessary knowledge, skills, and abilities to implement effective infection control practices, which then may influence their perceptions and provide motivation to change behavior.
TERMINOLOGY
- Healthcare-associated infection (HAI)
- An HAI is an infection acquired while receiving healthcare in any setting (e.g., hospital, long-term care facility, outpatient clinic, ambulatory setting, assisted living, or home care). These infections occur in patients who do not have infections and are not incubating an infection at the time of entry into the healthcare system but acquire them while receiving treatment for other conditions. Healthcare workers also can be the recipients of HAIs. Other common terms for HAIs are nosocomial (originating in a hospital) and iatrogenic (caused by medical treatment) (Monegro et al., 2023).
- Healthcare worker (HCW)
- Any person who has contact with patients, body fluids, or supplies used for patient care as part of their job. This includes physicians, nurses, occupational therapists, and physical therapists as well as administrative, environmental hygiene, and laboratory staff in medical facilities. HCWs also include interns, volunteers, and paid workers/employees who are involved in any aspect of healthcare in any setting. All HCWs are engaged in enhancing patient health (WHO, 2023a).
- Outbreak
- An outbreak is a sudden increase in the occurrence of a particular infectious disease from person to person or from an animal reservoir or other environmental source in a particular place and time. An epidemic is an outbreak in which a disease is actively spreading over a wide geographic area and affecting a high proportion of the population. A pandemic is an epidemic that has spread to multiple countries or regions of the world (WHO, 2023b).
- Surveillance
- Surveillance is the continuous, systematic collection, analysis, and interpretation of health-related data needed for the planning, implementation, and evaluation of public health practice. Monitoring and evaluating the impact of interventions occurs. It can serve as an early warning system for impending public health emergencies, document the impact of an intervention, or track progress toward specific goals (WHO, 2023c).
Healthcare-Associated Infections (HAIs)
The rate of HAIs is 1 in 31 patients in acute care hospitals and 1 in 43 patients in long-term care facilities (CDC, 2022a). In the United States, the Centers for Disease Control and Prevention (CDC) estimates that HAIs account for an estimated 1.7 million infections and 99,000 associated deaths each year.
COMMON TYPES OF HAIs
- Catheter-associated urinary tract infections (CAUTIs). These infections involve any part of the urinary system, including urethra, bladder, ureters, and kidneys, and result from incorrect insertion, failure to maintain asepsis, and leaving a catheter in place for too long. These make up 32% of all HAIs.
- Surgical site infections (SSIs). These can involve the skin, tissues, and organs under the skin, or implants such as material inserted or grafted into the body (e.g., prosthetic joints). These constitute 22% of HAIs.
- Central line–associated bloodstream infections (CLABSIs). These are bloodstream infections unrelated to an infection at another site that develops within 48 hours of central line placement. Of all HAIs, these are associated with increased care costs and mortality. These and other bloodstream infections add up to 14% of HAIs.
- Ventilator-associated events (VAEs) or pneumonias (VAPs). These events or pneumonias are caused by a wide variety of pathogens, can be polymicrobial, and can be due to multidrug-resistant organisms. These are 15% of all HAIs in the United States.
(Patient CareLink, 2023)
The impact of HAIs may be greater when they are due to drug-resistant organisms, which include:
- Methicillin-resistant Staphylococcus aureus (MRSA). This type of bacteria is resistant to many antibiotics.
- Clostridioides difficile (C. difficile) (formerly known as Clostridium difficile). When antibiotics are taken, “good” bacteria are destroyed for several months, during which time infection with C. difficile bacteria can cause life-threatening diarrhea.
- Carbapenem-resistant Enterobacteriaceae (CRE). This family of organisms, which includes Escherichia coli (E. coli) and Klebsiella pneumoniae, has a high level of antibiotic resistance.
(Monegro et al., 2023)
The improvement in reducing HAIs is mixed, depending on the type of infection being measured. The United States has made significant progress toward the collective goal of eliminating some HAIs and is safer now than it was in years past.
Cases of C. difficile decreased by 50% between 2015 and 2021. Other HAIs saw a 7%–21% reduction in in the same time period (CDC, 2021a). However, increases were documented for MRSA at 14%, VAEs at 12%, SSI-hysterectomy at 11%, CLABSIs at 7%, and CAUTIs at 5% between 2020 and 2021 (CDC, 2022c).
The presence of the COVID-19 virus also contributed to an unusual increase in infection rates. This new virus brought patients with existing central lines and urinary catheters into the hospital and/or caused newly immunocompromised patients to acquire these tubes as well as endotracheal tubes while hospitalized.
HAIs IN OUTPATIENT SETTINGS
Increasingly, healthcare delivery, including complex procedures, is being shifted to outpatient (ambulatory) settings. These settings often have limited capacity for oversight and infection control compared to hospital-based settings. Because patients with HAIs, including those caused by antibiotic-resistant organisms, often move between various types of healthcare facilities, prevention efforts must expand across the continuum of care.
Examples of outpatient settings include:
- Medical group practices
- Clinics at hospitals or other facilities
- Surgery centers
- Imaging centers
- Mental health centers
- Lab centers
- Physical therapy and rehabilitation facilities
- Chemotherapy and radiation therapy centers
- Dialysis centers
- Birthing centers
- Hospice homes
- Home care
Surveillance for infection in outpatient or ambulatory settings is inherently difficult, as detecting infections among outpatients typically requires retrospective reviews of medical records and/or prospective audits. However, intelligent information technology may serve as a meaningful tool. Such automated systems can be used to perform prospective surveillance for infections following outpatient procedures, such as a reference database designed to document SSIs in ambulatory surgery and linking institutional databases to detect bloodstream infections (Anderson & Kanafani, 2020; AHRQ, 2023).
ACCREDITATION FOR AMBULATORY HEALTHCARE FACILITIES
The Centers for Medicare and Medicaid Services has granted several organizations, along with itself, the authority to determine whether or not ambulatory healthcare facilities are in compliance with Medicare’s conditions for coverage and to provide accreditation for them. While there is no federal requirement for accreditation, some states and private payers require it. Organizations granted authority to accredit include:
- Accreditation Association for Ambulatory Health Care
- Accreditation Commission for Health Care, Inc.
- American Osteopathic Association/Healthcare Facilities Accreditation Program
- Center for Improvement in Healthcare Quality
- Community Health Accreditation Partner
- DNV GL-Healthcare
- National Dialysis Accreditation Commission
- The Compliance Team
- The Joint Commission
(CMS, 2020)
HAIs IN LONG-TERM CARE FACILITIES
Long-term care settings include nursing homes, skilled nursing facilities, and assisted living facilities. Over 4 million Americans are admitted to or live in nursing homes and skilled nursing facilities each year, and nearly 1 million live in assisted living facilities. While reporting is limited, the CDC (2020) provides the following data about infections in these facilities:
- 1 to 3 million serious infections occur each year.
- Infections include urinary tract infections, diarrheal diseases, antibiotic-resistant staph infections, and many others.
- Infections are a major cause of hospitalization and death.
As many as 380,000 people die of infections in long-term care facilities every year.
Development of Infection Control and Prevention Standards and Guidelines
Standards and guidelines are designed to proactively prevent the spread of infection in healthcare settings. The development of these standards and guidelines came about through the collaborative efforts of the Centers for Disease Control and Prevention, the Joint Commission, the World Health Organization, and the Occupational Safety and Health Administration.
Significant infection control challenges include:
- SARS-CoV-2 (COVID-19)
- SARS-CoV-1 (severe acute respiratory syndrome)
- HIV infection
- Lyme disease
- Escherichia coli
- Hantavirus
- Dengue fever
- West Nile virus
- Zika virus
Reemerging infectious diseases include:
- Tuberculosis
- Pertussis
- Influenza
- Pneumococcal disease
- Malaria
- Cholera
- Gonorrhea
Standards and guidelines for these and other infectious diseases include vaccinations, emergency medications, preventive care, and information learned about the possible risks to avoid or to prepare for when traveling (Johns Hopkins, 2023).
New York State Infection Control Requirements
Title 10, part 92, chapter 785, of the Official Compilation of Codes, Rules, and Regulations of New York established a requirement that certain healthcare professionals licensed in New York State must receive approved training in infection control and barrier precautions every four years unless otherwise exempted.
NEW YORK STATE PROFESSIONS REQUIRING INFECTION CONTROL TRAINING
- Dental hygienists
- Dentists
- Licensed Practical Nurses
- Registered Professional Nurses
- Medical residents
- Medical students
- Optometrists
- Physicians
- Physician assistants
- Physician assistant students
- Podiatrists
- Specialist assistants
The goal of New York State’s mandate is to:
- Assure that licensed, registered, or certified health professionals understand how bloodborne pathogens may be transmitted in the work environment from patient to healthcare worker, healthcare worker to patient, and patient to patient
- Apply current scientifically accepted infection prevention and control principles as appropriate for the specific work environment
- Minimize opportunity for transmission of pathogens to patients and healthcare workers
- Familiarize professionals with the law requiring this training and the professional misconduct charges that may result from failure to comply with the law
CORE ELEMENTS OF NEW YORK STATE INFECTION CONTROL TRAINING
Element I
Healthcare professionals have the responsibility to adhere to scientifically accepted principles and practices of infection control in all healthcare settings and to oversee and monitor the performance of those ancillary personnel for whom the professional is responsible.
Element II
Modes and mechanisms of transmission of pathogenic organisms in the healthcare setting and strategies for prevention and control
Element III
Use of engineering and work practice controls to reduce the opportunity for patient and healthcare worker exposure to potentially infectious material in all healthcare settings
Element IV
Selection and use of barriers and/or personal protective equipment for preventing patient and healthcare worker contact with potentially infectious material
Element V
Creation and maintenance of a safe environment for patient care in all healthcare settings through application of infection control principles and practices for cleaning, disinfection, and sterilization
Element VI
Prevention and control of infectious or communicable diseases in healthcare workers
Element VII
Sepsis awareness and education
(NYSDOH & NYSED, 2018)
This course explores each of the seven elements in detail, covering the basic principles of infection control and evidence-based infection control practices.
INFECTION CONTROL PRINCIPLES AND PRACTICES
ELEMENT I
Healthcare professionals have the responsibility to adhere to scientifically accepted principles and practices of infection control and to oversee and monitor the performance of those ancillary personnel for whom the professional is responsible.
Infection control practices can stop the spread of infection in healthcare settings.
Title 10, part 92 (Health), of the Official Compilation of Codes, Rules, and Regulations of New York, and the Rules of the Board of Regents, Part 29.2(a)(13), identify professionals who must receive infection control training, and defines unprofessional conduct in the area of infection prevention and control as the failure to use scientifically accepted infection control practices to prevent transmission of disease pathogens as appropriate to each profession, including:
- Cleaning and sterilization or disinfection of instruments, devices, materials, and work surfaces
- Utilization of personal protective equipment (PPE)
- Use of covers for contamination-prone equipment
- Safe handling of sharp instruments (“sharps”)
The National Institute for Occupational Safety and Health is the federal agency that provides scientifically sound infection control recommendations. Other professional organizations and accrediting agencies that provide guidelines, standards, and recommended practices for infection prevention and control in healthcare settings include:
- The Joint Commission
- American Hospital Association
- Association for Professionals in Infection Control and Epidemiology
- Society for Healthcare Epidemiology of America
- Centers for Disease Control and Prevention
- Det Norske Veritas Healthcare, Inc.
Consequences for Unprofessional Conduct
The consequences of failure to follow accepted standards of infection prevention and control include:
- Increased risk of adverse health outcomes for patients and healthcare workers
- Charges of professional misconduct
Patients, family members, or coworkers can file charges against a healthcare professional through their institution or directly to the New York State Department of Health, Office of Health Systems Management, which will investigate the complaint. Depending on the severity of misconduct, outcomes may include:
- Disciplinary action
- Revocation of professional license
- Professional liability
To be in compliance in order to avoid charges of unprofessional conduct, designated healthcare professionals must:
- Participate in required infection prevention and control training
- Adhere to accepted principles and practices of infection prevention and control
MODES AND MECHANISMS OF PATHOGEN TRANSMISSION: THE CHAIN OF INFECTION
ELEMENT II
Modes and mechanisms of transmission of pathogenic organisms in the healthcare setting and strategies for prevention and control
Epidemiology involves knowing how disease spreads and how it can be controlled. Infection can only spread when conditions are right. This set of conditions is referred to as the chain of infection, which consists of six links. When all the links are connected, infection spreads. Infection control and prevention training provides the knowledge and skills that healthcare professionals can use to break the links in the chain and prevent the occurrence of new infections. Thus, understanding the chain of infection is at the foundation of infection prevention (Infection Prevention and You, 2023).
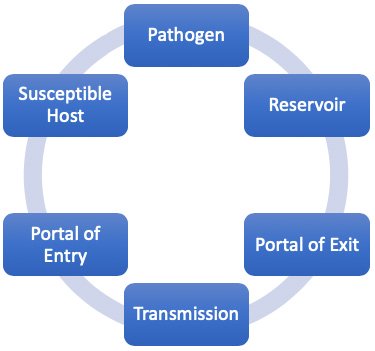
The six links in the chain of infection.
(Source: Wild Iris Medical Education, Inc.)
| Link | Examples |
|---|---|
| (Potter et al., 2023) | |
| 1. Pathogen: Microorganisms capable of causing disease or illness |
|
| 2. Reservoir: Places in which infectious agents live, grow, and reproduce |
|
| 3. Portal of exit: Ways in which an infectious agent leaves the reservoir |
|
| 4. Transmission: Ways in which the infectious agent is spread from reservoir to susceptible host |
|
| 5. Portal of entry: Ways in which the infectious agent enters the susceptible host |
|
| 6. Susceptible host: Individuals with traits that affect their susceptibility and severity of disease | Persons with:
|
Pathogens
A pathogen is any biological agent that can cause disease or illness in its host.
- Bacteria are single-celled organisms present everywhere, some of which can cause disease.
- Viruses are intracellular parasites, that is, they can only reproduce inside a living cell.
- Fungi are prevalent throughout the world, but only a few cause disease in healthy people.
- Prions are a form of infectious protein. Like viruses, prions are not considered living.
- Protozoa are single-celled microorganisms that are larger than bacteria.
- Helminths include roundworms, tapeworms, and flukes.
(OSU, 2020; CDC, 2022b)
Reservoirs
The next link in the chain of infection is the reservoir, the usual “habitat” in which the infectious agent (pathogen) lives and multiplies. It is defined as any person, animal, arthropod, plant, soil, substance, or combination of these on which the pathogen depends primarily for survival and where it reproduces itself in such a way that it can be transmitted to a susceptible host.
A reservoir, however, is not the same thing as the “source.” For example, in typhoid fever, the reservoir may be a person with the infection, but the source of infection may be the feces or urine of those who are infected or contaminated food, milk, or water (Potter et al., 2023).
HUMAN RESERVOIRS
The most important reservoir of infection for humans is other humans, and humans are also the most important reservoir for HAIs. The nose (nostrils, nares) may harbor bacteria and viruses. The skin is another natural reservoir for yeast and bacteria, and both healthcare workers and patients may carry pathogenic MRSA and Staphylococcus on their skin. The gastrointestinal tract is a reservoir for many different types of organisms, including viruses, bacteria, bacterial spores, and parasites.
People who are sick can release microbes into the environment through infected body fluids and substances. For example, sneezing releases influenza virus in secretions from the respiratory tract. Coughing releases tuberculosis bacteria from the lungs. Diarrhea releases C. difficile and many pathogens from the bowel. Exudates from skin lesions release Staphylococcus in pus from boils or herpesvirus from fluid in sores around the mouth, hands, or other body areas.
Two types of human or animal reservoirs are generally recognized. These are (1) symptomatic persons who have a disease (cases) and (2) carriers who are asymptomatic and can still transmit the disease. Carriers are individuals who have been colonized by a pathogen. Colonization is the presence of a microorganism on or in a host with growth and multiplication of the organism but no clinical expression or immune response from the host.
There are five types of carriers:
- Healthy or passive carrier: A person exposed to and harboring a pathogen but who has not become ill or has no symptoms
- Active carrier: A person exposed to and harboring a pathogen who may or may not exhibit signs and symptoms of infection
- Incubatory carrier: A person exposed to and harboring a pathogen in the beginning stages of the disease (e.g., the incubation period for HIV can last for many years before symptoms occur, but the person is able to transmit HIV to others during that time period)
- Intermittent carrier: A person exposed to and harboring a pathogen who can spread the disease in different places or at different intervals
- Convalescent carrier: A person who harbors the pathogen and, although in the recovery phase, is still infectious
(Merrill, 2021)
The important point to remember is that infectious agents are transmitted every day from people who are sick as well as from those who appear to be healthy. In fact, those individuals who are carriers may present more risk for disease transmission than those who are sick because:
- They are not aware of their infection.
- Their contacts are not aware of their infection.
- Their activities are not restricted by illness.
- They do not have symptoms and, therefore, do not seek treatment.
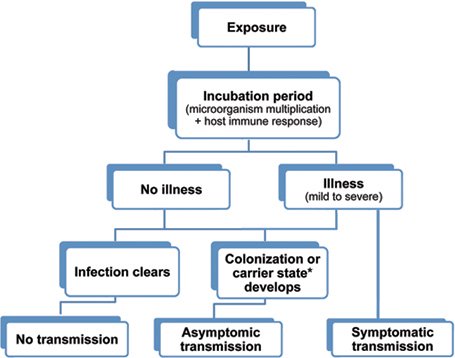
Possible outcomes of exposure to an infectious agent.
(Source: Wild Iris Medical Education, Inc.)
* The term carrier state is used to describe the presence of a microorganism on or in a host with growth and multiplication of the organism but no clinical expression or immune response from the host.
ANIMAL/INSECT RESERVOIRS
Animal reservoirs transmit infectious diseases from animal to animal, with humans as incidental hosts. An infectious disease caused by a pathogen transmitted from birds, rodents, reptiles, amphibians, insects, and other domestic and wild animals is called a zoonosis. A common way for these pathogens to spread is via a vector, usually a bite from a mosquito, mite, or tick. Examples of zoonoses include:
- Rabies (from bats, skunks, raccoons, and other mammals, transmitted directly through a bite)
- SARS (believed to originate from horseshoe bats that transmitted the virus to small mammals called civets, which are trapped and eaten, exposing humans to blood or organs during butchering or food preparation; now capable of being transmitted from human to human in respiratory secretions)
- Lyme disease (from deer mice to deer ticks to humans)
- Bubonic plague (from rats or prairie dogs to fleas to humans)
- West Nile virus (from birds to mosquito to humans)
- Zika (primarily from the Aedes aegypti mosquito to domestic animals and humans)
- Scabies (mites transmitted from humans to humans)
(Merrill, 2021)
ENVIRONMENTAL RESERVOIRS
Environmental reservoirs are certain environmental conditions or substances (e.g., food, feces, decaying organic matter) that are conducive to the growth of pathogens. Pathogens may survive in such a reservoir but may or may not multiply or cause disease. These reservoirs can include soil, water, and air, as well as inanimate objects, referred to as fomites, that convey infection because they have been contaminated by pathogenic organisms. Examples include facial tissues, doorknobs, telephones, bed linens, toilet seats, and clothing.
Environmental reservoirs in healthcare facilities can include:
- Contaminated medical devices (e.g., central venous catheters, urinary catheters, endoscopes, surgical instruments, ventilators, needles/sharps)
- Contaminated water sources
- Contaminated medications
- Air from heating, ventilation, or air conditioning systems
- Hospital textiles (e.g., linens, privacy curtains)
- Patient care equipment (blood pressure cuffs, gloves)
(Potter et al., 2023)
Portal of Exit
The portal of exit is the route (or routes) by which a pathogen leaves the reservoir.
| Portal | How the Pathogen Exits | Infectious Diseases |
|---|---|---|
| (Potter et al., 2023) | ||
| Respiratory tract | Coughing, sneezing | Influenza, tuberculosis, common cold, SARS, COVID-19 |
| Skin | Draining skin lesions or wounds | Scabies, staph infection, MRSA |
| Blood | Insect bite, needles, syringes | HIV/AIDS, hepatitis B, hepatitis C |
| Digestive tract | Feces, saliva | Hepatitis A, cholera, salmonella infection, parasites, typhoid |
| Genitourinary tract | Urine, semen, vaginal secretions | HIV/AIDS, herpes, cytomegalovirus |
| Placenta | Mother to fetus | Herpes, malaria, rubella |
Modes of Transmission
For an organism to travel from one person to another or from one place in the body to another it must have a way of getting there, or a mode of transmission. For any single agent, there are many different means by which it can be transmitted. The modes of HAI transmission include:
Contact transmission, the most important and frequent mode of HAI transmission, is divided into three subgroups: direct contact, indirect contact, and droplet.
- Direct contact transmission involves skin-to-skin direct contact and the physical transfer of pathogens between a susceptible host and an infected or colonized person. Examples include scabies, sexually transmitted diseases, mononucleosis, and MRSA. Direct contact can include:
- Childbirth
- Medical procedures
- Injections of drugs
- Airborne-propelled a short distance (3–6 feet) via droplets, coughing, or sneezing, and deposited on the host’s conjunctivae, nasal mucosa, or mouth
- Transfusion (blood)
- Transplacental
- Indirect contact transmission occurs by:
- Vehicle transmission involves contact of a susceptible host with a contaminated inanimate object (fomite), such as food, water, medications, contaminated instruments, patient-care equipment, needles, dressings, gloves, or hands. Contaminated hands are often responsible for transmission of HAIs.
- Vector-borne transmission usually refers to insects; however, a vector can be any living creature that transmits an infectious agent to humans. Vector-borne transmission is not a common source of HAIs.
- Airborne, long-distance transmission involves generation of aerosolized particles from droplet nuclei that remain infectious when suspended in air over long distances and time. Aerosolized particles may also be generated from biological waste products.
(Ather et al., 2020)
The mode of transmission is the weakest link in the chain of infection, and it is the only link that healthcare providers can hope to eliminate entirely. Therefore, a great many infection control efforts are aimed at avoiding carrying pathogens from the reservoir to the susceptible host.
Because people touch so many things with their hands, hand hygiene is still the single most important strategy for preventing the spread of infection.
HIGH-RISK SETTINGS FOR INFECTION TRANSMISSION
Every area of the healthcare facility and every type of patient care holds the potential for exposure to pathogens, but some settings and practices present greater risk than others. High-risk settings include:
- Intensive care units
- Burn units
- Pediatric units and newborn nurseries
- Operating rooms
- Long-term care facilities
Transmission risks within the various healthcare settings are influenced by the characteristics of the population (e.g., immunocompromised patients, exposure to indwelling devices and procedures), intensity of care, exposure to environmental sources, length of stay, and interaction among and between other patients as well as healthcare providers.
Portals of Entry
The term portal of entry refers to the anatomical route or routes by which a pathogen gains entry into a susceptible host. The portal of entry is often the same as the portal of exit from the reservoir.
| Portal | How the Pathogen Enters | Infectious Diseases |
|---|---|---|
| (Potter et al., 2023) | ||
| Skin | Conjunctivae, hair follicles, sweat ducts, cuts, nicks, abrasions, punctures, insect bites | Hookworm, tinea pedis, herpes simplex, folliculitis, sepsis |
| Respiratory tract | Inhalation | Influenza, tuberculosis, common cold, coronaviruses |
| Gastrointestinal tract | Food, drink, contaminated fingers | Diarrheal illnesses, salmonella infection, gastric and duodenal ulcers, gastroenteritis |
| Genitourinary tract | Skin or mucous membrane of penis, vagina, cervix, urethra, external genitalia | Cystitis, gonorrhea, chlamydia, genital herpes, HPV, HIV/AIDS |
| Across placenta to fetus | Vascular access | Zika, rubella, syphilis |
Medical and surgical procedures often introduce new portals or facilitate the entry of pathogens. Examples include IV catheters, surgical wounds, urinary catheters, endotracheal tubes, and percutaneous injuries. Healthcare workers may develop dermatitis from frequent handwashing or allergy to latex gloves. They may receive needlestick injuries that allow pathogens access to their bloodstream. Any invasive procedure may facilitate entry of pathogens into the host (Potter et al., 2023).
INVASIVE DEVICES
An invasive device provides a portal of entry for pathogens. It is a device that, in whole or part, penetrates inside the body either through a body orifice or through the body surface. Examples include:
- Vascular access devices
- Urinary catheters
- Wound drains
- Gastrostomy tubes
- Endotracheal or tracheostomy tubes
- Fracture fixation devices
- Traction pins
- Dental implants
- Joint prostheses
- Cardiac pacemakers
- Mammary implants
- Mechanical heart values
- Penile implants
Susceptible Host
The final link in the chain of infection is the susceptible host. In healthcare settings, susceptible hosts abound. Susceptibility to infections depends on genetic or constitutional factors, physiologic and immunological condition of the host, and virulence of the pathogen. Host factors that influence the outcome of an exposure include the presence or absence of natural barriers, the functional state of the immune system, and the presence or absence of an invasive device.
HOST NATURAL BARRIERS
There are many natural barriers against the penetration of pathogens into the human host. They are categorized as physical, mechanical, chemical, and cellular.
Lines of Defense
The first line of defense against the entry of pathogens includes physical, mechanical, and chemical barriers, which are considered functions of innate (natural or inborn) immunity.
- Physical barriers (or anatomical barriers) include the skin and associated accessories, such as nails and hair within the nose.
- Mechanical barriers include intact skin and mucous membranes. Coughing, sneezing, urinating, defecating, and vomiting are also mechanical barriers.
- Chemical barriers include tears, perspiration, sebum (oily substance produced by the skin), mucus, saliva, and gastrointestinal secretions. Tears contain active enzymes that attack bacteria. Mucus in the respiratory tract traps pathogens and contains enzymes that serve as antibiotics. The gastrointestinal tract contains various chemicals, including acid in the stomach.
The second line of defense comes into play when pathogens make it past the first line. Cellular defensive processes include:
- Phagocytes. Two types of white blood cells, macrophages and neutrophils, destroy and ingest pathogens that enter the body.
- Inflammation. Several types of white blood cells flood a localized area that has been invaded by pathogens, allowing for the removal of damaged and dead cells and beginning the repair process.
- Fever. Elevated temperature inhibits the growth of and is even lethal to some bacteria and viruses; it also facilitates the host’s immune response and increases the rate of tissue repair.
The third line of defense against invading pathogens is the immune system response, which involves lymphocytes.
- T cells send out an alarm and cause white blood cells to divide and multiply.
- B cells secrete antibodies that stick to antigens on the surface of pathogens and destroy them.
- Memory T and B cells store information about the invading pathogen to be used against a future invasion.
(InformedHealth.org, 2020)
The protective antibodies resulting from this process can be the result of:
- Past infection
- Vaccination or toxoid
- Transmission through the placenta from mother to child
- Administration of antitoxin or immunoglobulin
Factors Affecting a Host’s Natural Barriers
Several important factors affect a host’s susceptibility to infection:
- Age. The very young and the very old are more susceptible to infection. The older adult often has comorbid conditions such as diabetes, renal insufficiency, or a decrease in immune function, and the young do not as yet have an immune system as efficient as that of adults.
- Genetics. Genetic background causes variations in innate immunity, e.g., Alaska Natives, Native Americans, and Asians are more susceptible to tuberculosis than persons of other races/ethnicities.
- Stress level. Stress increases the release of cortisol from the adrenal cortex, causing a suppression of the inflammatory response, which facilitates infection.
- Nutritional status. The function of the cells that make up the first, second, and third lines of defense are dependent upon specific nutrients without which the system weakens.
- Current medical therapy. Patients undergoing chemotherapy or radiation are more susceptible to infections since these agents also destroy cells that make up the immune system. Transplant patients on immunosuppressant medications to prevent rejection are also more susceptible, as are patients taking corticosteroids.
- Preexisting disease. Patients with chronic diseases such as diabetes or AIDS are more susceptible.
- Sex. Anatomical differences of the genitourinary tract allow bacteria to more easily traverse the shorter female urethra to reach the bladder.
(JoVE, 2023)
INFECTIOUS AGENT FACTORS
It is only when a pathogen has been successful in establishing a site of infection in the host that disease occurs, and little damage will result if the pathogen is unable to spread to other parts of the body. There are a number of factors that are important in this process.
Specific to the pathogen itself are its:
- Infectivity, or the ability of an infectious agent to invade and replicate in a host
- Pathogenicity, or the capacity of the agent to cause disease
- Virulence, or the extent of disease that the pathogen can cause
Another important factor includes the number of organisms that are transferred to the host (the inoculum). Some organisms require only a few to cause disease, while others require many. The route of exposure, or the portal of entry of the pathogen, also influences the ability to cause infection, as does the duration, or amount of time the host is exposed to the pathogenic reservoir.
ENVIRONMENTAL FACTORS
Environmental factors are those extrinsic elements that affect the infectious agent and the opportunity for exposure. In a healthcare setting, these factors involve contamination of the environment and equipment.
Environmental contamination involves inanimate objects (fomites) such as air, water, food supply, floors, and surfaces around patients. Proper sanitation prevents the spread of infectious organisms from the environment to patients.
Contamination of equipment occurs when it is not cleaned and disinfected between patients. Equipment that has been contaminated can spread infectious agents from patient to patient.
METHODS FOR PREVENTING THE SPREAD OF PATHOGENS IN HEALTHCARE SETTINGS
Preventing the spread of pathogens involves breaking one of the links in the chain of infection, and the link most amenable to actions by healthcare workers is mode of transmission.
Standard Precautions
One of the most effective infection control practices is Standard Precautions. Standard Precautions are a set of infection control practices used to prevent transmission of pathogens and are based on the concept that all blood, body fluids, nonintact skin (including rashes), and mucous membranes may contain transmissible pathogens. Standard Precautions are implemented for all patient-care settings and include:
- Performing hand hygiene
- Using personal protective equipment (PPE) whenever there is an expectation of possible exposure to infectious material
- Following respiratory hygiene/cough etiquette principles
- Ensuring appropriate patient placement
- Proper handling and proper cleaning and disinfecting of patient-care equipment and instruments or devices
- Cleaning and disinfecting the environment appropriately
- Handling textiles and laundry carefully
- Following safe injection practices
- Wearing a surgical mask when performing lumbar punctures
- Ensuring healthcare worker safety, including handling of needles and other sharps
(Potter et al., 2023; CDC, 2016)
BLOOD AND OTHER POTENTIALLY INFECTIOUS MATERIALS (OPIM)
All occupational exposures to blood or other potentially infectious materials place healthcare providers at risk for infection with bloodborne pathogens. Standard Precautions are designed to eliminate exposure to blood and other potentially infectious materials.
The Occupational Safety and Health Administration (OSHA) defines blood as:
- Human blood
- Human blood components
- Products made from human blood
OPIM include:
- Semen
- Vaginal secretions
- Cerebrospinal fluid
- Synovial fluid
- Pleural fluid
- Pericardial fluid
- Peritoneal fluid
- Amniotic fluid
- Saliva in dental procedures
- Any body fluids that are visibly contaminated with blood
- All body fluids in situations where it is difficult or impossible to differentiate between them
- Any unfixed tissue or organ (other than intact skin) from a human (living or dead)
- Hepatitis B- and HIV-containing cell, tissue, or organ cultures, and HBV- or HIV-containing culture medium or other solutions
- Blood, organs, or other tissues from experimental animals infected with HBV or HIV
(OSHA, 2023a)
HAND HYGIENE
Hand hygiene is the single most important practice to reduce transmission of infectious agents and means cleaning hands by using handwashing with soap and water, antiseptic hand wash, antiseptic hand rub, or surgical hand antisepsis.
During delivery of healthcare, the CDC (2021c) advises healthcare workers to avoid unnecessary touching of surfaces in close proximity to the patient and requires performance of hand hygiene in accordance with the recommendations described below.
Wash hands with soap and water:
- When hands are visibly soiled
- After caring for a person with known or infectious diarrhea
- After known or suspected exposure to spores (e.g., B. anthracis, C. difficile outbreaks)
Unless hands are visibly soiled, an alcohol-based hand sanitizer is the method of choice for hand hygiene. Alcohol-based hand rubs come in gel, rinse, wipe, and foam form. They are less drying and have superior microbicidal activity in comparison to soap and water. Indications include:
- Immediately before having direct contact with a patient’s intact skin (e.g., taking a pulse)
- Before performing an aseptic task (e.g., placing an indwelling device) or handling invasive medical devices
- Before moving from work on a soiled body site to a clean body site on the same patient
- After touching a patient or the patient’s immediate environment
- After contact with blood, body fluids or excretions, mucous membranes, nonintact skin, or wound dressing
- After contact with inanimate objects in the immediate vicinity of the patient, including medical equipment
- Before donning gloves
- Immediately after glove removal
Skin and Nails
The CDC offers the following recommendations to maintain hand skin health:
- Use lotions and creams to prevent and decrease healthcare provider skin dryness related to frequent hand hygiene.
- Use only hand lotions approved by the healthcare facility so as to avoid interfering with hand sanitizing products.
Recommendations regarding fingernails state:
- Healthcare providers should not wear artificial fingernails or extensions when having direct contact with patients at high risk (e.g., those in the intensive care unit or operating room). Pathogens can live under artificial fingernails both before and after use of an alcohol-based hand sanitizer and handwashing.
- Keep natural nail tips less than 1/4 inch long.
(CDC, 2021c)
Studies regarding the issue of wearing nail polish remain inconclusive.
Some studies have shown that more pathogens are present on the skin underneath rings than comparable areas of skin on fingers without rings. Rings may also increase the risk of glove tears. Wearing a simple finger band and unchipped nail polish may be acceptable, although removal of all rings and wearing no nail polish may be the safest prevention option. However, further studies are needed to determine if wearing rings results in an increased spread of pathogens (CDC, 2021c).
FINGERNAIL HYGIENE
- Keep nails short, and trim them often.
- Scrub the underside of nails with soap and water (or a nail brush) every time they are washed.
- Clean any nail grooming tools before use.
- In commercial settings such as nail salons, sterilize nail grooming tools before use.
- Avoid biting or chewing nails.
- Avoid cutting cuticles, as they act as barriers to prevent infection.
- Never rip or bite a hangnail. Instead, clip it with a clean, sanitized nail trimmer.
(CDC, 2022d)
Hand Cleansing Techniques
Both the CDC and the World Health Organization provide guidelines in the techniques of handwashing as well as hand rub cleansing using an alcohol hand sanitizer.
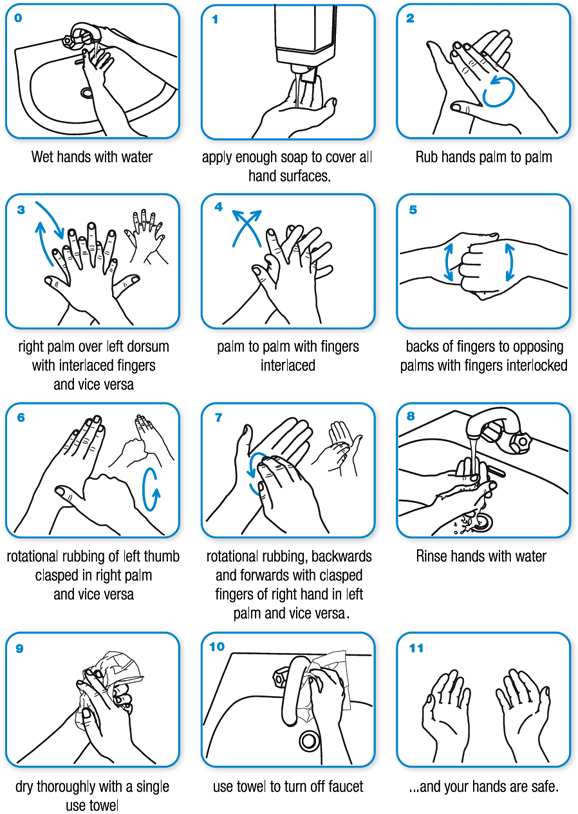
Handwashing requires 15 to 20 seconds to be effective (about as long as it takes to sing “Happy Birthday” twice).
Technique for handwashing with soap and water (WHO, 2020):
Technique for applying hand sanitizer correctly (CDC, 2023a):
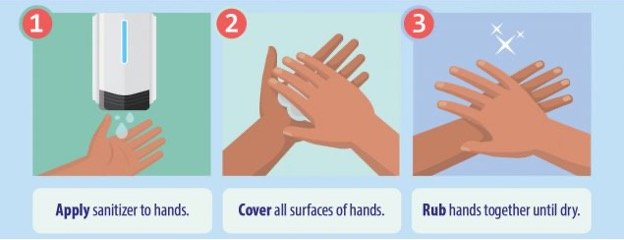
Sanitizers can quickly reduce the number of germs on hands in many situations. However:
- Sanitizers do not remove all types of germs.
- Hand sanitizers may not be as effective when hands are visibly dirty or greasy.
- Hand sanitizers might not remove harmful chemicals from hands, such as pesticides and heavy metals.
(CDC, 2023a)
Surgical Hand Antisepsis
Performing surgical hand antisepsis using either an antimicrobial soap or an alcohol-based hand sanitizer with persistent activity is recommended before donning sterile gloves when performing surgical procedures.
- Remove rings, watches, and bracelets before beginning to scrub.
- Clean under nails using a nail cleaner under running water.
- When using antimicrobial soap, scrub hands and forearms for 2–6 minutes. Long scrub times (e.g., 10 minutes) are not necessary.
- When using an alcohol-based surgical hand-scrub product with persistent activity, follow the manufacturer’s instructions.
- When using an alcohol-based surgical hand scrub, prewash hands and forearms with a nonantimicrobial soap and dry hands and forearms completely before donning sterile gloves.
- Double gloving is advised during invasive procedures that pose an increased risk of exposure to blood.
(CDC, 2021c)
GLOVES
The CDC recommendations state that gloves should be worn according to Standard Precautions, as described below:
- Make sure that gloves fit properly before performing any tasks.
- Wear disposable medical examination gloves when providing direct patient care.
- Wear disposable medical examination gloves or reusable utility gloves for cleaning the environment or medical equipment.
- Wear gloves whenever it can be reasonably anticipated that contact with blood or other potentially infectious materials, mucous membranes, nonintact skin, potentially contaminated skin, or contaminated equipment could occur.
- Wearing gloves is not a substitute for hand hygiene.
- Prior to a task that requires gloves, perform hand hygiene before donning them and before touching the patient or the patient environment.
- Change gloves and perform hand hygiene during patient care if:
- Gloves become damaged
- Gloves become visibly soiled with blood or body fluids following a task
- Moving from work on a soiled body site to a clean body site on the same patient or if another clinical indication for hand hygiene occurs
- Never wear the same pair of gloves in the care of more than one patient.
- Do not touch the face when wearing gloves.
- Do not wear gloves in the halls, except as approved by facility.
- Perform hand hygiene immediately after removing gloves.
(CDC, 2021c)
EYE PROTECTION, MASKS, FACE SHIELDS, AND GOWNS
Appropriate barriers include personal protective equipment used alone or in combination to protect the mucous membranes, airway, skin, and clothing from contact with infectious materials. These barriers include eye protection, face masks, and gowns.
- Wear eye protection (goggles and face shields) whenever there is a potential of a splash or spray of blood, respiratory secretions, or other body fluids. Personal eyeglasses and contact lenses are not considered adequate eye protection.
- Select masks, goggles, face shields, and combinations of each according to the need anticipated by the task to be performed.
- Wear a mask to protect patients from exposure to infectious agents carried in the mouth or nose of healthcare personnel.
- Wear a mask when placing a catheter or injecting material into the spinal canal or subdural space, or to perform intrathecal chemotherapy.
- Wear a fluid-resistant, nonsterile gown to protect skin and clothing during procedures or activities where contact with blood or body fluids is anticipated.
- Wear a new gown for the care of each individual patient.
- Don a gown prior to direct patient contact that may generate splashes or sprays of blood, body fluids, secretions, and excretions.
- Remove a gown and perform hand hygiene before leaving the patient’s environment.
- Do not reuse gowns, even for repeated contacts with the same patient.
(Potter et al., 2023)
RESPIRATORY HYGIENE/COUGH ETIQUETTE
To prevent transmission of all respiratory infections in healthcare settings, Standard Precautions require that the following infection control measures be implemented at the point of initial encounter with patients or accompanying individuals who have signs and symptoms of respiratory infection:
- Educate healthcare workers on the importance of source-control methods to contain respiratory secretions, especially during outbreaks of respiratory illness such as influenza, measles, or coronavirus.
- Post signs at entrances and in strategic places, such as elevators and cafeterias, in both ambulatory and inpatient settings, in languages appropriate to the population served, with instructions to patients and other persons on cough etiquette.
- Make the following supplies readily available:
- Tissues
- No-touch waste containers
- Hand hygiene supplies and instructions (alcohol-based hand rub, sinks when available)
- For patients with signs and symptoms of respiratory infection, offer a mask to contain their secretions.
- Separate patients with symptoms of respiratory infection from the general patient population by 6 feet.
- For healthcare workers, wear a surgical or procedural mask whenever in close proximity to a patient with signs or symptoms of respiratory infection.
(LabCE, 2023)
When coughing:
- Cover the mouth and nose when coughing and/or sneezing with a tissue; if no tissue is available, cough or sneeze into the elbow, not the hands.
- Immediately dispose of the tissue into the nearest waste container.
- Perform hand hygiene after coughing, sneezing, or using a facial tissue.
(LabCE, 2023)
PATIENT-CARE EQUIPMENT AND INSTRUMENTS/DEVICES
The following policies and procedures are recommended for containing, transporting, and handling patient-care equipment and instruments/devices that may be contaminated with blood or body fluids.
- Meticulously clean patient-care items with water and detergent or with water and enzymatic cleaners before high-level disinfection or sterilization procedures.
- Remove visible organic residue (e.g., blood and tissue) and inorganic salts with appropriate effective cleaning agents.
- Clean medical devices as soon as practical after use to avoid soiled materials drying onto the instruments. Dried or baked materials make the removal process more difficult and disinfection or sterilization less effective or ineffective.
- Wear PPE, such as gloves and gown, according to the level of expected contamination when handling patient-care equipment and instruments/devices that are visibly soiled or may have been in contact with blood or body fluids.
Progressively stronger substances are used to clean or disinfect medical instruments and devices depending on the equipment to be cleaned or the microorganisms to be eradicated. These can include:
- Intermediate disinfectants that are a chemical germicide, kill some viruses and bacteria, and can be used on medical devices that are visibly soiled or on intact skin
- High-level disinfectants that kill all living organisms and some spores
- Sterilization with extreme heat or chemicals
(Cumming, 2020)
CARE OF THE ENVIRONMENT
Policies and procedures for keeping the environment clean include using facility-approved disinfectants to clean patient equipment and to clean high-touch surfaces on a more frequent schedule, including:
- Bed rails
- IV poles
- Sink handles
- Bedside tables
- Counters where medications and supplies are prepared
- Edges of privacy curtains
- Patient-monitoring equipment (keyboards, control panels)
- Transport equipment (wheelchair handles)
- Call bells
- Doorknobs
- Light switches
- Surfaces in and around toilets in patients’ rooms
Spills of blood or other potentially infectious materials must be promptly cleaned and decontaminated following proper procedures and using protective gloves and other personal protective equipment appropriate for the task (ACSQHC, 2021).
In facilities providing healthcare to pediatric patients or that have waiting areas with child play toys, policies and procedures for cleaning and disinfecting toys at regular intervals should follow these principles:
- Select play toys that can easily be cleaned and disinfected and avoid use of stuffed, furry toys if they will be shared.
- Clean and disinfect large stationary toys (e.g., climbing equipment) at least weekly and whenever visibly soiled.
- If toys are likely to be put in the mouth, rinse with water after disinfection; alternatively, wash in a dishwasher.
- When a toy requires cleaning and disinfection, do so immediately or store in a designated, labeled container separate from toys that are clean and ready for use.
Clean and scrub with soap and water. Sanitize using a weaker bleach solution (bleach diluted in water) or spray to reduce the number of germs on a surface. Disinfect with stronger bleach solutions to remove germs and completely clean surfaces.
Include multiuse electronic equipment in environmental cleaning and disinfection policies, especially those items used by patients, those used during delivery of patient care, and mobile devices that are moved in and out of patient rooms frequently (CDC, 2023b).
TEXTILES AND LAUNDRY
Recommendations include:
- Handle used textiles and fabrics with minimum agitation to avoid contamination of air, surfaces, and persons.
- Bag or otherwise contain contaminated textiles and fabrics at the point of use.
- Use lead-resistant containment for textiles and fabrics contaminated with blood or body substances.
- If laundry chutes are used, ensure they are properly designed, maintained, and used so as to minimize dispersion of aerosols from contaminated laundry.
(CDC, 2023f; Decker, 2022)
PATIENT PLACEMENT
Include the potential for transmission of infectious agents in patient-placement decisions.
- Place patients who pose a risk for transmission to others in a single-patient room when available (e.g., those with uncontained secretions, excretions, or wound drainage; infants with suspected viral respiratory or gastrointestinal infections).
- Determine patient placement based on the following principles:
- Route(s) of transmission of known or suspected pathogen
- Risk factors for transmission in the infected patient
- Risk factors for adverse outcomes resulting from an HAI in other patients in the area or room being considered for patient placement
- Availability of single-patient rooms
- Patient options for room sharing (e.g., placing together [cohorting] patients with the same infection)
SAFE INJECTION PRACTICES
The following recommendations apply to the use of needles, cannulas that replace needles, and, where applicable, intravenous delivery systems.
- Use aseptic technique to avoid contamination of sterile injection equipment.
- Do not administer medications from a syringe to multiple patients. Needles, cannulas, and syringes are single-patient-use items; they should not be reused to access a medication or solution that might be used for a subsequent patient.
- Use fluid infusion and administration sets (i.e., intravenous bags, tubing, and connectors) for one patient only and dispose appropriately after use. Consider a syringe or needle/cannula to be contaminated after it has entered an IV bag or administration set.
- Use single-dose vials for parental medications whenever possible.
- Do not administer medications from single-dose vials or ampules to multiple patients or combine leftover contents for later use.
- If multidose vials must be used, use only a sterile needle/cannula and syringe to access them.
- Do not keep multidose vials in immediate patient-care areas; store as recommended by the manufacturer and discard if sterility is compromised.
- Do not use bags or bottles of intravenous solution as a common source of supply for multiple patients.
(CDC, 2023c)
(See also “Sharps- and Injection-Related Practices and Controls” later in this course.)
INFECTION CONTROL PRACTICES FOR LUMBAR PUNCTURE PROCEDURES
Healthcare workers should wear a surgical mask when placing a catheter or injecting material into the epidural or subdural spaces to prevent infection caused by the transfer through normal breathing or coughing of oral flora to the central nervous system of the patient during the procedure (CDC, 2023c; Johnson & Sexton, 2023).
Transmission-Based Precautions
In addition to Standard Precautions, which are used with all patients, patients with documented or suspected infection or colonization with highly transmissible or epidemiologically important pathogens require additional precautions known as transmission-based precautions. The duration of these precautions is to be extended for immunosuppressed patients with viral infections due to prolonged shedding of viral agents that may be transmitted to others. There are three types of transmission-based precautions: Contact, Droplet, and Airborne.
CONTACT PRECAUTIONS
Contact Precautions are designed to minimize transmission of organisms that are easily spread by contact with hands or objects. Conditions requiring Contact Precautions may include:
- Enteric infections (C. difficile, E. coli)
- Viral infections (rhinovirus, COVID-19)
- Scabies
- Impetigo
- Enteric infections
- Noncontained abscesses or decubitus ulcers (S. aureus, Group A Streptococcus)
(Anderson, 2020)
CDC Contact Precautions are summarized below.
Patient Placement
In acute care hospitals, place the patient in a single-patient room when available. When a single-patient room is not available:
- Prioritize patients with conditions that may facilitate transmission (e.g., stool incontinence) for single-patient-room placement.
- Place together in the same room (cohort) patients who are infected or colonized with the same pathogen and are suitable roommates.
- If necessary to place the patient in a room with a patient who is not infected or colonized with the same pathogen:
- Avoid placement in rooms with patients whose conditions increase risk of adverse outcome or may facilitate transmission (e.g., immunocompromised, open wounds, or have anticipated prolonged lengths of stay).
- Ensure physical separation of greater than 6 feet. Draw privacy curtain between beds.
- Change protective attire and perform hand hygiene between contacts with patients in the same room.
In long-term care and other residential settings, make decisions about placement on a case-by-case basis, balancing risk to other patients in the room, presence of risk factors increasing likelihood of transmission, and potential adverse psychological impact on infected or colonized patients.
In ambulatory settings, place patients in an examination room or cubicle as soon as possible (CDC, 2023c).
Use of Personal Protective Equipment
- Don gloves upon entry into the room or cubicle.
- Wear gloves whenever touching the patient’s intact skin or surfaces and articles in close proximity to the patient.
- Wear a gown when anticipating clothing will have direct contact with the patient or potentially contaminated environmental surfaces or equipment in close proximity to the patient.
- Don a gown upon entry and remove and perform hand hygiene before leaving the patient-care area.
- After removal of gown, ensure clothing and skin do not contact environmental surfaces in the patient-care area.
Patient Transport
- In acute care hospitals and long-term care and other residential settings, limit transport and movement of patients outside of the room to medically necessary purposes.
- If necessary to transport or move, ensure infected or colonized areas of the patient’s body are contained and covered.
- Remove and dispose of contaminated PPE and perform hand hygiene prior to transporting.
- Don clean PPE to handle the patient at the transport destination.
Patient-Care Equipment and Instruments/Devices
- Handle equipment and instruments/devices according to Standard Precautions.
- In acute care hospitals, long-term care, and other residential settings, use disposable equipment (e.g., blood pressure cuffs) or implement patient-dedicated use. If common use is unavoidable, clean and disinfect before use on another patient.
- Limit the amount of nondisposable patient-care equipment brought into the patient’s home. Whenever possible, leave equipment in the home until discharge from home services.
- If noncritical equipment (e.g., stethoscope) cannot remain, clean and disinfect items before taking them from the home using a low- to intermediate-level disinfectant. Alternatively, place in a plastic bag for transport and later cleaning and disinfection.
- In ambulatory settings, place contaminated reusable noncritical patient-care equipment in a plastic bag for transport to a soiled utility area for reprocessing.
- Ensure that rooms of patients are prioritized for frequent cleaning and disinfection (at least daily), with a focus on frequently touched surfaces (e.g., bed rails, over-bed table, bedside commode, lavatory, doorknobs) and equipment in the immediate vicinity of the patient.
(CDC, 2023c; WDHS, 2023)
DROPLET PRECAUTIONS
Droplet Precautions are designed to prevent transmission of diseases easily spread by large-particle droplets (>5 microns in size) produced when the patient coughs, sneezes, or talks, or during the performance of procedures. Conditions requiring Droplet Precautions may include:
- Neisseria meningitidis infection
- Mycoplasma pneumoniae infection
- Pertussis (whooping cough) (Bordetella pertussis)
- Influenza
- Rubella
- Mumps
- Adenovirus infection
- Parvovirus B19 infection
- Rhinovirus infection
- Certain coronavirus infections (e.g., MERS-CoV, SARS-CoV, and SARS-CoV-2)
(Anderson, 2020)
CDC Droplet Precautions are summarized below.
Patient Placement
In acute care hospitals, place the patient in a single-patient room when available. When a single room is not available:
- Prioritize patients who have excessive cough and sputum production for single-room placement.
- Place together in the same room (cohort) patients who are infected with the same pathogen.
- If necessary to place a patient in a room with another patient who does not have the same infection:
- Avoid placing in the same room with patients who have conditions that may increase risk of adverse outcome or may facilitate transmission (e.g., those who are immunocompromised or have anticipated prolonged lengths of stay).
- Ensure patients are physically separated greater than 6 feet and draw a privacy curtain between beds to minimize close contact.
- Change protective attire and perform hand hygiene between contact with patients in the same room, regardless of whether one patient or both patients are on Droplet Precautions.
In long-term care and other residential settings, make decisions on a case-by-case basis following consideration of infection risks to other patients in the room and available alternatives.
In ambulatory settings, place patients in an examination room or cubicle as soon as possible.
Instruct all patients to follow respiratory hygiene/cough etiquette.
Use of Personal Protective Equipment
Wear a mask upon entry into the patient room or cubicle.
Patient Transport
- In acute care hospitals, long-term care facilities, and other residential settings, limit transport and movement outside the room to medically necessary purposes.
- If transporting or moving is necessary, instruct the patient to wear a mask and follow respiratory hygiene/cough etiquette.
- No mask is required for persons transporting patients.
(CDC, 2023c)
AIRBORNE PRECAUTIONS
Airborne Precautions are designed to prevent transmission of small particles of respiratory secretions that contain infectious microbes over time and long distances. Airborne Precautions are the only type that requires a negative-pressure airborne infection isolation room (AIIR) with door kept closed and use of an N95 respirator.
Conditions requiring Airborne Precautions may include:
- Tuberculosis
- Varicella
- Measles
- Smallpox
- Certain coronavirus infections (e.g., MERS-CoV, SARS-CoV, and SARS-CoV-2)
- Ebola
(Anderson, 2020)
CDC Airborne Precautions are summarized below.
Patient Placement
- In acute care hospitals and long-term care settings, place patients in an AIIR that has been constructed in accordance with current guidelines.
- Keep the AIIR door closed except for entry and exit.
- When an AIIR is not available, transfer patient to a facility that has an available AIIR.
- In the event of an outbreak or exposure involving large numbers of patients requiring Airborne Precautions:
- Consult infection control professionals.
- Cohort patients presumed to have the same infection in areas away from other patients.
- Use temporary portable solutions (e.g., exhaust fan) to create negative pressure in the converted environment, and discharge air directly to the outside or through HEPA filters before it is introduced to other air spaces.
- In ambulatory settings:
- Develop a system (e.g., signage, triage) to identify patients requiring Airborne Precautions.
- Place in an AIIR as soon as possible. If not available, put a surgical mask on the patient, instruct in respiratory hygiene/cough etiquette, and place in an examination room. Once the patient leaves, the room should remain vacant for about 1 hour to allow full exchange of air.
- Instruct patients to wear a surgical mask and observe respiratory hygiene/cough etiquette. Once in an AIIR, mask may be removed.
- Restrict susceptible healthcare personnel from entering rooms of patients known to have measles (rubeola), varicella (chicken pox), disseminate zoster, or smallpox if other immune healthcare personnel are available.
- Respiratory protection is recommended for all healthcare personnel, vaccinated and unvaccinated.
Use of Personal Protective Equipment
- Wear a fit-tested NIOSH-approved N95 or higher-level respirator personal mask when entering the room or home of a patient with known or confirmed infectious pulmonary tuberculosis and when procedures for treating tuberculosis skin lesions are performed that would aerosolize viable organisms. It is essential to wear medical gloves while removing an N95 respirator personal mask.
Patient Transport
- In acute care hospitals, long-term care, and other residential settings, limit transport and movement outside of the room to medically necessary purposes.
- If transport or movement is necessary, instruct patients to wear a surgical mask and observe respiratory hygiene/cough etiquette. Cover infectious skin lesions.
- Transporting healthcare personnel do not need to wear a mask or respirator during transport if patient is wearing a mask and infectious skin lesions are covered.
Exposure Management
- Healthcare workers are encouraged to be vaccinated against common childhood illnesses to prevent outbreaks in vulnerable populations such as neonates.
- Annual influenza vaccination reduces the incidence of institutional outbreaks.
- Healthcare workers should receive periodic boosters against pertussis.
- Immunize or provide appropriate immune globulin to susceptible persons as soon as possible following unprotected exposure to a patient with measles, varicella, or smallpox.
(CDC, 2023c)
Infection Prevention and Control Practices for COVID-19
The CDC and OSHA recommend using additional infection prevention and control practices along with Standard and transmission-based precautions when caring for patients with suspected or confirmed SARS-CoV-2 or COVID-19 infection.
The infectious period includes the time between 2 days before symptoms started until 10 days after symptoms stop or a 5-day negative test; or on day 5 or later with no fever without the help of a fever-reducing medication.
Recommended prevention and control practices include, but are not limited to:
- Implement telehealth and nursing-directed triage protocols.
- Screen and triage everyone entering a healthcare facility for signs and symptoms of COVID-19.
- Perform screening assessments of all admitted patients on a daily basis.
- Implement universal source control measures (face coverings or face mask) for everyone in a healthcare facility working with infected patients.
- Ensure all healthcare personnel wear eye protection in addition to a face mask during patient-care encounters to ensure protection, where indicated and per current facility policy.
- Encourage physical distancing (6 feet) between people wherever possible.
- Limit visitors to only those essential for patients’ physical and emotional well-being and care.
- Quarantine an infected employee and allow their return to the workplace only when cleared according to the above restrictions.
- Give notice to employees of any incidences of possible close exposure to another person who possibly has COVID-19.
- Maximize the amount of outside air whenever possible.
- Aerosolizing procedures, which may promote the spread of potentially infectious material, should be considered as to risks and benefits whenever possible.
(OSHA, 2023b)
Due to the rapidly growing body of knowledge concerning SARS-CoV-2 infection, it is recommended that all healthcare professionals review the most recent infection control guidelines provided by the CDC. (See also “Resources” at the end of this course.)
ENGINEERING AND WORK PRACTICE CONTROLS
ELEMENT III
Use of engineering and work practice controls to reduce the opportunity for patient and healthcare worker exposure to potentially infectious material in all healthcare settings
In addition to the precautions described above, other practices and controls can be employed to prevent and control infection. These include:
- Engineering controls
- Work practice controls
- Environmental controls
Types of Practices and Controls
Engineering controls are measures that protect workers by removing hazardous conditions or by placing a barrier between the worker and the hazard. Examples include:
- Sharps disposal containers
- Self-sheathing needles
- Sharps with engineered sharps injury protections
- Needleless systems
According to the Occupational Safety and Health Administration (OSHA, 2020b), engineering controls shall be examined and maintained or replaced on a regular schedule to ensure their effectiveness.
ENGINEERING CONTROL DEVICE EXAMPLES
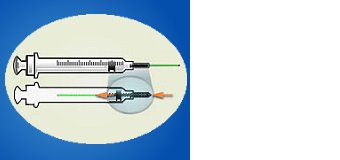
Retractable needle.
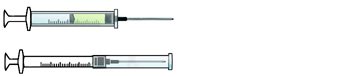
Self-resheathing needle.

Resheathing disposable scalpel.

Phlebotomy needle with hinged shield as an add-on safety feature.
(OSHA, 2020)
Work practice controls reduce the likelihood of exposure to pathogens by changing the way a task is performed, such as:
- Practices for handling and disposing of contaminated sharps
- Handling specimens
- Handling laundry
- Cleaning contaminated surfaces and items
- Performing hand hygiene
(CDC, 2023c; OSHA, 2023a)
Environmental controls help prevent the transmission of infection by reducing the concentration of pathogens in the environment. Such measures include but are not limited to:
- Appropriate ventilation (including air filtration systems)
- Environmental cleaning (housekeeping)
- Cleaning and disinfecting strategies (including food service areas)
- Cleaning, disinfection, and sterilization of patient-care equipment
- Proper linen and laundry management
- Disposal of regulated medical waste
(CDC, 2023c)
Sharps- and Injection-Related Practices and Controls
Engineering, work practice, and environmental controls have all been developed to prevent and control the spread of infection related to the use of needles and other sharps in the healthcare setting.
SHARPS HANDLING
OSHA requirements for handling sharps state that contaminated sharps are needles, blades (such as scalpels), scissors, and other medical instruments and objects that can puncture the skin. Contaminated sharps must be properly disposed of immediately or as soon as possible in containers that are closable, puncture resistant, leakproof on the sides and bottom, and color-coded or labeled with a biohazard symbol.
- Discard needle/syringe units without attempting to recap the needle whenever possible.
- If a needle must be recapped, never use both hands. Use the single-hand “scoop” method by placing the cap on a horizontal surface, gently sliding the needle into the cap with the same hand, tipping the needle up to allow the cap to slide down over the needle, and securing the cap over the needle with the same hand.
- Never break or shear needles.
- To move or pick up needles, use a mechanical device or tool, such as forceps, pliers, or broom and dustpan.
- Use blunt-tip suture needles to decrease risk of percutaneous injury.
- Use only sharps with a safety mechanism attached, and activate the safety mechanism as soon as the device has been used.
- Use luer-lock syringes whenever possible to promote a secure connection between the needle and the syringe.
- Substitute plastic material for glass wherever possible.
- Dispose of needles in labeled sharps containers only. Sharps containers must be accessible and maintained upright.
- When transporting sharps containers, close the containers immediately before removal or replacement to prevent spillage or protrusion of contents during handling or transport.
- Fill a sharps container up to the fill line or two thirds full. Do not overfill the container.
(CU, 2023)
SAFE INJECTION PRACTICES
The following recommendations apply to the use of needles, cannulas that replace needles, and, where applicable, intravenous delivery systems.
- Draw up medications in a designated clean medication preparation area that is not adjacent to potential sources of contamination, including sinks or other water sources.
- Perform hand hygiene prior to preparing medications.
- Access parenteral medications in an aseptic manner, using a new sterile syringe and sterile needle.
- Prepare an injection as close as possible to the time of administration to prevent compromised sterility.
- Always enter a medication vial with a sterile needle and sterile syringe, even when obtaining additional doses of medication for the same patient, and discard after use.
- Use a syringe and needle only once to administer a medication to a single patient.
- Discard medications according to the manufacturer’s expiration date (even if not opened) and whenever sterility is compromised or questionable.
- If a multidose vial has been opened or accessed, the vial should be dated and discarded within 28 days unless manufacturer specifies a different length of time or date.
- Single-dose vials that have been opened or accessed should be discarded according to the manufacturer’s time specifications or at the end of the case/procedure for which they are being used. Do not store for future use.
(OSHA, 2020, 2023a)
BARRIERS AND PERSONAL PROTECTIVE EQUIPMENT
ELEMENT IV
Selection and use of barriers and/or personal protective equipment for preventing patient and healthcare worker contact with potentially infectious material
Personal protective equipment (PPE) includes a variety of barriers and respirators used alone or in combination to protect mucous membranes, airways, skin, and clothing from contact with infectious agents. The selection of PPE is based on the nature of the patient interaction and/or the likely mode or modes of transmission.
Types of Personal Protective Equipment
PPE must be readily accessible to employees and available in appropriate sizes. It is important to know which type of PPE is available at work and where it is stored. To protect themselves, healthcare providers must have a barrier between themselves and any potentially infectious material.
Types of PPE used in healthcare settings include:
- Gloves
- Gowns/aprons/coveralls
- Face/mask and eye protection
- Head coverings
- Boots/shoe covers
- Respirators
Factors that influence the selection of appropriate PPE include:
- Type of exposure anticipated:
- Blood or body fluid splash/spray vs. touch
- Contact with minimal bleeding/drainage/body substances
- Contact with large-volume bleeding/drainage/body substances that are likely to soak through the contact area
- Category of isolation precautions (Contact, Droplet, Airborne)
- Durability and appropriateness for the task. This affects whether a gown or fluid-proof apron is selected, or, if a gown is selected, whether it must be fluid-resistant, fluid-proof, or neither.
- Fit of the equipment. The employer must ensure that all PPE is available in sizes appropriate for the workforce to be protected.
(See also “Standard Precautions” earlier in this course for additional information related to PPE.)
GLOVES
Gloves are the most common type of PPE. They are used for patient care as well as environmental service. Gloves can be sterile or nonsterile and single use or reusable. Because of allergy concerns, latex products have been eliminated in many facilities, and materials used for gloves are mostly synthetics such as vinyl or nitrile.
Gloves can protect both patients and healthcare personnel from exposure to infectious material that may be carried on the hands. Nonsterile disposable medical gloves are available for routine patient care. The selection of glove type for nonsurgical use is based on the task that is to be performed and anticipated contact with chemicals and chemotherapeutic agents.
For contact with blood and body fluids during nonsurgical patient care, a single pair of gloves provides adequate barrier protection. However, there is considerable variability among gloves, and studies have shown repeatedly that vinyl gloves have higher failure rates than latex or nitrile gloves. For this reason, either latex or nitrile gloves are preferable for clinical procedures that require manual dexterity and/or will involve more than brief patient contact.
Most patient-care activities require the use of a single pair of nonsterile gloves. Vinyl gloves are frequently available and work well if patient contact is limited. However, some gloves do not provide a snug fit on the hand, especially around the wrist, and should be not used if extensive contact is likely. Gloves should not tear or damage easily and must stand up to the task.
Sterile surgical gloves are worn when performing invasive patient procedures. At times, two pairs of gloves may be worn during surgical procedures for additional protection.
Heavier, reusable utility gloves are indicated for non-patient-care activities such as handling or cleaning contaminated equipment or surfaces. Environmental service personnel often wear reusable heavy-duty gloves to work with caustic disinfectants.
Proper glove use includes:
- Performing hand hygiene before putting on gloves
- Putting gloves on last when being worn in combination with other PPE
- Working from clean to dirty
- Limiting touch contamination (e.g., adjusting eyeglasses, touching light switches, etc.) when wearing gloves that have been in contact with a patient
- Changing gloves during the care of a single patient to prevent cross-contamination of body sites or if patient interaction also involves touching portable keyboards or other mobile equipment that is transported from room to room
- Changing gloves during use if torn or when heavily soiled and after use on each patient
- Discarding gloves between patients and disposing of gloves in the proper receptacle
- Performing hand hygiene following glove removal
- Never washing or reusing disposable gloves
(CDC, 2023c; IPC, 2021)
GOWNS/APRONS
Clinical and laboratory coats or jackets worn over personal clothing are not considered PPE.
Isolation gowns are preferred, but aprons occasionally are used where limited contamination is expected. Gowns must fully cover the torso, fit comfortably over the body, and have long sleeves that fit snugly at the wrist.
The type of protection is based on the nature of the patient interaction, including the anticipated degree of contact with infectious material and potential for blood and body fluid penetration of the barrier. Clean gowns are generally used for isolation precautions. Sterile gowns are needed only when performing invasive procedures.
For prevention of transmission of an infectious agent, donning of both gown and gloves upon room entry is indicated. Gowns are always worn in combination with gloves and with other PPE when indicated, and are usually the first piece of PPE to be donned. Full coverage of arms and body front from neck to mid-thigh or below ensures that clothing and exposed body areas are protected.
FACE/MASK AND EYE PROTECTION
Face/mask and eye protection are used during patient-care activities that are likely to generate splashes or sprays of blood, body fluids, secretions, or excretions. Two mask types are available for use: surgical masks and procedure or isolation masks.
Masks are used for three primary purposes:
- Worn by healthcare personnel to protect from contact with infectious material from patients
- Worn by healthcare personnel who are engaged in procedures requiring sterile technique to protect patients from exposure to infectious agents carried in the healthcare worker’s mouth or nose
- Placed on patients to limit potential dissemination of infectious respiratory secretions from the patient to others
Eye protection depends on the circumstances of exposure, other PPE used, and personal vision needs. Goggles protect the eyes and should fit over and around them snugly. Personal prescription glasses are not a substitute for goggles. Some goggles have antifog features that improve clarity, and many styles of goggles fit adequately over prescription lenses with minimal gaps.
Face shields protect the face, nose, mouth, and eyes. A face shield should cover the forehead, extend below the chin, and wrap around the sides of the face. Face shields can be worn along with face masks.
Removal of a face shield, goggles, and mask can be performed safely after removing gloves and performing hand hygiene (IPC, 2021).
HEAD COVERINGS
Head coverings such as surgical caps are worn when gross contamination is expected, such as during orthopedic surgery or autopsies.
BOOTS/SHOE COVERS
Theater boots are waterproof boots worn by surgical personnel as a protective measure from contamination with blood and other body fluids. Shoe covers protect the wearer from accidental spills and body fluids.
RESPIRATORY PROTECTION
Respiratory protection includes both surgical and standard N95 or higher-level respirators. Respirators require medical evaluation to determine if it is safe for the healthcare worker to wear one and to fit the worker with the appropriate respirator size and type. Healthcare workers are responsible for performing a user-seal check (formerly called a fit check) each time a respirator is donned to minimize air leakage around the face piece.
A surgical mask is recommended only for use by healthcare personnel who need protection from both airborne and fluid hazards such as splashes and sprays.
The CDC currently recommends standard N95 or higher-level respirators for healthcare personnel who are exposed to patients with suspected or confirmed tuberculosis and COVID-19 (CDC, 2023d).
Donning PPE
There are various ways to put on PPE. Training and practice using the healthcare facility’s procedure is critical. The CDC recommends that PPE be put on (donned) in the following sequence:
- Gown
- Mask or N95 filtering respirator
- Face shield or goggles
- Gloves
How to put on a gown:
- Select appropriate type and size.
- Perform hand hygiene using hand sanitizer.
- Put on with opening in the back.
- Secure at neck and waist.
- If gown is too small, use two gowns, with the first tied in front and the second tied in back.
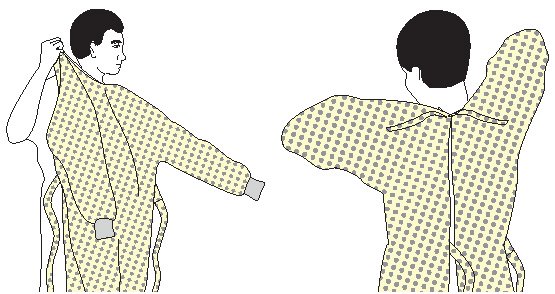
How to put on a respirator:
- Respirator straps should be placed on the crown of the head (top strap) and base of the neck (bottom strap). Perform a user seal check each time putting on the respirator.
How to put on a mask:
- Place over nose, mouth, and chin.
- Fit flexible nose piece over bridge of nose using both hands. Do not bend or tent. Do not pinch the nosepiece with one hand.
- Ensure face mask extends under the chin.
- Mask ties should be secured on the crown of the head (top tie) and base of the neck. If the mask has loops, hook them appropriately around the ears.
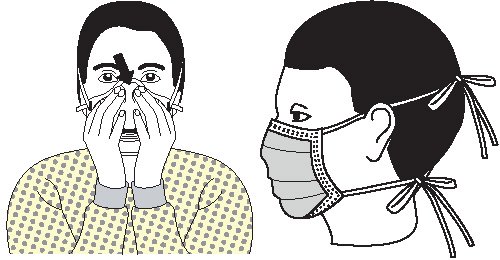
How to put on goggles and face shield:
- Place over face and eyes.
- Adjust to fit.

How to put on gloves:
- Select correct type and size.
- Insert hands into gloves.
- Extend gloves over isolation gown cuffs (wrists).
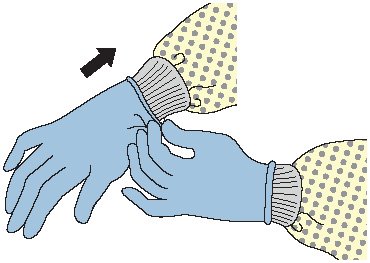
(CDC, n.d.)
Doffing PPE
More than one doffing method may be acceptable. Training and practice using the healthcare facility’s procedure is critical. The following is one example of doffing recommended by the CDC.
Contaminated PPE should be removed in the following sequence:
- Gloves
- Gown
- Hand hygiene and exit patient room
- Face shield or goggles
- Mask or respirator
- Hand hygiene
How to remove gloves:
- Grasp outside edge near wrist.
- Peel away from hand, turning glove inside out.
- Hold in opposite gloved hand.
- Slide ungloved finger under wrist of remaining glove.
- Peel off from inside, creating a bag for both gloves.
- Discard.
- Perform hand hygiene if contamination occurs.
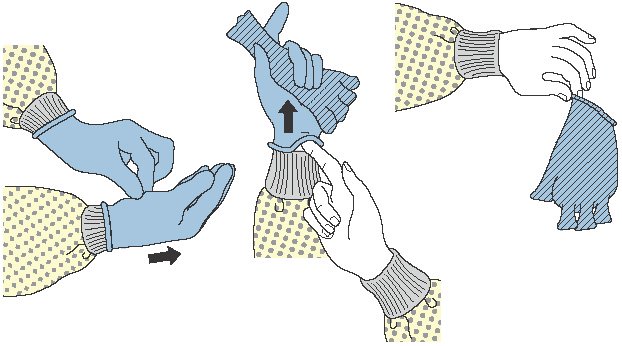
How to remove gown:
- Consider gown front and sleeves to be contaminated.
- Unfasten ties. (Some gown ties can be broken rather than untied.)
- Peel gown away from neck and shoulders, touching inside of gown only.
- Turn contaminated outside toward the inside.
- Fold or roll into a bundle and discard.
- Perform hand hygiene before exiting the patient area.
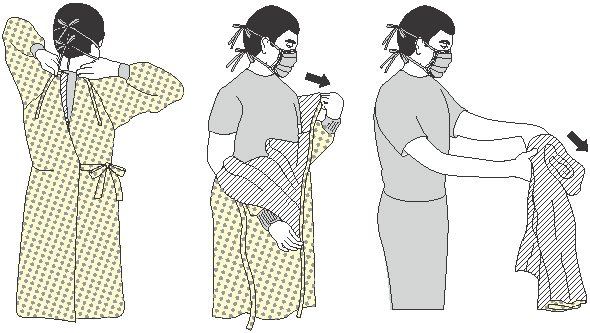
How to remove goggles or face shield:
- Consider outside of goggles or face shield to be contaminated.
- Grasp earpieces or headpieces from the back with ungloved hands by lifting head band or earpieces.
- Lift away from face.
- Place in designated receptacle for reprocessing or disposal.
- Perform hand hygiene if contamination occurs.
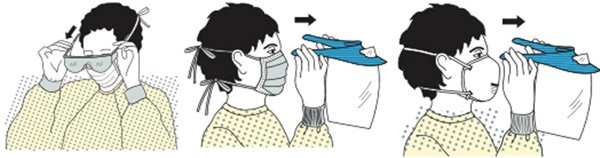
How to remove mask or respirator:
- Consider front of mask or respirator to be contaminated; do not touch.
- Respirator: Remove the bottom strap of the respirator by touching only the strap and bringing it carefully over the head. Grasp the top strap and bring it carefully over the head. Then pull the respirator away from the face without touching the front of it.
- Face mask: Carefully untie (or unhook from the ears) and pull away from face without touching the front.
- Discard.
- Perform hand hygiene.
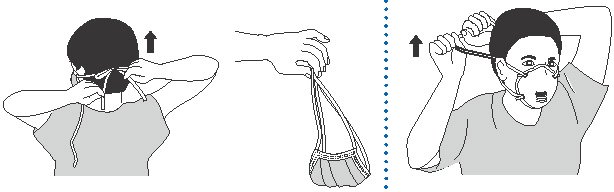
PERSONAL PROTECTIVE EQUIPMENT AND SARS-CoV-2 (COVID-19) PANDEMIC
- Healthcare personnel should adhere to Standard and transmission-based precautions when caring for patients with SARS-CoV-2 infection.
- Transmission-based precautions for SARS-CoV-2 require the use of a filtering facepiece respirator (e.g., N95).
- A study has found that SARS-CoV-2 is able to survive on materials such as plastic, stainless steel, and cardboard for up to 72 hours.
- A respirator mask can be decontaminated between uses by storing it between uses in a dry, dark place such as a paper bag.
- N95 masks can be reused until they become soiled, damaged, or difficult to breathe through.
- Ultraviolet germicidal irradiation, vaporous hydrogen peroxide, and moist heat have shown the most promise as methods to decontaminate respirators.
- Reuse of respirators should be practiced only where shortages exist.
(Drummond, 2022)
(See also “Resources” at the end of this course for a link to the latest CDC guidance on PPE and COVID-19.)
A SAFE ENVIRONMENT: CLEANING, DISINFECTING, AND STERILIZING
ELEMENT V
Creation and maintenance of a safe environment for patient care in all healthcare settings through application of infection control principles and practices for cleaning, disinfection, and sterilization
The healthcare environment can easily become contaminated with pathogens. The potential for contamination exists in every area of the hospital or other healthcare facility. Contaminated patient-care equipment (e.g., wet or soiled dressings), invasive devices that were used in diagnosis and treatment (e.g., surgical instruments or endoscopes), and environmental surfaces (e.g., doorknobs, floors, toilets) can act as vehicles for the transmission of infection to healthcare workers and/or patients. In addition, contamination depends on:
- The potential for external contamination (e.g., presence of hinges, crevices)
- The potential for internal contamination (e.g., presence of lumens)
- The physical composition, design, or configuration of an instrument, medical device, equipment, or environmental surface
General Environmental Surface Cleaning
Transmission of infections to either staff or patients is largely via hand contact with a surface. Thus, cleaning and disinfecting environmental surfaces is fundamental to reducing the incidence of HAIs.
The number and type of pathogens present on environmental surfaces are affected by:
- Number of people in the environment
- Amount of activity
- Amount of moisture
- Presence of material able to support microbial growth
- Rate at which organisms suspected in the air are removed
- Type of surface and orientation (horizontal or vertical)
Cleaning is the first step of any sterilization or disinfection process and requires the physical action of scrubbing with detergents and surfactants and rinsing with water.
Cleaning is followed by disinfection. The following are factors that influence the choice of disinfection procedure for environmental surfaces:
- The nature of the item to be disinfected
- The number of microorganisms present
- The innate resistance of those microorganisms to the inactivating effects of a germicide
- The amount of organic soil present
- The type and concentration of germicide used
- The duration and temperature of germicide contact
Environmental surfaces can be divided into medical equipment surfaces (e.g., knobs or handles on equipment) and housekeeping surfaces (e.g., floors, walls, and tabletops).
Manufacturers of medical equipment provide care and maintenance instructions specific to their equipment. These instructions include information about:
- The equipment’s compatibility with chemical germicides
- Whether the equipment is water-resistant or can be safely immersed for cleaning
- How the equipment should be decontaminated if servicing is required
Use barrier protective coverings as appropriate for equipment surfaces that are:
- Touched frequently with gloved hands during the delivery of patient care
- Likely to become contaminated with blood or body substances
- Difficult to clean (e.g., computer keyboards)
Most, if not all, housekeeping surfaces (e.g., floors, walls, tabletops) must be cleaned on a regular basis using only soap and water or a detergent/disinfectant, depending on the nature, type, and degree of contamination. Spills must be cleaned up promptly. Walls, blinds, and window curtains in patient-care areas are cleaned when visibly dusty or soiled.
High-touch housekeeping surfaces in patient-care areas (e.g., doorknobs, bed rails, light switches, wall areas around the toilet in the patient’s room, and the edges of privacy curtains) are cleaned and/or disinfected more frequently than surfaces with minimal hand contact.
Disinfectant/detergent formulations registered by the Environmental Protection Agency (EPA) are used for environmental surface cleaning, but the most important step is the physical removal of microorganisms and soil by scrubbing and/or wiping.
A one-step process and an EPA-registered hospital disinfectant/detergent designed for general housekeeping purposes should be used in patient-care areas when:
- The nature of the soil on these surfaces is uncertain
- The presence or absence of multidrug-resistant organisms on such surfaces is uncertain
Follow facility policies and procedures for effective handling and use of mops, cloths, and solutions (ADA, 2022; CDC, 2023c, 2023e).
Cleaning Immunosuppressed-Patient Areas
In areas where immunosuppressed patients are cared for, cleaning/disinfecting involves:
- Wet dusting of horizontal surfaces daily with cleaning cloths premoistened with detergent or an EPA-registered hospital detergent/disinfectant
- Avoiding the use of cleaning equipment or methods that disperse dust or produce mists or aerosol
- Equipping vacuums with HEPA filters
- Regular cleaning and maintenance of equipment to ensure efficient particle removal
- Closing the doors of immunocompromised patients’ rooms when vacuuming, waxing, or buffing corridor floors to minimize exposure to airborne dust
(CDC, 2023e)
Cleaning Up Blood Spills
All environmental and working surfaces must be promptly cleaned and decontaminated after contact with blood or OPIM. Protective gloves and other appropriate PPE should be worn, and an appropriate disinfectant should be used.
After putting on personal protective equipment:
- If the spill contains large amounts of blood or body fluids, clean the visible matter with disposable absorbent material, and discard in an appropriate, labeled container.
- Swab the area with a cloth or paper towels moderately wetted with disinfectant and allow the surface to dry.
- Use EPA-registered hospital disinfectants labeled tuberculocidal or registered germicides with specific label claims for HIV or HBV.
- Use an EPA-registered sodium hypochlorite product; if not available, general versions (e.g., household chlorine bleach) may be used.
- Use a 1:100 solution of hypochlorite product or chlorine bleach to decontaminate nonporous surfaces after cleaning a spill in patient-care settings.
(CDC, 2023e)
Reprocessing Healthcare Equipment and Devices
Depending on the intended use, reusable medical and surgical equipment and devices must undergo reprocessing that involves:
- Cleaning alone, for noncritical items
- Cleaning followed by disinfection, for semicritical items
- Cleaning followed by sterilization, for critical items
| Classification/Intended Use | Goal of Process | Appropriate Process |
|---|---|---|
| (CDC, 2023e; Cumming, 2020; JST Medical, 2023) | ||
| Noncritical items
Objects that come into contact with intact skin but not mucous membranes, and high-touch surfaces, e.g.:
|
Clean of:
|
|
| Semicritical items Items that make contact, directly or indirectly, with intact mucous membranes or nonintact skin, e.g.:
|
Free of all microorganisms, with exception of high numbers of bacterial spores | High-level disinfection (thermal, chemical) |
| Critical items Items entering sterile tissue, body cavity, vascular system, nonintact mucous membranes, e.g., surgical instruments |
Free of all microorganisms, including bacterial spores |
|
CLEANING
Cleaning involves removal of foreign material (e.g., soil, organic material) from objects and is normally accomplished using water with detergents or enzymatic products. Thorough cleaning is required before high-level disinfection and sterilization because inorganic and organic material remaining on the surfaces of instruments interfere with the effectiveness of these processes. If soiled materials dry or bake onto the instruments, the removal process becomes more difficult and the disinfection or sterilization process less effective or ineffective.
Perform either manual cleaning (using friction) or mechanical cleaning (using ultrasonic cleaners, washer-disinfector, or washer-sterilizer).
When a washer-disinfector is used, care should be taken in loading instruments: hinged instruments should be opened fully to allow adequate contact with the detergent solution; stacking of instruments in washers should be avoided; and instruments should be disassembled as much as possible.
There are no real-time tests that can be used in a clinical setting to verify cleaning. The only way to ensure adequate cleaning is to conduct a reprocessing verification test (e.g., microbiologic sampling), but this is not routinely recommended (Cumming, 2020; Potter et al., 2023).
DISINFECTION
Disinfection describes a process that eliminates many or all pathogenic microorganisms (except bacterial spores) on inanimate objects. In healthcare settings, objects usually are disinfected by liquid chemicals or wet pasteurization. Factors that can affect the effectiveness of either method include:
- Organic and inorganic load present
- Type and level of microbial contamination
- Concentrations of an exposure time to the germicide
- Physical nature of the object
- Presence of biofilm
- Temperature and pH of the disinfection process
- Relative humidity of the sterilization process
There are three levels of disinfection, as described in the table below.
| Level | Disinfection/Process |
|---|---|
| (Cumming, 2020) | |
| Low |
|
| Intermediate |
|
| High |
|
STERILIZATION
Sterilization destroys all microorganisms on the surface of an article or in a fluid to prevent disease transmission. Medical devices that have contact with sterile body tissues or fluids must be sterile when used. The use of inadequately sterilized items represents a high risk of transmitting pathogens; however, documented transmission of pathogens associated with an inadequately sterilized critical item is exceedingly rare.
FDA-approved sterilization methods are described in the table below.
| Sterilization Process | Description | Uses |
|---|---|---|
| (Dias et al., 2023; Potter et al., 2023) | ||
| Steam under pressure (moist heat) |
|
|
| Flash steam | Modification of conventional steam sterilization where item is placed on an open tray or in a container for rapid penetration of steam |
|
| Low-temperature ethylene oxide (ETO) gas | Parameters:
|
|
| Hydrogen peroxide gas plasma | Inactivates microorganisms by the combined use of hydrogen peroxide gas and the generation of free radicals | High temperature– and humidity-intolerant materials and devices, including:
|
| Peracetic acid |
|
Medical and surgical instruments (e.g., endoscopes, arthroscopes) |
| Dry heat | Static or forced air penetrates material | Materials damaged by moist heat or impenetrable to moist heat (e.g., powders, sharp instruments, petroleum products) |
MONITORING REPROCESSING EFFECTIVENESS
Effectiveness of reprocessing depends on:
- Thorough cleaning before either disinfection or sterilization
- Choice of the right disinfectant product
- Presence of organic matter (inadequate cleaning), which can inactivate many disinfectants
- Use of mechanical scrubbing. In general, biofilms are not readily removed by chemicals alone but require mechanical scrubbing. (Biofilms are constructed by some bacteria to protect themselves from hostile environments such as disinfectants. An example of a biofilm is the film on teeth in the morning, not removed by mouthwash, requiring brushing.)
Monitoring disinfection is essential to document the effectiveness of reprocessing. Factors to be documented include:
- Activity (concentration) of the disinfectant
- Contact time with internal and external components
- Recordkeeping and tracking of equipment usage and reprocessing
- Handling and storage after disinfection to prevent contamination
Monitoring sterilization involves maintaining records of each sterilizer load, routinely evaluating the sterilizing conditions, and indirectly evaluating the microbiologic status of the processed items. This is accomplished by using a combination of mechanical, chemical, and biological indicators.
- Mechanical monitors for both steam sterilization and ETO sterilization provide data on cycle time, temperature, and pressure. However, two essential elements for ETO sterilization (gas and humidity) cannot be monitored in healthcare ETO sterilizers.
- Chemical indicators are affixed on the outside of each pack to indicate that the item has been exposed to the sterilization process. They do not, however, prove sterilization has been achieved. A chemical indicator should also be placed on the inside of each pack to verify sterilant penetration. Chemical indicators are either heat- or chemical-sensitive inks that change color when one or more sterilization parameters are present. Chemical indicators should be used in conjunction with biological indicators.
- Biological indicators are considered to be the closest to ideal because they measure the sterilization process directly by using a preparation of the most resistant pathogen (Bacillus spores) as an indicator of sterility. These preparations are added to a carrier and then packaged. Biological indicators specifically designed for monitoring flash sterilization are now available, and a new rapid-readout ETO biological indicator has been designed for rapid and reliable monitoring of the process.
Periodic infection control rounds to areas using sterilizers for the purpose of standardizing use may identify correctable variances in operator competence, documentation of sterilization records, sterilizer maintenance and wrapping, and load numbering of packs (Schrubbe, 2020).
PACKAGING, STORAGE, AND HANDLING OF PROCESSED ITEMS
Written and illustrated procedures for preparation of items to be packaged should be readily available and used by personnel when packing procedures are performed. Sterility standards should follow the manufacturer’s recommendations (e.g., a maximum relative humidity in the storage area of 60% and a temperature of no more than 72–78 °F [22–26 °C]).
Once items are cleaned, dried, and inspected, those requiring sterilization must be wrapped or placed in rigid containers and arranged in instrument trays/baskets according to guidelines.
There are several choices in packaging methods to maintain sterility of surgical instruments, including:
- Rigid containers
- Peel-open pouches, (e.g., self-sealed or heat-sealed plastic and paper pouches)
- Roll stock or reels (i.e., paper-plastic combinations of tubing designed to allow the user to cut and seal the ends to form a pouch)
- Sterilization wraps (woven and nonwoven)
The packing material must:
- Allow penetration of the sterilant
- Provide protection against contamination during handling
- Provide an effective barrier to microbial penetration
- Maintain the sterility of the processed item after sterilization
Packages are inspected to ensure sterility. If they do not pass, they are returned for correction and reprocessing.
Wrapped surgical trays remain sterile for varying periods depending on the type of material used to wrap the trays. Safe storage times for sterile packs vary with the porosity of the wrapper and storage conditions. The central surgical area where sterile supplies are stored must be free from excess humidity, heat, cold, dirt, dust, moisture, and contamination. Sterile supplies can be stored with nonsterile supplies but must be clearly labeled.
Any sterilized item should not be used after the expiration date has been exceeded or if the sterilized package is wet, torn, or punctured. This practice recognizes that the product should remain sterile until some event causes it to become contaminated (e.g., tear in packaging, packaging becomes wet, or seal is broken).
Following the sterilization process, handling of medical and surgical devices must use aseptic technique in order to prevent contamination.
Any healthcare facility must be able to show evidence of a written policy that demonstrates knowledge of evidence-based guidelines and national standards (TJC, 2022).
REPROCESSING AND REUSE OF SINGLE-USE DEVICES
Reusing single-use medical devices (SUDs) has been occurring since the late 1970s. Single-use medical devices can be reprocessed within healthcare organizations or by outside vendors (third-party reprocessors). Specifically, the FDA requires third-party reprocessors to meet the same criteria for the reprocessed devices as the original equipment manufacturer.
The public health risk presented by a reprocessed SUD varies. Some that are low risk when used only one time may present an increased risk to a patient upon reprocessing. Others that are low risk when used for the first time may remain low risk after reprocessing, provided the reprocessor conducts cleaning and sterilization/disinfection in the appropriate manner.
WHEN DISINFECTION/STERILIZATION IS PERFORMED ELSEWHERE
According to the NYS Department of Health, those health professionals who practice in settings where handling, cleaning, and reprocessing equipment, instruments, or medical devices are performed elsewhere (such as a dedicated Sterile Processing Department) should:
- Understand core concepts and principles, including:
- Standard/Universal Precautions (e.g., wearing of PPE)
- Cleaning, disinfection, and sterilization (see above)
- Safe practices for handling instruments, medical devices, and equipment in the area of professional practice
- Physical separation of patient-care areas from cleaning and reprocessing areas
- Verify with those responsible for reprocessing what steps are necessary prior to submission:
- Precleaning
- Soaking
New York healthcare professionals who have primary or supervisory responsibilities for equipment, instrument, or medical device reprocessing (such as Sterile Processing Department staff or clinics and physician practices where medical equipment is reprocessed on-site) should:
- Understand the core concepts and principals identified in #1 above
- Determine appropriate reprocessing practices, taking into consideration:
- Selection of appropriate methods:
- Antimicrobial efficacy
- Time constraints and requirements for various methods
- Compatibility among equipment and material
- Corrosiveness
- Penetrability
- Leaching
- Disintegration
- Heat tolerance
- Moisture sensitivity
- Toxicity
- Occupational health risks
- Environmental hazards
- Abatement methods
- Monitoring exposures
- Potential for patient toxicity/allergy
- Residual effect
- Antibacterial residue
- Potential for patient toxicity/allergy
- Ease of use
- Need for specialized equipment
- Special training requirements
- Stability
- Concentration
- Potency
- Efficacy of use
- Effect of organic material
- Odor
- Cost
- Monitoring
- Frequency
- FDA regulations for reprocessing single use devices
(NYSDOH, 2018)
- Selection of appropriate methods:
Waste Management
Treatment of regulated medical waste reduces the microbial load in or on the waste and renders the byproducts safe for further handling and disposal. Prior to 1997 and a change in the Environmental Protection Agency (EPA) standards, 90% of all medical waste was incinerated. Regulated medical waste treatment methods now include:
- Chemical disinfection
- Grinding/shredding/disinfection methods
- Energy-based technologies (e.g., microwave, radio-wave treatments)
- Disinfection/encapsulation methods
Medical wastes require careful disposal and containment. Occupational Safety and Health Administration (OSHA) requirements are designed to protect workers who generate medical waste and who manage the wastes from point of generation to disposal. Personnel responsible for waste management must receive appropriate training in handling and disposal methods in accordance with facility policy.
State medical waste regulations specify appropriate treatment for each category of regulated waste. Major categories of medical waste requiring special handling and disposal precautions include:
- Microbiology laboratory wastes (e.g., cultures and stocks of microorganisms)
- Bulk blood, blood products, blood, and bloody body fluid specimens
- Pathology and anatomy waste
- Sharps (e.g., needles, broken glass, and scalpels) that can transmit disease (e.g., human immunodeficiency virus, hepatitis C)
NEW YORK STATE REGULATED MEDICAL WASTE (RMW)
New York State provides regulatory oversight of regulated medical waste. It has adopted a comprehensive regulatory framework covering all aspects of handling, storage, treatment, and disposal of this waste, and has stated its expectations with respect to differing levels of disinfection and sterilization methods and agents based on the area of professional practice, setting, and scope of responsibilities.
In accordance with these laws and regulations, both the New York State Department of Health (DOH) and the Department of Environmental Conservation (DEC) jointly administer New York State’s RMW program.
The DEC has oversight authority for all storage, treatment, and destruction processes located onsite of facilities not under DOH jurisdiction; for off-site transport of RMW; for all generators, tracking, and responding to illegal disposal incidents; and for all off-site storage, transfer, treatment, and disposal facilities (NYSDEC, 2020).
CONTAINERS AND DISPOSAL METHODS
In addition to any state rules for disposing of regulated waste, CDC recommendations state that regulated waste must be placed in containers. These are recommendations only since the CDC is not a regulatory agency. The EPA has not enforced waste management regulations since the Medical Waste Tracking Act of 1988 expired in 1991. Waste disposal regulations are primarily controlled by the individual state environmental protection agencies (EPA, 2023a).
It is recommended that the containers be:
- Closable
- Constructed to contain all contents and prevent leakage of fluids during handling, storage, transport, or shipping
- Labeled or color-coded
- Closed prior to removal to prevent spillage or protrusion of contents during handling, storage, transport, or shipping
If outside contamination of the regulated waste container occurs, it must be placed in a second container meeting the above standards.
Sharps containers must be puncture resistant and located as close as possible to point of use. The container must be labeled with the universal biohazard symbol and the word biohazard or be color-coded red. Sharps containers must be maintained upright throughout use, replaced routinely, and not be allowed to overfill. Also, containers must be:
- Capable of maintaining impermeability after waste treatment
- Closed immediately prior to removal or replacement to prevent spillage or protrusion of contents during handling, storage, transport, or shipping
- Placed in a secondary container if leakage is possible; the second container must be:
- Closeable
- Constructed to contain all contents and prevent leakage during handling, storage, transport, or shipping
- Labeled or color-coded
- Reusable containers must not be opened, emptied, or cleaned manually or in any other manner that would expose employees to risk of percutaneous injury (OSHA, 2023b).
- On-site incineration is an option for microbiologic, pathologic, and anatomic waste.
- Waste generated in isolation areas should be handled using the same methods used for waste from other patient-care areas.
- Containers with small amounts of blood remaining after laboratory procedures, suction fluids, or bulk blood can either be inactivated or carefully poured down a utility sink drain or toilet. No evidence indicates that bloodborne diseases have been transmitted from contact with raw or treated sewage.
- If treatment options are not available at the site of waste generation, transport in closed, impervious containers to the on-site treatment location or to another facility for treatment as appropriate.
- Store regulated medical wastes awaiting treatment in a properly ventilated area inaccessible to vertebrate pests. Use waste containers that prevent development of noxious odors.
- Regulated waste that has been decontaminated need not be labeled or color-coded.
(Bio-MED, 2023)
Solid wastes can be disposed of in landfills or incinerators.
Of all the categories of regulated medical waste, microbiologic wastes pose the greatest potential for infectious disease transmission, and sharps pose the greatest risk for injuries (EPA, 2023b).
WARNING LABELS
Warning labels that include the universal biohazard symbol, followed by the term biohazard, must be included on:
- Bags and containers of regulated waste
- Refrigerators and freezers containing blood or other potentially infectious materials (OPIM)
- Other containers used to transport or ship blood or OPIM
- Contaminated equipment that is to be serviced or shipped (must also contain a statement relating to which portions of the equipment remain contaminated)
These labels are fluorescent orange, red, or orange-red. Bags used to dispose of regulated waste must be red or orange-red, and they too must have the biohazard symbol in a contrasting color readily visible upon them.
Red bags or red containers may be substituted for the biohazard label.

Biohazard warning label. (Source: OSHA, 2020b.)
Linens and Laundry Management
The risk of actual disease transmission from soiled laundry is negligible. However, the hands of healthcare workers may be contaminated by contact with patient bed linens. Thus, common-sense hygienic practices for handling, processing, and storage of textiles are recommended. These practices include:
- Do not shake items or handle them in any way that may aerosolize the infectious agents.
- Avoid contact of one’s own body and personal clothing with the soiled items being handled.
- Wear gloves and other protective equipment, as appropriate, when handling contaminated laundry.
- Contain soiled items in a laundry bag or designated bin at the location where they were used, minimizing leakage.
- Do not sort or rinse textiles in the location of use.
- Label or color-code bags or containers for contaminated waste.
- If laundry chutes are used:
- Ensure that laundry bags are securely closed before they are placed in the chute.
- Do not place loose items in the laundry chute.
- For textiles heavily contaminated with blood or other body fluids, bag and transport in a manner that will prevent leakage.
- Do not use dry cleaning for routine laundering in healthcare facilities.
- For clean textiles, handle, transport, and store by methods that will ensure their cleanliness.
- If healthcare facilities require the use of uniforms, they should either make provisions to launder them or provide information to the employee regarding infection control and cleaning guidelines for the item based on the tasks being performed at the facility.
(CDC, 2023c, 2023f)
OSHA’s Bloodborne Pathogens Standard requires employers to ensure that employees who have contact with contaminated laundry wear protective gloves and other appropriate PPE.
Employers are responsible for laundering reusable PPE. Work clothes such as uniforms are not considered to be PPE. Provided gowns or other PPE should be used to prevent soiling of uniforms.
Training healthcare workers who are responsible for housekeeping and management of linen and waste in appropriate infection control for their particular duties is essential for safe patient care (OSHA, 2023a).
INFECTIOUS DISEASES AND OCCUPATIONAL HEALTH STRATEGIES
ELEMENT VI
Prevention and management of infectious and communicable diseases in healthcare workers
Because healthcare workers have contact with patients and infectious material, and because vulnerable patients will be exposed to healthcare workers, healthcare organizations utilize various occupational health strategies to assess, prevent, and control infections and communicable diseases.
Occupational health services provide or refer potential healthcare employees for preplacement medical evaluation prior to taking on job duties and for periodic and episodic medical evaluations during the course of employment.
Preplacement assessments are done in order to:
- Document the employee’s baseline health status
- Implement measures to reduce the employee’s risk of acquiring or transmitting infections in the healthcare settings, such as:
- Ensuring the individual has evidence of immunity to vaccine-preventable diseases, as recommended by the Advisory Committee on Immunization Practices
- Conducting tuberculosis screening, as required by OSHA
- Offering hepatitis B immunization before starting work, as required by the OSHA Bloodborne Pathogens Standard
- Providing or referring for medical clearance for respirator fit-testing, training, and medical reevaluations, as required by the OSHA Respiratory Protection Standard
- Assess job placement and provide “clearance for duty”
- Inform the healthcare worker about occupational health services and expectations and confidentiality of health information
Periodic medical evaluations are done in order to:
- Provide additional doses of recommended vaccines
- Perform or refer for indicated follow-up testing
- Conduct periodic screening for tuberculosis as recommended by CDC
- Provide or refer for periodic respirator fit testing
Episodic medical evaluations are done in order to:
- Evaluate and manage potentially infectious exposures and illnesses
- Evaluate and manage new health conditions that may affect risk of acquiring or transmitting infections or ability to perform job functions
- Provide preplacement medical evaluation for those who are changing job duties
- Survey healthcare personnel for exposures and/or illness during outbreaks of infectious diseases in healthcare settings
(CDC, 2019)
Healthcare Workers and Communicable Diseases
Healthcare workers are responsible for reporting to their supervisor or occupational health service when they have any signs or symptoms of a communicable disease. Symptoms requiring immediate evaluation by a licensed medical professional and possible restriction from patient-care activities and return-to-work clearance may include:
- Fever or chills
- Sore throat
- Cough
- Shortness of breath or difficulty breathing
- Rash
- Vesicular lesions
- Draining wounds
- New loss of taste or smell
- Vomiting
- Diarrhea
(CDC, 2023d; NYSDOH, 2018)
Employees who report symptoms of illness should be removed from duty and medically evaluated to determine their ability to work and the duration of work restrictions.
Reporting of suspected or confirmed communicable diseases is mandated under the New York State Sanitary Code (10NYCRR 2.10). Although physicians have primary responsibility for reporting, school nurses, laboratory directors, infection control practitioners, day care center directors, healthcare facilities, state institutions, and any other individuals/locations providing healthcare services are also required to report communicable diseases.
Reports should be made to the local health department in the county in which the patient resides and must be submitted within 24 hours of diagnosis. However, some diseases warrant prompt action and must be reported immediately to local health departments by phone (NYSDOH, 2016). (See “Resources” at the end of this course for a link to New York’s “Communicable Disease Reporting Requirements.”)
STRATEGIES FOR PREVENTION AND CONTROL OF BLOODBORNE PATHOGEN TRANSMISSION
Healthcare workers who have or may be infected with hepatitis B virus (HBV), hepatitis C virus (HCV), or HIV should be evaluated for the ability to work safely. This evaluation is based on the premise that HBV, HCV, or HIV infection alone is not sufficient justification to limit the worker’s professional duties.
Factors that may bear on the ability of the healthcare worker to provide healthcare include:
- Physical or mental condition that may interfere with ability to perform assigned tasks or regular duties
- Lack of compliance with established guidelines for prevention of disease transmission and/or documentation or evidence of previous transmission of bloodborne pathogens
- Lack of infection prevention and control techniques related to performance of procedures (e.g., poor hand hygiene or failure to follow Standard Precautions)
- Any health condition that would pose a significant risk to others
Notification of patients exposed to the blood of a healthcare worker should be based on documentation of an injury to a healthcare worker or negligent practice. In such cases, the patient should be advised to receive testing for potential bloodborne pathogen exposure (OSHA, 2023a).
Healthcare facilities are required to establish a mechanism for evaluating healthcare workers with HBV, HCV, or HIV infection. However, this does not include involuntary screening of employees for HBV, HCV, or HIV. New York State Public Health Law § 2781 requires verbally advising an individual before an HIV-related test is performed and allows for the individual to object.
Institutional evaluation of individual workers known to be infected with HBV, HCV, or HIV shall be based on New York State DOH criteria and shall involve consultation with experts who can provide a balanced perspective. Such experts can include:
- An infectious disease physician and/or hospital epidemiologist with an understanding of HBV, HCV, or HIV
- A representative from the infected healthcare worker’s practice area
- The personal physician of the infected worker
All matters related to evaluation must be handled confidentially.
All HIV-infected healthcare workers are entitled to protection under the NYS HIV Confidentiality Law. Such workers are not required to disclose their status to patients or employers.
IMMUNIZATIONS
Vaccinating healthcare workers protects both themselves and patients. For those who work directly with patients or handle material that could spread infection, the CDC recommends several vaccines, described in the table below (CDC, 2023i).
Influenza
The CDC conducts studies each year to determine how well the influenza vaccine protects against flu illness. While effectiveness can vary, recent studies show that the 2022–2023 vaccine reduced the risk by 75% in the pediatric population, with 45% less likely to visit an emergency room with flu symptoms. Among adults, 18–64-year-olds were 43% less likely to be hospitalized with the flu, while those over 65 were 35% less so (CDC, 2023k).
The CDC, the Advisory Committee on Immunization Practices (ACIP), and the Healthcare Infection Control Practices Advisory Committee recommend that all U.S. healthcare workers be vaccinated annually against influenza.
From October 2022 through April 2023, between 27–54 million people in the United States were infected with influenza. Between 300,000 and 650,000 individuals were sufficiently ill to require hospitalization, and there were 19,000–58,000 deaths due to influenza. Because the flu is not required to be reported, these numbers are not representative of the total number of cases of flu during this 18-month period. These figures are considered to be lower than usual because people were taking precautions during the COVID-19 pandemic that protected them from contracting the flu (CDC, 2023g).
New York Codes, Rules, and Regulations, Title 10, Section 2.59, requires all healthcare and residential facilities and agencies to document the influenza vaccination status of all personnel for the current influenza season in each individual’s personnel record or other appropriate record. During the influenza season, all healthcare and residential facilities and agencies shall ensure that all personnel not vaccinated against influenza for the current influenza season wear a surgical or procedure mask while in areas where patients or residents are typically present.
This law requires long-term care facilities, adult homes, adult day healthcare facilities, and enriched housing programs to provide or arrange for influenza vaccination for all residents and employees every year. The law also requires these types of facilities to provide or arrange for pneumococcal vaccinations for all residents and employees for whom the vaccine is recommended per guidelines issued by ACIP (NYS, 2014).
Hepatitis B
Federal law requires that all employees whose jobs involve participation in tasks or activities with potential exposure to blood or OPIM be offered hepatitis B vaccination. The vaccination is free, safe, and highly protective. This vaccine is given in three doses. Serologic testing after vaccination (to verify that the vaccination was effective) is recommended.
The vaccination schedule most often used is three intramuscular injections, the second and third doses administered 1 and 6 months, respectively, after the first dose (CDC, 2023h).
Other Vaccines
Vaccinations recommended by the CDC for healthcare workers who do not have evidence of immunity are shown below.
The New York State of Code of Rules and Regulations currently requires that healthcare workers demonstrate immunity or be vaccinated against measles, rubella, and diseases that can be effectively controlled by one or two doses of a highly efficacious vaccine (NYS, 2023a).
| Vaccine | Recommendations in Brief |
|---|---|
| (CDC, 2023i) | |
| Hepatitis B |
|
| Influenza |
|
| MMR (measles, mumps, and rubella) |
|
| Varicella (chicken pox) |
|
| Tetanus, diphtheria, and pertussis (Td/Tdap) |
|
| Meningococcal |
|
| COVID-19 |
|
Bloodborne Pathogens Training
OSHA (2020b) requires employers to provide bloodborne pathogens training for all workers who may come into contact with blood and OPIM in their jobs.
- This training includes information on bloodborne pathogens and diseases, methods used to minimize risk and control occupational exposure, hepatitis B vaccine, and medical evaluation and postexposure follow-up procedures.
- Employers must offer this training on initial assignment, at least annually thereafter, and when new or modified tasks or procedures affect a worker’s occupational exposure.
- HIV and HBV laboratory and production facility workers must receive specialized initial training in addition to the training provided to all workers with occupational exposure. Workers must have the opportunity to ask the trainer questions. Training must be presented at an educational level and in a language that workers understand.
Although HBV and HIV are specifically identified in the OSHA Bloodborne Pathogens Standard, bloodborne pathogens include any pathogen present in human blood or OPIM that can infect and cause disease in people exposed to the pathogen. There are approximately 20 additional pathogens that can be transmitted by blood, including:
- HCV
- Malaria
- West Nile virus
- Syphilis
- Babesiosis
- Brucellosis
- Leptospirosis
- Arboviral infections
- Relapsing fever
- Creutzfeldt-Jakob disease (although not a microorganism)
- Adult T-cell leukemia/lymphoma (caused by human T-lymphotropic virus type 1 [HTLV-1])
- HTLV-1-associated myelopathy
- Diseases associated with HTLV-2
- Ebola (also known as Ebola hemorrhagic fever)
- Zika viral infection
It is yet unknown whether other nonrespiratory body fluids from an infected person, including blood, vomit, urine, breast milk, or semen, can contain viable infectious SARS-CoV-2 (Denault & Gardner, 2023; NIEHS, 2023).
To prevent transmission of bloodborne pathogens to healthcare workers, the CDC recommends:
- Strict adherence to sharps safety guidelines and Standard Precautions
- Hepatitis B vaccination of healthcare workers
- Postexposure prophylaxis and counseling in the event of exposure incident
(NIEHS, 2023)
Exposure Control Plan
OSHA’s Bloodborne Pathogens Standard (OSHA, 2020b) requires employers to:
- Establish a written exposure control plan designed to eliminate or minimize employee exposure to bloodborne pathogens. Employers must:
- Prepare an exposure determination that contains a list of job classifications in which all workers have occupational exposure and a list of job classifications in which some workers have occupational exposure, along with a list of the tasks and procedures performed by those workers that could result in exposure
- Ensure that a copy of the exposure control plan is accessible to employees
- Update the exposure control plan at least annually to reflect changes in tasks, procedures, and positions that affect occupational exposure, and also technological changes implemented to eliminate or reduce occupational exposure. Employers must:
- Annually document in the plan that they have considered and begun using appropriate, commercially available, and effective safer medical devices designed to eliminate or minimize occupational exposure
- Document that they have solicited input from frontline workers in identifying, evaluating, and selecting effective engineering and work practice controls
The exposure control plan is a key document to assist in implementing and ensuring compliance with OSHA standards, detailing information about the ways an employer provides a safe and healthy work environment, including:
- Who is responsible for implementing the plan
- Determination of employee exposure incidents
- Methods of exposure control, such as Standard Precautions; environmental, engineering, and work practice controls; PPE; and housekeeping methods
- Hepatitis B vaccination programs
- Postexposure evaluation and follow-up, as well as the procedures for evaluating the circumstances surrounding an exposure incident
- Communication of hazards to employees
- Training and recordkeeping
Employers are required to implement these preventive measures to reduce or eliminate the risk of exposure to bloodborne pathogens (MFASCO, 2023).
EMERGENCY STEPS FOLLOWING AN OCCUPATIONAL EXPOSURE
If an occupational exposure to blood or other body fluids occurs, the following steps must immediately be taken:
- Clean the contaminated area thoroughly with soap and water.
- Wash needlestick injuries, cuts, and exposed skin with soap and water.
- Flush out any splashes of blood and OPIM to the mouth and nose with water.
- If the eyes are involved, irrigate with clean water, saline, or sterile irrigants for 20 minutes.
- Seek immediate follow-up care as identified in the facility’s exposure control plan.
Medical treatment should include an immediate postexposure evaluation, prophylaxis treatment, and appropriate follow-up care, all of which should be conducted by a physician at no cost to the employee.
All incidents should be reported and documentation completed as soon as possible. However, medical treatment should not be delayed in order to fill out paperwork.
An exposure incident report includes the following:
- The time, date, and location of the exposure
- An account of all the people involved, including the exposed person, names of their first aid providers, and, if possible, the name of the source individual
- The circumstances of the exposure, any actions taken after the exposure, and any other information required by your employer
(ProBloodborne, 2023)
EMPLOYER FOLLOW-UP
Following an exposure incident, the employer is required to:
- Perform a timely evaluation of the circumstances surrounding the exposure incident to find ways of preventing such a situation from occurring again
- Identify the source individual (unless the employer can establish that identification is not possible or prohibited by state or local law), and determine the source’s HBV and HIV infectivity status
- If the status of the source individual is not already known, test the source’s blood as soon as possible, provided the source individual consents
- If the source individual does not consent, establish that legally required consent cannot be obtained
- If state or local law allows testing without the source person’s consent, test the individual’s blood if it is available
- Make the results of the tests available to the exposed worker and inform the worker of the laws and regulations concerning disclosure of the source’s identity and infectivity status
- Provide a timely written report of the above information
Medical care as the result of an exposure is provided by the employer at no charge to the healthcare worker. All test records are confidential. The healthcare worker must be given a copy of the healthcare professional’s written opinion within 15 days after the medical evaluation is finished. Postexposure prophylaxis may be administered if medically necessary, as recommended by the U.S. Public Health Service. The healthcare worker should also be offered counseling that includes recommendations for transmission and prevention of HIV (OSHA, 2020b).
Postexposure Prophylaxis (PEP)
The CDC and the Clinician Consultation Center offer guidelines for occupational postexposure prophylaxis.
HEPATITIS B
Following an exposure to HBV, prophylaxis can prevent HBV infection and subsequent development of chronic liver infection. The central component of postexposure prophylaxis is hepatitis B vaccine. In certain circumstances, hepatitis B immune globulin is recommended in addition to vaccine for added protection.
HEPATITIS C
Below are CDC guidelines for testing source patients and healthcare personnel (HCP) potentially exposed to hepatitis C virus. A source patient or HCP who is positive for HCV RNA should be referred to care.
Postexposure prophylaxis with direct-acting antivirals (DAAs) is not recommended for occupational exposures to hepatitis C. This is due to the low risk of hepatitis C infection after exposure (0%–0.2%), the effectiveness of DAAs in curing hepatitis C infections, the high cost of DAA treatments, and lack of research supporting the use of DAAs for hepatitis C postexposure prophylaxis (Corcoran, 2021).
Source-Patient Testing
- Testing of the source patient may follow option A (preferred), which is testing with a nucleic acid test (NAT) for hepatitis C virus (HCV) RNA, or option B, which is testing for anti-HCV with reflex to an NAT if positive.
- If a source patient is known or suspected to have recent behaviors that increase risk for HCV acquisition (e.g., injection drug use within the previous 4 months) or if risk cannot be reliably assessed, initial testing should include a NAT.
- Follow-up testing of health care personnel (HCP) is recommended if the source patient is HCV RNA positive, anti-HCV positive with RNA status unknown, or cannot be tested.
Healthcare Personnel Testing
- Baseline testing of HCP for anti-HCV with reflex to a NAT if positive should be conducted as soon as possible (preferably within 48 hours) after the exposure and may be simultaneous with source-patient testing.
- If follow-up testing* of HCP is recommended based on the source-patient’s status, test with a NAT at 3–6 weeks postexposure.
- If the HCP is NAT negative at 3–6 weeks postexposure, a final test* for anti-HCV at 4–6 months post exposure is recommended.
* Follow-up testing of HCP is also warranted when concerns exist about specimen integrity, including handling and storage conditions that might have compromised source-patient test results, or if they exhibit any clinical signs of HCV infection (Moorman et al., 2020).
HIV
Occupational exposures require urgent medical evaluation. Baseline HIV testing of the exposed worker should be done even if the exposed worker refuses PEP treatment.
PEP should be initiated as soon as possible, ideally within 2 hours of exposure. A first dose of PEP should be offered while evaluation is underway and should not be delayed while awaiting information about the source person or results of the exposed worker’s baseline HIV test. HIV can be established as soon as 24–36 hours after exposure. PEP is not recommended >72 hours after exposure.
Whether the exposed worker accepts or declines PEP treatment, if postexposure evaluation shows that PEP is indicated, repeat HIV testing should be done at 4 and 12 weeks. If test results at 12 weeks are negative, HIV can reasonably be excluded in relation to an occupational exposure.
The preferred HIV three-drug occupational PEP regimen is Truvada (emtricitabine plus tenofovir) orally once a day plus raltegravir orally twice a day or dolutegravir once a day for a duration of 28 days. If source-person testing is found to be negative for HIV, PEP can be discontinued before 28 days (CDC, 2023j).
SARS-CoV-2/CORONAVIRUS
Higher-risk exposures generally involve exposure of the healthcare worker’s eyes, nose, or mouth to material potentially containing SARS-CoV-2. It is considered higher risk if the healthcare worker is not wearing a respirator, or, if wearing a mask, if the person with COVID is not wearing a mask. It is also considered high risk if a healthcare worker is not wearing a face shield and the person with COVID is not wearing a mask. Close contact of 15 minutes or longer is considered a prolonged exposure, but any duration should be considered prolonged if the exposure occurs during performance of an aerosol-generating procedure.
A record of healthcare workers exposed to the virus should be maintained and should include whether or not the worker was:
- Wearing a respirator or mask
- Wearing eye protection if the source person was not wearing a surgical-grade mask
- Wearing all recommended PPE while performing an aerosol-generating procedure
Healthcare workers who are exposed to COVID-positive individuals do not need to implement work restrictions if they exhibit no symptoms and test negative for COVID at 24 and 48 hours after the exposure, unless the healthcare worker is moderately to severely immunocompromised or works with patients who are moderately to severely immunocompromised. If a work restriction is indicated, healthcare workers can return to work after 7 days if they are asymptomatic and test negative. They must be afebrile for 24 hours without the assistance of antipyretic medication. If fever or symptoms develop, they should immediately contact occupational health to arrange for medical evaluation and testing (CDC, 2022e).
A systematic review of 17 resources regarding hydroxychloroquine, lopinavir-ritonavir (LPV/r), angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), vitamin D, arbidol, thymosin drugs, and Xin guan no.1 (XG.1, a Chinese formula medicine) showed little if any benefit as PEP agents to treat SARS-CoV-2 (Seyed Alinaghi et al., 2023).
EBOLA VIRUS
There are two developed monoclonal antibody treatments recommended by the WHO to treat Ebola: mAb114 (Ansuvimab, Ebanga) and REGN-EB3 (Inmazeb). It is considered that these have contributed to reducing the fatality from Ebola from 90% to 50% of all cases (WHO, 2023d).
ZIKA VIRUS
Healthcare workers who believe an occupational exposure to Zika virus has occurred should report it immediately to their supervisor and follow their employer’s procedures. This usually involves contacting the occupational health office for an assessment of the exposure with consideration of all bloodborne pathogens.
If it is determined that an occupational exposure did occur, testing might be indicated; however, this needs to be determined individually along with public health authorities and will depend on the type of exposure, infectious status of the source patient, and individual healthcare personnel factors, including pregnancy status.
Prevention involves control of the Aedes mosquito, considered to be the primary vector. Although there is ongoing research for prevention and treatment for the Zika virus, there is currently none available. The disease is treated symptomatically for fever, rash, conjunctivitis, malaise, headache, and muscle and joint pain (WHO, 2023e).
SEPSIS
ELEMENT VII
Sepsis awareness and education
Scope of the Problem
Sepsis is the body’s severe response to an infection. Sepsis occurs when a person has an infection that triggers an extreme systemic response throughout the body. Without timely recognition and intervention, sepsis can rapidly lead to tissue damage, organ failure, and death.
Sepsis can range from mild to more severe. As sepsis worsens, blood flow to vital organs becomes impaired, leading to organ failure or tissue death. Blood clots can form in the arms, legs, fingers, and toes, resulting in gangrene and amputation.
Sepsis can progress to septic shock when the systemic infection leads to dangerously low blood pressure. Septic shock is more likely than sepsis to cause death. Most people recover from mild sepsis, but the average mortality rate for septic shock is 30%–40%. Also, an episode of severe sepsis may place the patient at higher risk for future infection.
Each year, at least 1.7 million adults in the United States develop sepsis, and on average 21% of septic patients die from sepsis (CDC, 2023k). As many as 87% of sepsis cases begin in the community, and up to 50% of sepsis survivors are left with long-term physical and/or psychological effects (Mayo Clinic, 2023; Sepsis Alliance, 2022).
In New York State, approximately 50,000 patients are impacted each year, and on average almost 30% of septic patients die from sepsis (NYS, 2019).
NEW YORK STATE SEPSIS CARE IMPROVEMENT INITIATIVE
The New York State Sepsis Care Improvement Initiative was begun in 2013 to improve early recognition and treatment of patients with severe sepsis and septic shock. This was partly due to the death of Rory Staunton, a previously healthy 12-year-old who died of septic shock after obtaining a simple abrasion on his arm. The bacterial skin infection that developed went undetected, and he was misdiagnosed as having a stomach virus. Within 48 hours of his injury, he died of streptococcal sepsis (SIDM, 2023).
Rory’s Regulations, mandated in 2013, require all hospitals to develop protocols for sepsis recognition and treatment that provide for:
- Screening and early recognition for patients with sepsis, severe sepsis, and septic shock
- A process to identify and document individuals appropriate for treatment through severe sepsis protocols
- Guidelines for treatment, including for early delivery of antibiotics
(NYS, 2023b)
Since 2014, the initiative has been a resource for quality improvement in sepsis care with the goal of improving early detection, providing timely interventions, and reducing overall mortality. Data are collected in an annual public report containing the results from each reporting hospital in New York State for:
- Use of evidence-informed sepsis protocols to identify and treat adults and children with sepsis
- Adherence to time-dependent key interventions
- Development of a methodology for evaluating risk adjustment mortality rates for each hospital
(NYS, 2019)
Causes of Sepsis
Sepsis is caused by infection—bacterial, viral, fungal, or parasitic. Anyone can get an infection, and almost any infection can lead to sepsis. The most likely include:
- Pneumonias
- Infections of the digestive system
- Infections of the urinary tract
- Bacteremia (bloodstream infections)
People who are higher risk for sepsis include:
- Adults 65 or older
- People with chronic medical conditions (e.g., diabetes, lung disease, cancer, kidney disease)
- Pregnant women
- People with weakened immune systems (e.g., human immunodeficiency virus)
- Children younger than 1 year
- Those with wounds or injuries, such as burns
- Those with invasive devices (e.g., central lines, endotracheal tubes)
- Those who previously received antibiotics or corticosteroids in the last 90 days
(Cleveland Clinic, 2023; Mayo Clinic, 2023)
Early Recognition of Sepsis
There is no one sign or symptom of sepsis. Rather, sepsis can be recognized by a combination of symptoms, including any of the following:
- Tachycardia
- Fever usually >101 °F, shivering, or feeling very cold
- Change in mental status
- Rapid, shallow breathing
- Lightheadedness
- Symptoms specific to source of infection (i.e., dysuria, worsening cough)
Symptoms of Septic Shock
Septic shock is a later, often final, stage of sepsis. The following may occur quickly after the early signs of sepsis if it is not recognized and treatment started immediately:
- Confusion or disorientation, difficult to rouse
- Severe hypotension
- Inability to stand up
- Clammy or sweaty skin
- Extreme sleepiness
(Mayo Clinic, 2023)
Diagnosing Sepsis
Sepsis is diagnosed based on history, physical findings, and laboratory and other tests. Since the signs and symptoms of sepsis are the same as in many other conditions, sepsis may be hard to diagnose in its early stage.
Several tests may be ordered in order to determine underlying infection. A blood sample may be drawn to test for:
- Evidence of infection (peripheral blood cultures from two different sites)
- Clotting problems
- Abnormal liver or kidney function
- Impaired gas exchange
- Electrolyte imbalances
- Serum lactate (essential to determine adequate tissue perfusion)
Depending on symptoms, other tests may be done on one or more body fluids including:
- Urine
- Wound secretions
- Respiratory secretions
If the site of infection is not obvious, one or more of the following imaging tests may be done to target a suspected site of infection:
- Chest, abdominal, or extremity X-ray
- CT scan of abdomen or head
- Abdominal ultrasound
- MRI
A lumbar puncture test is indicated when there is clinical evidence or suspicion of either meningitis or encephalitis.
A venous or arterial blood lactate level should be drawn early with suspected infection and sepsis, in the presence of mildly abnormal vital signs. A high lactate level places the patient at increased risk of adverse outcomes.
DART is a sepsis scoring tool used to plan for early recognition, assessment, and treatment for sepsis in order to prevent it from becoming septic shock:
- Detect: identify sepsis early, measure lactate
- Act: give a crystalloid bolus to start and reassess, start antibiotics early, and get source cultures quickly
- Reassess: repeat lactate measurement, reassess patient after fluid bolus
- Titrate: monitor patient response, address ongoing hypotension
(ACEP, 2023; Mayo Clinic, 2023)
Principles of Sepsis Treatment
Patients with sepsis and septic shock require admission to the hospital. Early and aggressive treatment boosts a patient’s chances of surviving sepsis. Management principles of sepsis and septic shock include:
- Early recognition
- Early and adequate antibiotic therapy
- Treating the source of infection with antimicrobial therapy, surgery, or both
- Early hemodynamic resuscitation and continued support
- Proper ventilator management with low tidal volume in patients with acute respiratory distress syndrome
- Renal dialysis, if indicated
(Cleveland Clinic, 2023; Sepsis Alliance, 2022)
Patient Education and Prevention
Sepsis and septic shock have serious consequences. Therefore, it is imperative that patients and families are educated about what it is and how to prevent it from happening to them. Since the best way to prevent sepsis is to block pathogens from entering the body, education should include such measures as:
- Practicing good hygiene, such as handwashing
- Keeping scrapes and cuts clean and covered until healed
- Getting recommended vaccinations
- Having any serious cut or animal or human bite examined by a primary care provider
- Managing chronic medical conditions
- Recognizing early signs and symptoms of worsening infection and sepsis
- Seeking immediate care if signs and symptoms are present
(Cleveland Clinic, 2023)
CONCLUSION
During the past four decades, healthcare-associated infections (HAIs) have emerged as a significant risk to patient and healthcare provider safety. In order to ensure both patient and healthcare worker safety, infection control and prevention strategies are required in all healthcare settings. Outcomes of infection control programs should be continually assessed and reported for their effectiveness.
Healthcare workers must understand the chain of infection as it applies to basic infection prevention and control concepts and their role in breaking the links of the chain to prevent HAIs. Effective infection control programs include an emphasis on Standard and transmission-based precautions, along with updates on the most current recommendations for PPE, work practices, and engineering controls.
As seen in recent infectious disease outbreaks, such as the SARS-CoV-2/coronavirus pandemic, there is a need to recognize unique situations requiring enhanced infection control precautions and to respond in a timely and efficient manner. A swift response on the part of scientists and healthcare workers, together with accurate, large-scale public education, can help keep people safe.
RESOURCES
Communicable disease reporting requirements (NY State Department of Health)
Demonstration of donning (putting on) and doffing (taking off) personal protective equipment (video) (CDC)
Healthcare-associated infections (CDC)
Infection control (NY State Department of Health)
Interim infection prevention and control recommendations during the COVID-19 pandemic (CDC)
OSHA Bloodborne Pathogens Standard
Postexposure prophylaxis (PEP) consultation
888-448-4911
REFERENCES
NOTE: Complete URLs for references retrieved from online sources are provided in the PDF of this course.
American Dental Association (ADA). (2022). Infection control and sterilization. https://www.ada.org
Agency for Healthcare Research and Quality (AHRQ). (2023). Multiple settings. https://www.ahrq.gov
American College of Emergency Physicians (ACEP). (2023). Sepsis. https://poctools.acep.org
Anderson D. (2020). Infection prevention: Precautions for preventing transmission of infection. UpToDate. https://www.uptodate.com
Anderson D & Kanafani Z. (2020). Infection control in the outpatient setting. UpToDate. https://www.uptodate.com
Ather B, Mirza T, & Edemekong P. (2020). Airborne precautions. StatPearls. https://www.ncbi.nlm.nih.gov
Australian Commission on Safety and Quality in Health Care (ACSQHC). (2021). Environmental cleaning: Information for cleaners. https://www.safetyandquality.gov
Bio-MED. (2023). OSHA and medical waste. https://www.getbiomed.com
Centers for Disease Control and Prevention (CDC). (n.d.). Sequence for donning and doffing personal protective equipment (PPE). https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2023a). Hand hygiene in healthcare settings. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2023b). How to clean and disinfect early care and education centers. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2023c). Isolation precautions. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2023d). Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2023e). Environmental cleaning procedures. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2023f). Preventing HAIs: Appendix D—Linen and laundry management. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2023g). 2022–2023 U.S. flu season: Preliminary in-season burden estimates. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2023h). Hepatitis B VIS (interim). https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2023i). Vaccine information for adults. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2023j). How do I prescribe PEP? https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2023k). CDC launches new effort aimed at strengthening survival and recovery rates for all sepsis patients. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2022a). HAIs and antibiotic use prevalence survey. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2022b). About parasites. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2022c). HAI data: Data portal. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2022d). Nail hygiene. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2022e). Interim guidance for managing healthcare personnel with SARS-CoV-2 infection or exposure to SARS-CoV-2. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021a). FAQs about HAI progress report. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021b). Strategies for sharps disposal container use during supply shortages for managers and purchase. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021c). Hand hygiene in healthcare settings: Healthcare providers. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2020). Nursing homes and assisting living (long-term care facilities [LTCFs]). https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2019). Medical evaluations. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2016). Standard precautions for all patient care. https://www.cdc.gov
Centers for Medicare and Medicaid Services (CMS). (2020). CMS-approved accrediting organizations. https://www.cms.gov
Cleveland Clinic. (2023). Sepsis. https://my.clevelandclinic.org
Corcoran MA. (2021). Management of health care personnel exposed to HCV. Hepatitis C Online. https://www.hepatitisc.uw.edu
Cornell University (CU). (2023). Sharps handling. https://ehs.cornell.edu
Cumming C. (2020). Medical device cleaning, disinfection, & sterilization 101. https://www.mindflowdesign.com
Decker BK. (2022). Role of health care laundry in infection prevention. Infection Control Today, 26(6). https://www.infectioncontroltoday.com
Denault D & Gardner H. (2023). OSHA bloodborne pathogen standards. Library of Medicine. https://pubmed.ncbi.nlm.nih.gov
Dias R, Sousa D, Lourinho R, & Maurício R. (2023). Peracetic acid as a disinfectant for wastewater reuse—Regulation (EU) 2020/741 application on a pilot-scale. Environmental Monitoring and Assessment, 195, article 697. https://doi.org/10.1007/s10661-023-11313-7
Drummond C. (2022). How many times can you reuse your N95 mask? Verywellhealth. https://www.verywellhealth.com
Environmental Protection Agency (EPA). (2023a). Who regulates medical wastes? https://www.epa.gov
Environmental Protection Agency (EPA). (2023b). Waste management. https://www.epa.gov
Infection Prevention and You. (2023). Break the chain of infection. https://infectionpreventionandyou.org
Infection Prevention Control (IPC). (2021). Community infection prevention and control policy for domiciliary care staff. https://www.infectionpreventioncontrol.co.uk/content/uploads/2021/05/DC-08-PPE-April-2021-Version-2.00.pdf
InformedHealth.org. (2020). The innate and adaptive immune systems. for Quality and Efficiency in Health Care. https://www.ncbi.nlm.nih.gov
Johns Hopkins. (2023). Emerging infectious diseases. https://www.hopkinsmedicine.org
Johnson KS & Sexton DJ. (2023). Technique, indications, contraindications, and complications in adults. UpToDate. https://www.uptodate.com
JoVE. (2023). Factors affecting risk of infection. https://www.jove.com
JST Medical. (2023). What is a critical, semi-critical, and non-critical instrument? https://www.autoclave.nz/pages/what-is-a-critical-semi-critical-and-non-critical-instrumen
LabCE. (2023). Respiratory hygiene/cough etiquette. https://www.labce.com
Mayo Clinic. (2023). Sepsis. https://www.mayoclinic.org
Merrill R. (2021). Introduction to epidemiology (8th ed.). Jones & Bartlett Learning.
Michigan First Aid Company (MFASCO). (2023). 7 elements of a successful bloodborne pathogens exposure control plan. https://www.mfasco.com
Monegro AF, Muppidi V, & Regunath H. (2023). Hospital-acquired infections. StatPearls. https://www.ncbi.nlm.nih.gov
Moorman AC, de Perio MA, Goldschmidt R, et al. (2020). Testing and clinical management of health care personnel potentially exposed to hepatitis C virus—CDC guidance, United States. MMWR, 69(6),1–8. http://dx.doi.org/10.15585/mmwr.rr6906a1external icon
National Institute of Environmental Health Sciences (NIEHS). (2023). Can SARS-CoV-2 be spread by blood? https://tools.niehs.nih.gov
New York State (NYS). (2023a). Codes, rules, and regulations. Volume A (title 10), part 405.3. Administration. https://regs.health.ny.gov
New York State (NYS). (2023b). Codes, rules, and regulations. Volume C (title 10), chapter V, subchapter A, article 2, part 405, title: section 405.4—medical staff. https://regs.health.ny.gov
New York State (NYS). (2014). Codes, rules, and regulations. Volume A (title 10), part 2, section 2.59. Prevention of influenza transmission by healthcare and residential facility and agency personnel. https://regs.health.ny.gov
New York State (NYS). (2019). New York State report on sepsis care improvement initiative: Hospital quality performance. https://www.health.ny.gov
New York State Department of Environmental Conservation (NYSDEC). (2020). Regulated medical waste. https://www.dec.ny.gov
New York State Department of Health (NYSDOH) and State Education Department. (2018). Infection control training syllabus. https://www.health.ny.gov
New York State Department of Health (NYSDOH). (2016). Reporting requirements. https://www.health.ny.gov
Occupational Safety and Health Administration (OSHA), U.S. Department of Labor. (2023a). Bloodborne pathogens and needlestick prevention. https://www.osha.gov
Occupational Safety and Health Administration (OSHA), U.S. Department of Labor. (2023b). Control and prevention. https://www.osha.gov
Occupational Safety and Health Administration (OSHA), U.S. Department of Labor. (2020a). Needlestick/sharps injuries. https://www.osha.gov
Occupational Safety and Health Administration (OSHA), U.S. Department of Labor. (2020b). OSHA bloodborne pathogens standard. http://www.osha.gov
Oregon State University (OSU). (2020). Ch. 1: An invisible world. In Allied health microbiology. OpenStax. https://open.oregonstate.edu
Patient CareLink. (2023). Healthcare-acquired infections (HAIs). https://www.patientcarelink.org
Potter PA, Perry A, Stockert P, & Hall A. (2023). Fundamentals of nursing (11th ed.). Elsevier.
ProBloodborne. (2023). Exposure incident and reporting. https://www.probloodborne.com
Schrubbe K. (2020). Sterilization monitoring: An important quality assurance process. Science and Health, Technology, and Gadgets. https://www.docseducation.com
Selanders L. (2023). Florence Nightingale. Encyclopedia Britannica. https://www.britannica.com
Sepsis Alliance. (2022). What is sepsis? https://www.sepsis.org
Seyed Alinaghi S, Karimi A, Pashaei Z, Shobeiri P, Janfaza N, et al. (2023). Post-exposure prophylaxis for COVID-19: A systematic review. Infectious Disorders Drug Targets. 23(5), e130423215723. https://doi.org/10.2174/1871526523666230413082721
Society to Improve Diagnosis in Medicine (SIDM). (2023). From a small scrape to sepsis. https://www.improvediagnosis.org
The Joint Commission (TJC). (2022). Temperature and humidity requirements: Guidance for storage of sterile supplies: Do we need to monitor the temperature and humidity of the rooms where sterile supplies are stored? https://www.jointcommission.org
Wisconsin Department of Health Services (WDHS). (2023). Infection control and prevention: Transmission-based precautions. https://www.dhs.wisconsin.gov
World Health Organization (WHO). (2023a). Health workforce. https://www.who.int/health-topics/health-workforce#tab=tab_1
World Health Organization (WHO). (2023b). Disease outbreaks. https://www.emro.who.int/health-topics/disease-outbreaks/index.html
World Health Organization (WHO). (2023c). Surveillance in emergencies. https://www.who.int/emergencies/surveillance
World Health Organization (WHO). (2023d). Ebola virus disease. https://www.who.int/news-room/fact-sheets/detail/ebola-virus-disease
World Health Organization (WHO). (2023e). Zika virus. https://www.who.int/news-room/fact-sheets/detail/zika-virus
World Health Organization (WHO). (2020). Clean care is safer care. https://www.who.int/gpsc/clean_hands_protection/en/
Zoltán I. (2023). Ignaz Semmelweis. Encyclopedia Britannica. https://www.britannica.com






Customer Rating
4.9 / 3267 ratings