Stroke
Comprehensive Acute Stroke Care
Online Continuing Education Course
Course Description
Stroke education for nurses and other healthcare professionals. This stroke training course includes the NIH Stroke Scale (NIHSS) assessment, stroke symptoms, types of stroke, pathophysiology, diagnosis, prehospital and ED evaluation, treatment options. The course provides an emphasis on acute care and initial rehabilitation for patients who have experienced a stroke.
Course Price: $49.00
Contact Hours: 9
Pharmacotherapeutic Hours: 0.5
Course updated on
December 2, 2025
"The course was very interesting and easy to follow. Since I work at a Certified Stroke Center of Excellence, this course will be very beneficial to me in my practice going forward." - Tyler, RN in Louisana
"Very useful and most complete resource for a stroke unit." - Erlinda, RN in California
"One of the best-written CEU's I have taken. Will look forward to taking more programs. Very well explained, and I love the format and the algorithm." - Dorothy, RN in Florida
"This is an excellent learning activity, and I would highly recommend it. I much prefer this reading format to modules. I was able to complete it in less time due to my background in stroke care." - Cheryl, RN in New Hampshire
Stroke
Comprehensive Acute Stroke Care
Copyright © 2025 Wild Iris Medical Education, Inc. All Rights Reserved.
LEARNING OUTCOME AND OBJECTIVES: Upon completion of this continuing education course, you will demonstrate an understanding of the anatomic alterations, pathophysiology, diagnosis, evaluation, and treatment options, emphasizing acute care and initial rehabilitation, for patients who have experienced a stroke. Specific learning objectives to address potential knowledge gaps include:
- Review stroke epidemiology.
- Identify risk factors, triggers, and effects of stroke.
- Discuss major classifications, including pathophysiology and clinical presentation.
- Describe the components of prehospital and emergency department evaluation and management.
- Discuss the guidelines for early treatment and management of patients with acute stroke.
- Identify the complications and associated interventions that may occur during the intensive care unit (ICU) care of acute stroke patients.
- Summarize hospital nursing management for stroke patients beyond 24 hours.
- Identify assessment, interventions, and goals of physical, occupational, and speech-language stroke rehabilitation in the acute setting.
- List actions to be taken in the prevention of secondary stroke.
TABLE OF CONTENTS
- Introduction
- Epidemiology
- What Is a Stroke?
- Types of Stroke
- Prehospital Management of Acute Stroke
- Emergency Department Stroke Evaluation and Management
- Early Treatment and Acute Stroke Management
- Preventing and Managing Complications Post 24 Hours
- Nursing Care Beyond 24 Hours
- Rehabilitation in the Acute Stroke Setting
- Discharge from the Hospital
- Conclusion
- Resources
- References
INTRODUCTION
A stroke—also called a cerebrovascular accident (CVA) or a brain attack—is a reduction or interruption of the flow of blood through an artery to one or more areas of the brain within the territory supplied by that artery. The end result is varying degrees of neurologic and/or cognitive malfunction lasting longer than 24 hours. A very severe stroke can cause sudden death.
Stroke is a medical emergency, and for persons experiencing a stroke, the difference between recovery and disability or death is measured in hours. For healthcare professionals, it is imperative that an understanding of stroke and the ways to take action become part of day-to-day practice. Providers are responsible for improving their skills along the continuum of care from prehospital/acute stroke to poststroke education. In addition, educating patients about stroke prevention and recognition of stroke should be part of every provider’s practice.
EPIDEMIOLOGY
- Globally, 1 in 4 adults over the age of 25 will have a stroke in their lifetime. 12.2 million people worldwide will have their first stroke this year, and 6.5 million people will die as a result. Over 100 million people globally have experienced a stroke.
- Stroke is the second leading cause of death among noncommunicable disorders (NCDs) and the third leading cause of death and disability combined in the world.
- Every 40 seconds, someone has a stroke in the United States, totaling more than 795,000 people. About 610,000 of these are first or new strokes, and about 185,000 (nearly 1 in 4) strokes are in people who have had a previous stroke.
- Each day, 453 individuals die of stroke in the United States, one every 3 minutes and 14 seconds. Between 2012 and 2022, stroke deaths in the United States increased 28.7%.
(AHA, 2025a; CDC, 2024d; Feigin et al., 2025; WSO, 2025)
By Age
Stroke risk increases with age, but a stroke can occur at any age. Although it is more common among older people, a stroke can happen to anyone at any time, including teenagers, children, newborns, and unborn babies. Roughly 53% of strokes occur in people under age 70 worldwide, and every year about 70,000 Americans under the age of 45 have strokes. About 10% to 15% of strokes occur in children and adults under age 45, and that number is rising.
The risk of stroke in children is greater in the first year of life and during the periods right before and right after birth. The cause of most of these perinatal strokes remains unknown. Warning signs are often missed in children because there is a lack of awareness that strokes can happen in this age group (ASA, 2025d; CHASA, 2025; Sutter Health, 2024; WSO, 2025).
By Gender
Women face a disproportionate burden of stroke mortality and disability. One in five women in the United States experience stroke, with 55,000 more women than men having a stroke each year. Stroke is the third leading cause of death for women and kills over 90,000 women a year. Among women, Black women have the highest prevalence of stroke.
Unique risk factors for women include:
- Oral contraceptive use, especially in women with high blood pressure
- Pregnancy, with pregnant women being three times more likely to have a stroke than nonpregnant women of the same age
- Preeclampsia, which doubles the risk of stroke later in life
- Hormone replacement therapy (HRT)
- Combination of migraine headaches with aura (more common in women) and smoking
- Atrial fibrillation, which increases the risk of stroke fivefold
(AHA, 2025d)
By Race and Ethnicity
Black people have nearly twice the risk of a first stroke as White people. Strokes among African Americans tend to occur earlier in life, and these individuals are more likely to become disabled from a stroke. Research indicates over two thirds of Black Americans have at least one risk factor for stroke, which can include:
- Hypertension (present in over 50% of Black adults)
- High prevalence of overweight or obesity (present in almost 70% of Black men and 80% of Black women)
- High prevalence of diabetes
- High sodium intake (African Americans may have a gene that greatly increases sensitivity to salt and its effects)
- Prevalence of high levels of LDL (“bad”) cholesterol (present in nearly 25% of Black people)
- Sickle cell anemia (the most common genetic disorder among African Americans)
- Smoking (seen in over 14% of Black adults, increasing the risk of stroke two- to fourfold)
- Not exercising regularly (fewer than half of African American adults meet the weekly goal of moderate or vigorous activity)
- Stress
Hispanic Americans and American Indians/Alaska Natives are also at greater risk than White people for having a stroke but are at less risk than Black people. The rate of stroke at younger ages is higher in Hispanics than in the rest of the population.
Stroke mortality rates are higher in Black Americans, Native Americans, Alaska Natives, Hispanics, Native Hawaiians, and other Pacific Islanders, compared with White Americans. Although stroke death rates have declined over the decades among all other races/ethnicities, Hispanics have seen an increase (ASA, 2023a; Levine et al., 2020; Madsen et al., 2024; NIH, 2025).
By Geographic Location
The southeastern United States is often referred to as the stroke belt because of its higher stroke incidence and mortality rate. This area includes a higher proportion of rural residents relative to other regions, and nationally, residents of rural regions have a higher stroke incidence compared with those living in more urban areas.
Data suggest that contributors to the higher incidence of stroke in this region may include a larger proportion of Black people and of people with a higher prevalence of traditional stroke risk factors, a higher rate of inflammation and infection, and lower socioeconomic status. Environmental exposures and lifestyle choices are thought to play a lesser role. While substantial progress has been made in gaining an understanding of the contributors to the stroke belt, much work remains to understand this disparity (Liuzzo et al., 2024).
The 15 states with the highest mortality rate for stroke in 2022 were:
- Delaware
- Mississippi
- Alabama
- Louisiana
- North Carolina
- Oregon
- Ohio
- Tennessee
- Arkansas
- South Carolina
- Florida
- Michigan
- Georgia
- Maryland
- West Virginia
The 15 states with the highest percentage of stroke diagnosis in adults from 2020 to 2022 were:
- Mississippi
- Tennessee
- Alabama
- Louisiana
- Kentucky
- Arkansas
- West Virginia
- Oklahoma
- North Carolina
- Ohio
- Georgia
- Missouri
- South Carolina
- Indiana
- Pennsylvania
(Statista, 2024, 2025)
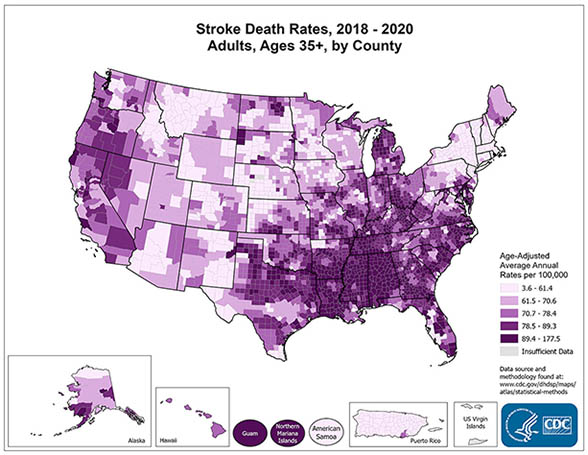
(Source: National Center for Chronic Disease Prevention and Health Promotion.)
WHAT IS A STROKE?
What is a stroke and how does it happen? For many, a stroke happens suddenly, without any warning, and because the brain controls everything the body does, it can affect how the person moves, feels, thinks, and communicates. Any stroke can be life-changing, and it can happen to anyone of any age. The experience of stroke is different for everyone, but some effects are more common than others.
Risk Factors for Stroke
Stroke risk factors have been categorized as either nonmodifiable or modifiable.
NONMODIFIABLE RISK FACTORS
- Age. The incidence of stroke increases with age for both males and females.
- Gender. Women have more strokes than men, and stroke kills more women than men. Factors increasing risk include pregnancy (especially high blood pressure during and after pregnancy); history of preeclampsia/eclampsia, gestational diabetes, endometriosis, premature ovarian failure, or early menopause; oral contraceptive use (especially when combined with smoking); postmenopausal hormone therapy; and changes in hormonal status.
- Race/ethnicity. Disparities among racial and ethnic groups are well documented (see above).
- Genetics. Family history of stroke in a parent, grandparent, sister, or brother, especially before reaching age 65, increases stroke risk. Some strokes are also caused by genetic disorders such as CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy), which occurs when thickening of blood vessel walls blocks the flow of blood to the brain.
- Fibromuscular dysplasia (FMD). This medical disorder involves fibrous tissue growth in artery walls, which causes them to narrow.
- Brain aneurysms or arteriovenous malformations (AVMs). Aneurysms are bulges in an artery that can stretch and burst, and AVMs are tangles of faulty arteries and veins that can rupture.
- Viral infections or conditions that cause inflammation. Examples may include lupus, rheumatoid arthritis, and COVID-19, which damage the heart muscle and causes arrhythmias.
- Personal past history of stroke, transient ischemic attack (TIA), or heart attack. A TIA is a brief episode of stroke-like symptoms (lasting under 24 hours but with no permanent damage or disability); it increases the risk of stroke by 10 times.
(ASA, 2025e; Kesav et al., 2023; NHLBI, 2023)
MODIFIABLE RISK FACTORS
- Hypertension. Elevated blood pressure is the leading cause of stroke and the most important controllable risk factor for stroke.
- Diabetes. While treatable, diabetes increases the risk of stroke.
- Cigarette smoking. Cigarette smoking is a major risk factor. Nicotine and carbon monoxide damage the cardiovascular system. Use of birth control pills combined with cigarette smoking can greatly increase the risk. Secondhand smoke has also been identified as an independent risk factor. Smoking may double stroke risk for African Americans.
- Marijuana. Smoking, eating, or vaping marijuana increases the risk of stroke. Inhaling marijuana and smoking cigarettes have a similar risk of stroke, since the toxins found in marijuana are similar to those in tobacco cigarettes.
- Cardiac causes. Atrial fibrillation and atrial cardiopathy are associated with cardioembolic strokes. Sleep disorders are also associated with increased stroke risk.
- Dyslipidemia. High total and LDH cholesterol are risk factors for stroke. HDL levels influence development of hemorrhagic but not ischemic stroke.
- Sedentary behavior. The relationship between physical activity and stroke may be due to its association with decreased blood pressure, reduction in diabetes mellitus, and reduction in excess body weight.
- Diet/nutrition. Diet influences the risks of stroke and other stroke risk factors, including diabetes mellitus, hypertension, and dyslipidemia. Some components are well known, such as salt intake (increased hypertension) and potassium intake (associated with decreased stroke risk).
- Obesity/waist-to-hip ratio. Obesity is related to other stroke risk factors such as hypertension and diabetes mellitus. The major contributor to risk is waist-to-hip ratio rather than overall increased weight as indicated by body mass index.
- Medications. Certain drugs have been shown to increase risk for stroke, especially when used in combination with other risk factors for stroke. These may include anticoagulants and fibrinolytic agents, which increase the risk for bleeding.
- Polluted air. Increasing evidence suggests that living or working in areas with air pollution is an emerging risk factor for stroke.
- Alcohol consumption. The relationship of alcohol to stroke risk depends on the stroke type. Alcohol consumption has a more direct linear relationship with hemorrhagic stroke, and consuming even small amounts of alcohol seems to increase risk of hemorrhages. Heavy drinking is associated with an increased risk of ischemic stroke.
- Other unhealthy lifestyle habits. Getting too much sleep (more than 9 hours) and using illegal drugs (such as cocaine) are associated with increased risk of stroke.
- Infections. Data suggest that chronic exposure to common bacterial and viral infections is a risk factor for stroke and may act as a trigger for stroke. Research has found that the risk of stroke increased after respiratory tract infection but was reduced after vaccination against influenza, pneumococcal infection, and tetanus.
- Genetics. Although most often considered a nonmodifiable risk factor, genetic therapies may change this in the future. Some may be modifiable because environmental factors may interact with genetic mutation, e.g., a person predisposed to diabetes or hypertension could reduce risk through lifestyle modifications. Some may already be modifiable, if not curable, e.g., those with sickle cell anemia can be treated with exchange transfusions to reduce stroke risk.
- Other medical conditions. These may include bleeding disorders, high red blood cell count, sleep apnea, kidney disease, or migraine headaches with aura.
(AHA 2024; ASA 2023b; CDC, 2024b, 2024d; NHLBI, 2023; Sabih et al., 2023)
ANSWERING PATIENT QUESTIONS
Q: My mother died of a stroke. Am I likely to have a stroke, too? What about my children?
A: People whose parents, grandparents, brothers, or sisters had a stroke have a higher risk of stroke themselves. It is therefore of great importance to reduce your chances of having a stroke and thus protect your children against stroke in the future. The following lifestyle measures can help to reduce the risk of stroke.
- Keep your blood pressure in a healthy range. High blood pressure can cause a stroke. People can have high blood pressure without knowing it, so get your blood pressure checked. If you have high blood pressure, follow your healthcare provider’s recommendations.
- Stop smoking. Cigarette smokers have a greater risk of having a stroke, and cigarette smokers who are also taking birth control pills have an even higher risk. Ask your provider to suggest a plan to cease tobacco use.
- Control your diabetes. People with diabetes have a higher risk of having a stroke. Follow your provider’s recommendations for controlling your blood sugar levels.
- Keep your cholesterol level low. High blood cholesterol makes a person more likely to develop atherosclerosis, and atherosclerosis is a major cause of strokes. Get your cholesterol level checked. If your cholesterol levels are unhealthy, follow your provider’s recommendations for your diet and take any medications that are prescribed.
- Keep your weight low. Obesity is another condition that will increase your risk of developing a stroke. Losing weight is difficult, so ask your provider for help in making a realistic weight loss plan.
- Stay active. Regular exercise lowers your risk of developing a stroke.
Stroke Triggers
Although there is a good understanding of the major stroke risk factors listed above, what triggers a stroke to occur at a particular point in time remains to be understood. A new area of investigation in stroke epidemiology involves the determination of such stroke triggers. At this time, they may include:
- Infection, particularly of the urinary tract
- Acute anger or emotional upset, which is associated with the onset of ischemic stroke and intracranial hemorrhage
- Heavy physical exertion, which is associated with intracranial hemorrhage
(Reeve et al., 2025; Wegener, 2022)
PATHOLOGIES UNDERLYING STROKES
The primary pathologies underlying stroke are heart or blood vessel diseases, and the secondary manifestations in the brain are the result of one or more of these underlying diseases or risk factors.
Associated heart conditions may include:
- Atrial arrhythmias (fibrillation, flutter, paroxysmal atrial fibrillation)
- Rheumatic mitral or aortic valve disease
- Bioprosthetic and mechanical heart valves
- Atrial or ventricular thrombus
- Sinus node dysfunction
- Sustained atrial flutter
- Recent myocardial infarction (within one month)
- Chronic myocardial infarction together with ejection fraction less than 28%
- Symptomatic congestive heart failure with ejection fraction less than 30%
- Dilated cardiomyopathy
- Fibrous nonbacterial endocarditis in persons with systemic lupus (i.e., Libman-Sacks endocarditis), antiphospholipid syndrome, and cancer (marantic endocarditis)
- Infective endocarditis
- Papillary fibroelastoma
- Left atrial myxoma
- Coronary artery bypass graft (CABG) surgery
Associated blood vessel diseases may include:
- Atherosclerosis
- Hypertension
- Essential thrombocytosis
- Heparin-induced thrombocytopenia
- Noninflammatory blood vessel disorders
- Fibromuscular dysplasia
- Vasospasm after subarachnoid hemorrhage
- Reversible cerebral vasoconstriction syndromes
- Radiation-induced vasculopathy
- Moyamoya disease
- Fabry disease
- Inflammatory blood vessel disorders
- Primary angiitis of the central nervous system (PACNS)
- Giant cell arteritis (GCA)
- Cerebral vasculitis related to infection, toxins, or neoplasms
- Hematological disorders
- Inherited and acquired blood clotting disorders
- Prothrombotic disorders
- Polycythemia vera
- Genetic mutations causing disorders of the coagulation system
- Antiphospholipid antibody syndrome (APS)
- Sickle cell disease
- Hyperhomocysteinemia
- Thrombotic thrombocytopenic purpura (TTP)
Other associated pathologies may include:
- Migraine-related stroke
- Cerebral venous thrombosis
(ASA, 2023c; Caplan, 2024)
Effects of a Stroke
A stroke can have profound effects on the body as well as the mind and emotions. The effects of a stroke depend on several factors, including the location of the obstruction or hemorrhage and how much brain tissue has been affected.
Because one side of the brain controls the opposite side of the body, a stroke affecting one side of the brain will cause neurologic complications on the opposite side of the body. A stroke occurring in the left side of the brain will result in some or all of the following:
- Weakness, numbness, stiffness, or paralysis on the right side of the body
- Speech/language problems (aphasia/dysphasia)
- Slow, cautious behavioral style
- Abstract thinking
- Cognitive changes (memory loss)
A stroke occurring in the right side of the brain will result in some or all of the following:
- Weakness, numbness, stiffness, or paralysis on the left side of the body
- Vision problems
- Quick, inquisitive behavioral style
- Spatial thinking or imagery
- Cognitive changes (memory loss)
When a stroke occurs in the brainstem, depending on how severe the injury is, both sides of the body may be affected, and the person may be left in what is referred to as a locked-in state. When this occurs, the patient is able to think and is cognitively intact, but there is paralysis of all movements except vertical gaze and eyelid opening. These individuals are able to communicate with eye movements.
Damage to the brain following a stroke can result in many cognitive changes, such as the following types of memory loss:
- Memory of names, stories, and information having to do with language (verbal)
- Memory of shapes, faces, routes, and things seen (visual)
- Memory for skills or trouble learning new things (information)
Along with losing memory following a stroke, a person may develop vascular dementia, which causes problems with reasoning, planning, judgment, or other thought processes.
It is common for emotional and behavioral changes to occur as a result of a stroke. A stroke can impact mood and outlook. Mood disorders such as depression and anxiety are common, with anxiety affecting 20% of stroke survivors.
Pseudobulbar affect (PBA) is a neurologic condition that can be caused by a stroke. It is also known as emotional lability, reflex crying, and involuntary emotional expression disorder, among other names. In PBA, there is a disconnect between the frontal lobe (which controls emotions) and the cerebellum and brainstem (where reflexes are mediated). The effects are uncontrollable and can occur without an emotional trigger. PBA is characterized by a mismatch between feelings and expression, such as laughing at a funeral or crying at something that is funny (ASA, 2025a).
ANATOMY AND PHYSIOLOGY OF CEREBRAL CIRCULATION
In order to function normally, the brain depends on receiving adequate oxygen and nutrients through a network of blood vessels. Two major sets of vessels supply blood to the brain. The anterior circulation of the brain is supplied by the right and left common carotid arteries, and the posterior portion of the brain is supplied by the right and left vertebral arteries. Every minute, about 600 to 700 mL of blood flows through the carotid arteries and their branches, and 100 to 200 mL flows through the vertebral-basilar system.
The right common carotid artery originates from the bifurcation of the brachiocephalic trunk, while the left common carotid artery originates directly from the aortic arch. Each then branches to form the external and internal carotid arteries. The external carotid arteries supply blood to the face and scalp, and the internal carotid arteries supply blood to most of the anterior portion of the cerebrum.
The vertebral arteries arise from the subclavian arteries and run alongside the medulla, giving rise to branches that supply the cervical spinal cord as well as the brainstem. They end by fusing to form the basilar artery. The vertebra-basilar arteries supply the posterior two fifths of the cerebrum, part of the cerebellum, and the brainstem.
The anterior and posterior circulations communicate through a circular anastomosis of arteries called the circle of Willis, which is located at the base of the brain and serves as an effective collateral circulation, protecting against ischemia in the event of vessel disease or damage in one or more areas (UMass Chan Medical School, n.d.).
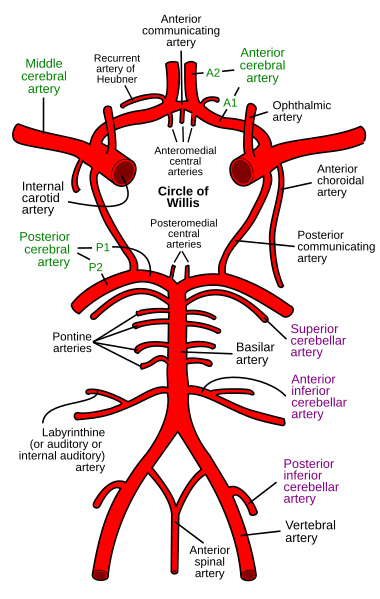
Major arteries of the brain and the brain stem (inferior view). (Source: Rhcastilhos, Public Domain.)
TYPES OF STROKE
The two major categories of stroke—ischemic and hemorrhagic—are diametrically opposite conditions, each resulting from underlying pathophysiologic states. Two other subtypes of stroke are transient ischemic attack (TIA) and cryptogenic stroke.
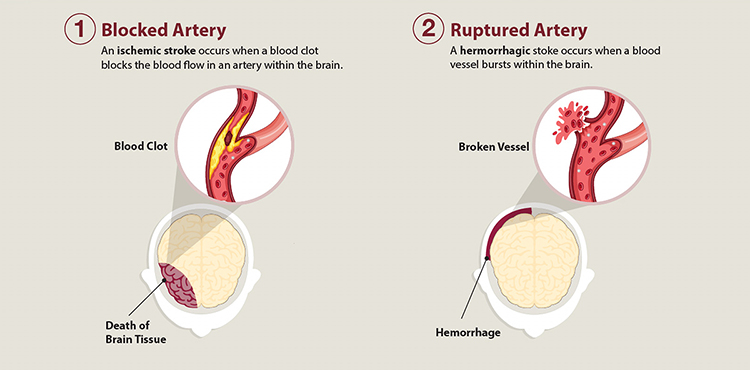
Two primary stroke types: ischemic and hemorrhagic. (Source: CDC.)
Ischemic Stroke
Most strokes (87%) are ischemic. They are characterized by the sudden loss of blood circulation to a specific area of the brain caused by an occlusion of a cerebral artery, resulting in a corresponding loss of neurologic function. Ischemia results in the loss of oxygen and nutrients to the brain cells, and local blood flow is limited to any residual flow in the major arterial source plus the collateral supply, if any (ASA, 2025a; CDC, 2024d).
The etiology of ischemic stroke is due to either a thrombotic or embolic event.
- Thrombotic stroke. Thrombotic strokes are responsible for about half of all strokes (Shmerling, 2025). Cerebral thromboses are clots that form in the cerebral arterial tree. Blood clots usually form in arteries that are damaged by atherosclerotic plaque but may also be due to arterial dissection or fibromuscular dysplasia, or to an inflammatory condition. There are two types of thrombotic stroke: large vessel thrombosis and small artery thrombosis (lacunar infarction).
- Embolic stroke. Cerebral emboli occur due to clots or other debris (such as pieces of plaque) arising from outside the cerebral arterial tree that block arterial access to a particular brain region. A main cause of embolism is atrial fibrillation, and the most common source of the clot is the valves or chambers of the heart. Other less frequent causes include venous, septic, air, or fat emboli.
- Systemic hypoperfusion. This is a more general circulatory problem that manifests itself in the brain and other organs and may be the result of cardiac pump failure or reduced cardiac output related to acute myocardial ischemia, pulmonary embolism, pericardial effusion, or bleeding (ASA, 2025a; Caplan, 2024; Lui et al., 2025).

Embolic stroke due to a thrombus formed in the atrium. (Source: NHLBI. Public domain.)
ANSWERING PATIENT QUESTIONS
Q: What is the difference between a stroke, a brain attack, and a cerebrovascular accident (CVA)?
A: These are three different names for the same thing.
Q: What is a stroke? What are the different types?
A: There are two main types of stroke: ischemic and hemorrhagic.
The most common type of stroke is ischemic. In an ischemic stroke, a brain artery becomes blocked, most commonly by a blood clot. The region of the brain normally supplied by that artery no longer gets enough blood, and that part of the brain becomes starved for oxygen and sugar. Without oxygen and sugar, nerve cells stop working, so the affected region of the brain can no longer perform its particular functions, such as moving an arm or a leg.
A less common type of stroke is hemorrhagic, which means “bleeding.” In a hemorrhagic stroke, an artery is torn, and blood begins to leak out and form a pool in or surrounding the brain. When the blood is leaking out of the artery, it is not carrying sufficient oxygen and sugar to the region that it normally supplies, and the person has the same problems as in an ischemic stroke. In addition, in a hemorrhagic stroke, the pool of blood expands and pushes on the neighboring blood vessels and brain cells. The pressure of the expanding pool of blood causes additional brain damage.
Q: I have heart disease, and my primary care provider said I might have a stroke. How can heart disease affect the brain?
A: Most strokes are caused by clots that become stuck inside arteries in the head and then cut off the supply of blood to the brain.
Both heart disease and strokes can be caused by atherosclerosis. Just as in a stroke, heart attacks and attacks of chest pain (called angina) are often caused by blood clots. Blood clots in the heart usually come from atherosclerosis. Atherosclerosis is a disease that can affect all the large arteries in the body, and some clots formed by atherosclerosis can be swept into the brain. Therefore, if a person has blood clots in their heart, then they also have a chance of getting blood clots elsewhere, such as in their brain.
Another relation between heart disease and strokes has to do with problems in the rhythm of the heartbeat. Irregular heart rhythms can cause blood clots. One particular heart rhythm irregularity, called atrial fibrillation, is notorious for putting a person at risk for a stroke. If you have atrial fibrillation, ask your primary care provider (PCP) how you can reduce your chance of getting a stroke. Also ask your PCP to teach you the warning signs of a stroke.
PATHOPHYSIOLOGY OF ISCHEMIC STROKE
Interruption of blood flow through an intracranial artery leads to deprivation of oxygen and glucose in the supplied vascular territory. This initiates a cascade of events at a cellular level that, if circulation is not reestablished in time, will lead to cell death, mostly through liquefactive necrosis (Sharma, 2025c).
Following a stroke, the affected areas of the brain that receive blood flow of less than 10 mL per 100 grams of tissue per minute are referred to collectively as the core. The cells in the core are presumed to die within minutes of stroke onset. Zones of decreased or marginal perfusion with less than 25 mL per 100 grams of tissue per minute are collectively called the ischemic penumbra. The tissue in the penumbra can remain viable for several hours because of this marginal tissue perfusion (Jauch, 2024).
ISCHEMIC PENUMBRA PROVIDES EARLY THERAPEUTIC WINDOW
After an ischemic stroke in which the ischemic penumbra has not yet been damaged structurally, permanent structural damage may be prevented if prompt restoration of perfusion in the penumbra can be restored. Collateral and residual blood flow can preserve neurons in the penumbra and border areas. Certain treatments, such as intravenous thrombolysis with tissue plasminogen activator (tPA), mechanical thrombectomy, and therapeutic hypothermia, can reduce the amount of damage that is irreversible (Lui et al., 2025; Rahmati, 2024).
Evolution of Ischemic Stroke
The temporal evolution of an ischemic stroke occurs in five stages (see table below).
| Stage | Time frame | Description |
|---|---|---|
| Early hyperacute | 0–6 hours | Growth of the lesion |
| Late hyperacute | 6–24 hours | Swelling develops and intracranial pressure increases |
| Acute | 24 hours–1 week | Extended volumes of critically hypoperfused cortical tissue |
| Subacute | 1–3 weeks | Swelling starts to subside |
| Chronic | >3 weeks | Residual swelling passes and healing begins |
Microscopically, there is also a temporal neuronal evolution of an ischemic stroke. Immediately following an ischemic stroke, the histologic appearance of neuronal tissue begins to change.
- 0–5 minutes: Irreversible neuronal injury occurs.
- 12–24 hours: Red neurons are the first pathologic microscopic finding post ischemic stroke. These are indicative of acute neuronal injury and subsequent apoptosis (cell death triggered by normal processes) or necrosis (cell death triggered by external factors). The red color comes from acidophilic cytoplasmic granules of eosinophilic neurons. These neurons can persist in the ischemic penumbra for 2–6 months.
- 24–72 hours: Neuronal cell necrosis and acute inflammatory response are seen.
- 1–3 days: Liquefactive tissue necrosis and neutrophilic infiltration are seen. The brain’s necrotic tissue becomes liquified due to proteolytic enzymes released by microglial cells.
- 3–5 days: Microglia (a macrophage specific to the CNS) become activated around the dying neuron cells to clean cell debris and release mediators of inflammation.
- 1–2 weeks: Gliosis occurs, creating more and larger glial cells that protect and support nerve cells and maintain homeostasis. These new cells can cause scars in the brain that impact how the body functions. Vascular proliferation is also seen. Reactive astrocytes stimulate angiogenesis, which induces vascular proliferation by secreting vascular endothelial growth factor.
- 2 weeks: Glial scars are seen, resulting from a healing process formed to separate and sanction off damaged tissue. Pericytes (cells present along the walls of capillaries) appear to contribute to glial scar formation.
- Over 1 month: Necrotic tissue will be completely removed, and a cystic cavity surrounded by a glial scar will be formed.
(Sharma, 2025c; Picmonic, 2025)
CLINICAL PRESENTATION OF ACUTE ISCHEMIC STROKE (AIS)
Ischemic strokes typically give rise to specific (focal) and often painless neurologic symptoms. Onset is abrupt and may progressively evolve over 24–48 hours. Most patients are involved in normal daily activities and notice these common symptoms:
- Sudden numbness or weakness of the face, arm, or leg, especially involving one side of the body
- Sudden confusion or trouble speaking or understanding
- A change in the vision of one or both eyes that occurs suddenly with no known cause
- A quick onset of dizziness, loss of coordination/balance, or other problems walking
- Sudden, severe headache with no known cause
Some patients may also experience a sudden loss of consciousness, fainting or a seizure without a known cause, and vomiting or fever that occurs within minutes or hours that cannot be explained by another cause. In large vessel ischemic stroke, headache may occur prior to, during, or following stroke onset.
The effects of an AIS may cause additional symptoms in women, including:
- Face, arm, or leg pain
- Hiccups or nausea
- Chest pain or palpitations
- Shortness of breath
(Cedars Sinai, 2025; Lui et al., 2025)
ISCHEMIC STROKE SYNDROMES
Specific neurologic functions are dependent on specialized brain regions, with each artery primarily supplying a particular region. Thus, occlusion in particular branches of the major cerebral arteries produces characteristic stroke syndromes, which are symptom complexes caused by impaired blood supply to specific areas of the brain. These syndromes help clinicians to infer which brain areas have been damaged in a specific patient’s stroke.
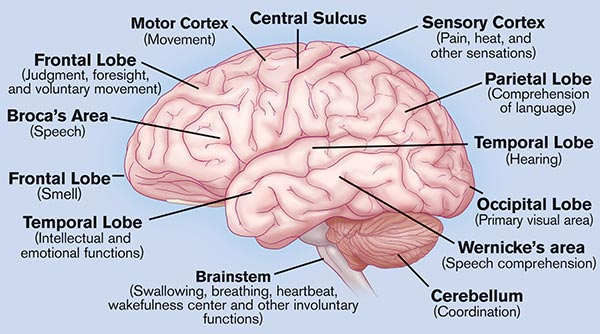
Brain regions and associated neurologic functions. (Source: Activase Image Library.)
Anterior Cerebral Artery (ACA) Stroke Syndrome
The anterior cerebral artery provides blood to the medial portion of the brain: frontal, prefrontal, primary motor, primary sensory, and supplemental motor cortices. The clinical features of this syndrome include:
- Contralateral hemiparesis and hemiplegia of lower limbs
- Contralateral sensory loss of lower limbs
- Urinary incontinence
- Fecal incontinence
- Abulia (apathy)
- Akinetic mutism (a rare condition of pathologically slowed or nearly absent bodily movement accompanied by loss of speech)
- Transcortical motor aphasia (nonfluent speech, comprehension intact, and can repeat phrases)
- Anosmia (loss of smell) related to olfactory bulb infarction
- Alien hand syndrome (in which patient thinks the left hand is not part of their body and that they have no control over its movement)
- Gait apraxia (disequilibrium and inability to lift the feet off the floor)
- Dysarthria (inability to control muscles used in speech)
- Aphasia (inability to understand or express speech)
(FlintRehab, 2025; Strokenetwork, 2025; Yartsev, 2023)
Internal Carotid Artery Syndrome
The internal carotid arteries are part of the anterior circulation supplying blood to the forebrain. The clinical features of this syndrome include:
- Amaurosis fugax (transient monocular or binocular visual loss)
- Affects circulation to the middle cerebral artery, anterior cerebral artery, and posterior cerebral artery, resulting in the presentation of middle cerebral artery (MCA) syndrome, anterior cerebral artery (ACA) syndrome, and posterior cerebral artery (PCA) syndrome
(Murphy, 2025)
Middle Cerebral Artery (MCA) Stroke Syndrome
Two thirds of ischemic strokes occur in the MCA because of the size of the territory and the direct flow from the internal carotid artery into the middle cerebral artery. Clinical presentation will depend on the extent of the infarct and hemispheric dominance.
If the infarct occurs in the left hemisphere (i.e., dominant), the resulting signs and symptoms can include:
- Right motor and sensory deficit (face and arm/leg/foot)
- Complete hemiplegia if internal capsule involved
- Right homonymous hemianopia (a visual field loss deficit in the same halves of the visual field of each eye)
- Dysarthria
- Aphasia, fluent and nonfluent
- Alexia (inability to read)
- Agraphia (inability to write)
- Acalculia (inability to do simple arithmetic)
- Apraxia (inability to perform a familiar movement on command, even though the command is understood and there is willingness to perform the movement)
If the infarct occurs in the right hemisphere (i.e., nondominant), the following signs and symptoms can occur:
- Contralateral hemiparesis
- Contralateral sensory loss
- Left hemianopsia (blindness or reduced vision in half the visual field)
- Dysarthria
- Hemispatial inattention (contralateral neglect of the left side of the environment)
- Anosognosia (lack of insight)
- Asomatognosia (the impression that one’s own body has ceased to exist)
- Loss of prosody of speech (intonation, stress pattern, loudness variations, pausing, and rhythm)
- Flat affect
(FlintRehab, 2025; Jichici, 2024; Murphy, 2025; Strokenetwork, 2025; Yartsev, 2023)
Posterior Cerebral Artery (PCA) Stroke Syndrome
The PCA supplies blood to multiple brain regions: occipital lobe, inferomedial temporal lobe, a large portion of the thalamus, upper brainstem, and midbrain. Clinical features for each region include:
Occipital:
- Contralateral homonymous hemianopia (a visual field defect involving either the two right or two left halves of the visual fields of both eyes)
- Visual disturbances, such as color anomia
- Hallucinations
- Sensory loss
- Strabismus (eye misalignment)
- Ptosis (droopy eyelid)
- Mydriasis (enlarged pupil) with abnormal reaction to light
- Choreoathetosis (involuntary movements with twisting and writhing)
- Spontaneous pain
- Cranial nerve III palsy (impaired eye movements and pupillary reaction to light)
- Motor deficit
- Cortical blindness (bilateral lesions)
- Anosognosia (unawareness or denial of blindness)
Medial temporal lobe:
- Deficits in long- and short-term memory
- Behavior alterations (agitation, anger, paranoia)
Thalamus:
- Contralateral sensory loss
- Aphasia (if dominant side involvement)
- Executive dysfunction
- Decreased level of consciousness
- Memory impairment
(Murphy, 2025; Strokenetwork, 2025)
Brainstem Stroke Syndrome
There are many brainstem stroke syndromes. Brainstem infarcts affect the midbrain, pons, and medulla oblongata, which can cause a varied range of symptoms. Early diagnosis is a must, as brainstem infarction is associated with high mortality and morbidity. Some of the clinical features seen are:
- Crossed sensory and motor findings (e.g., ipsilateral face and contralateral body numbness)
- Gaze-evoked nystagmus (rapid involvement eye movements)
- Ataxia and vertigo
- Limb dysmetria (lack of coordination of movement typified by the undershoot or overshoot of intended position with hand, arm, leg, or eye)
- Diplopia and eye movement abnormalities
- Dysarthria
- Dysphagia
- Tongue deviation
- Deafness (very rare)
- Locked-in syndrome
(Strokenetwork, 2025)
Cerebellar Stroke Syndrome
Cerebellar strokes are rare and, if left untreated, can be life-threatening. Cerebellar strokes can cause:
- Ataxia
- Nystagmus
- Vertigo
- Nausea, vomiting
- Dysarthria
- Headache
- Rapid deterioration in consciousness
(Strokenetwork, 2025)
Lacunar Stroke Syndrome
Lacunar stroke is common and most of the time asymptomatic but can cause significant morbidity and mortality. It is due to occlusion of the small branches of the cerebral vessels from the circle of Willis. The characteristic pattern of lacunar strokes may include:
- Pure motor hemiparesis
- Absence or abnormal sensation of contralateral face, arm, and leg
- Ataxic hemiparesis
- Clumsy hand syndrome (dysarthria)
- Contralateral facial weakness with dysarthria and dysphagia
- Contralateral hand weakness, ataxia, and sometimes weakness in the arm or leg
(Strokenetwork, 2025)
Vertebral Artery Stroke Syndrome
These strokes are relatively uncommon but are a disproportionate cause of morbidity and mortality due to discrete symptoms that resemble nonstroke medical conditions. The most common symptoms experienced by patients include:
- Disequilibrium
- Vertigo
- Diplopia
- Cortical blindness (total or partial loss of vision in a normal-appearing eye)
- Alternating paresthesia
- Tinnitus
- Dysphasia
- Dysarthria
- Quadriplegia
- Drop attacks (sudden fall without warning)
- Ataxia
- Perioral numbness
- Bilateral sensorimotor deficit
- Respiratory dysfunction
- Consciousness impairment/coma
Other signs and symptoms may include:
- Unilateral limb weakness
- Dysarthria
- Dysphagia
- Headache
- Nausea and vomiting
- Gait ataxia
- Nystagmus
- Perioral numbness
(Cleveland Clinic, 2022; Morasch, 2024)
Basilar Artery Stroke Syndrome
Patients with acute occlusion of the basilar artery will present with sudden and dramatic neurologic impairment, the exact characteristics dependent upon the site of occlusion:
- Sudden death/sudden loss of consciousness
- Visual and oculomotor deficits
- Behavioral abnormalities
- Somnolence, hallucinations, and dreamlike behavior
- Locked-in syndrome
- Quadriparesis
- Respiratory muscle paralysis
- Preserved consciousness
- Preserved ocular movements
(Ikram & Zafar, 2023; Sharma, 2025a)
CRYPTOGENIC STROKE
Cryptogenic strokes comprise 25% to 40% of ischemic strokes. Cryptogenic strokes are those in which a comprehensive evaluation cannot define the cause. Most cryptogenic strokes produce symptoms similar to those of strokes known to be caused by emboli; nonetheless, the strokes are labeled cryptogenic if available tests cannot document the specific cause (Prabhakaran & Ibeh, 2025).
Transient Ischemic Attack (TIA)
A transient ischemic attack, sometimes called a ministroke, is a warning sign of a future stroke. It is different from the major types of stroke because blood flow to the brain is blocked for only a short time—usually no more than five minutes. For this reason, a TIA is often dismissed and not taken seriously.
The brief interruption of blood flow in a TIA is most often caused by thrombosis to part of the brain, spinal cord, or retinas. TIA may cause temporary stroke-like symptoms but does not damage brain cells or cause permanent disability. There is brief neurologic dysfunction, with clinical symptoms typically lasting less than one hour and without evidence of acute infarction.
More than one third of people who have a TIA and don’t receive treatment have a major stroke within one year, and as many as 10% to 15% of people will have a major stroke within three months of a TIA (CDC, 2024a; Rost & Voetsch, 2025).
CLINICAL PRESENTATION OF TIA
A person experiencing a transient ischemic attack may have one or more of the following signs or symptoms:
- Weakness or numbness in the arms and/or legs, usually on one side of the body
- Dysphasia
- Dysarthria
- Dizziness
- Sudden vision changes
- Paresthesias (tingling)
- Abnormal taste and/or smells
- Confusion
- Sudden trouble walking, loss of balance or coordination
- Altered consciousness and loss of consciousness
(Panuganti et al., 2023)
Hemorrhagic Stroke
Intracranial bleeding caused by a blood vessel within the cranium that has leaked or ruptured is called a hemorrhagic stroke. Hemorrhagic strokes are less common than ischemic strokes, making up about 13% of all strokes. Risk of death related to each bleed is 10% to 15%, and the chance of permanent brain damage is 20% to 30%. There are two types of hemorrhagic strokes.
- Intracerebral hemorrhage (ICH), the most common type, occurs when a blood vessel within the brain ruptures.
- Subarachnoid hemorrhage (SAH) refers to bleeding in the subarachnoid space, the area between the brain and the meninges that cover it.
(ASA, 2025f)
PATHOPHYSIOLOGY OF HEMORRHAGIC STROKE
An intracranial hemorrhage is most commonly the result of hypertension. Bleeding occurs suddenly and rapidly. There are usually no warning signs, and bleeding can be severe enough to cause coma or death. Subarachnoid hemorrhage is often due to an aneurysm or an arteriovenous malformation (AVM) but can also be caused by trauma.
An aneurysm is a ballooning of a weakened region of a blood vessel. If untreated, it continues to weaken until it ruptures and bleeds into the brain. Berry (saccular) aneurysms are the most common type of intracranial aneurysms (90%), and most of these are located in the circle of Willis.
Ruptured brain aneurysms are fatal in about 50% of cases, and approximately 15% of people with a ruptured aneurysm die before reaching the hospital. Of those who survive, about 66% experience some permanent neurologic deficit. Brain aneurysms are most prevalent in people ages 35 to 60 but can occur in children as well (BAF, 2025; Cohen-Gadol, 2024).
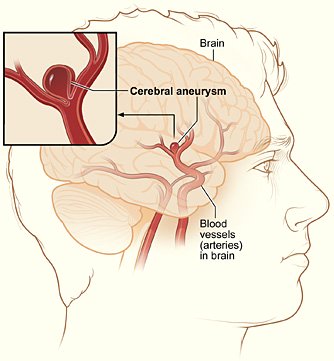
A typical location of a cerebral aneurysm in the arteries that supply blood to the brain. (Source: NIH. Public domain.)
Arteriovenous malformations (AVMs) are dilated tangled blood vessels in which the arterial blood flows directly into the venous system, bypassing the capillary bed within the brain tissue or on its surface. Brain AVMs are usually congenital but not hereditary. AVMs do not grow or change much, although the vessels involved may dilate over time and eventually burst. Risk of death related to each bleed is 10% to 15%, and the chance of permanent brain damage is 20% to 30% (ASA, 2025c).

Cranial arteriovenous malformation. (Source: © Michel Royon, CC BY-SA 3.0.)
There are different mechanisms of brain injury in intracranial hemorrhage, including:
- Primary mechanical injury to the brain parenchyma occurs via hematoma expansion and perilesional edema. This can increase intracranial pressure, which can cause reduced cerebral perfusion and ischemic injury. In very large ICH, cerebral herniation can occur, compressing the brainstem and often causing secondary hemorrhage in the midbrain and pons.
- Secondary brain injury is caused by the breakdown of the blood-brain barrier after the initial hemorrhage, including excitotoxic and inflammatory processes.
(Rordorf & McDonald, 2025b)
ANSWERING PATIENT QUESTIONS
Q: Can a stroke be stopped?
A: A stroke is the set of symptoms that follow when a brain artery is blocked or bleeding. The brain can often recover if the cause of the stroke can be reversed and fresh blood can be gotten to the blood-starved areas soon enough.
When the underlying problem is a blocked artery, the stroke symptoms will sometimes lessen or even disappear if the obstructing clot is removed or dissolved quickly enough. In the case of bleeding arteries, these will sometimes stop bleeding on their own, and sometimes they can be coaxed to slow down or stop. If the bleeding can be stopped, the stroke symptoms will sometimes lessen.
CLINICAL PRESENTATION OF HEMORRHAGIC STROKE
Symptoms of intracerebral hemorrhage often begin with a sudden headache occurring during activity. However, headache may be mild or absent in the older adult. Loss of consciousness is common, often within seconds or a few minutes. Nausea, vomiting, delirium, and focal or generalized seizures are common.
Neurologic deficits usually are sudden and progressive. Large hemorrhages, when occurring in the hemispheres, cause hemiparesis; when occurring in the posterior fossa, they cause cerebellar or brainstem deficits, such as stertorous (low-pitched, nonmusical) breathing, pinpoint pupils, coma, conjugate eye deviation, or ophthalmoplegia (extraocular muscle paralysis).
A large intracerebral hemorrhage is fatal within a few days in approximately half of patients. In those who survive, consciousness returns and neurologic deficits gradually diminish to different degrees as the blood is resorbed. Some patients may have only a few neurologic deficits due to the fact that hemorrhage is less destructive to the brain tissue than an infarct.
Small hemorrhages may result in focal deficits with no impairment of consciousness and with minimal or no headache or nausea, and they may mimic ischemic stroke (Alexandrov & Krishnaiah, 2025a).
Symptoms of subarachnoid hemorrhage begin abruptly with a severe headache (often referred to as a thunderclap headache or WHOML, “worst headache of my life”), which peaks within seconds. Loss of consciousness may follow, usually immediately, but sometimes not for several hours.
Severe neurologic deficits may develop and become irreversible within minutes or a few hours. Sensorium may be impaired, and patients may become restless. Seizures are possible.
Usually, the neck is not stiff initially, unless the cerebellar tonsils herniate. However, within 24 hours, chemical meningitis causes moderate to marked meningismus, vomiting, and sometimes bilateral extensor plantar responses. Heart or respiratory rate is often abnormal.
Fever, continued headaches, and confusion are common during the first 5 to 10 days. Secondary hydrocephalus may cause headache, obtundation, and motor deficits that persist for weeks (Alexandrov & Kirshnaiah, 2025b).
PREHOSPITAL MANAGEMENT OF ACUTE STROKE
Because fast recognition and treatment of a stroke can reduce the possibility of death and long-term disabilities, the American Heart Association developed the stroke chain of survival. This chain involves eight links or steps that should be taken by patients, family members, and prehospital and emergency room personnel in caring for stroke patients. This approach can be an effective way to make certain that appropriate care is delivered as rapidly as possible, increasing the odds for a full recovery. The eight links include:
- 1. Detection: Rapid recognition of stroke symptoms
- 2. Dispatch: Early activation and dispatch of EMS
- 3. Delivery: Rapid EMS identification, management, and transport
- 4. Door: Adhere to all door delivery times (e.g., door to needle in less than 60 minutes)
- 5. Data: Rapid triage, evaluation, and management in emergency department (ED)
- 6. Decision: Stroke expertise and therapy selection
- 7. Drug/device: Fibrinolytic therapy, intra-arterial strategies
- 8. Disposition: Rapid admission to the stroke unit or critical care unit
Prehospital management of acute stroke involves the first three links of the chain: detection, dispatch, and delivery (NHCPS, 2025).
The Role of Patients and Bystanders
The role of patients and bystanders involves the first two links in the stroke chain of survival:
- Detection: Recognizing a stroke
- Dispatch: Responding by calling 911
RECOGNIZING A POTENTIAL STROKE
Recognizing that a stroke may be taking place is the first step in caring for the patient, so public education and information is required in order to increase recognition of potential strokes. This information should include the following symptoms:
- Sudden numbness or weakness of face, arm, or leg, especially on one side of the body
- Sudden confusion or trouble speaking or understanding
- Sudden trouble seeing in one or both eyes
- Sudden trouble walking, dizziness, loss of balance or coordination
- Sudden severe headache with no known cause
(NINDS, 2025)

Classic signs of a stroke. (Source: NIH/NINDS. Public domain.)
If they are experiencing any of these symptoms, patients should call 911 or get someone else to call 911 (see below).
BARRIERS TO RECOGNIZING A STROKE IN ONESELF
Even people who know the warning signs may not realize they are having a stroke. Some factors contributing to this problem are:
- Stroke can change a person’s level of consciousness.
- Stroke can make a person confused.
- Stroke victims can misunderstand the seriousness of their bodies’ signals; for instance, pain is a major symptom of illness, but most strokes are painless.
- Stroke victims with damage to their nondominant parietal lobe can lose the ability to recognize that they are ill (anosagnosia).
- The person may be in denial.
For these reasons, it is often a family member or bystander who first realizes that a medical problem is occurring. The public should understand that if there is the possibility that someone is having a stroke, they should not hesitate—they should call 911 immediately (NINDS, 2025).
RESPONDING BY CALLING 911
People often wonder what first aid to give to a stroke victim. The best first aid is professional transport to a hospital, and bringing an emergency medical service (EMS) team to the patient is the most important action to take for a stroke victim.
In an emergency, people often believe that time is being lost by waiting for an EMS team to arrive, and so family members or bystanders often hurriedly drive patients to the hospital. In fact, patients usually get to the appropriate hospital more quickly if they use the EMS system by calling 911. EMS teams are trained to choose the most appropriate hospital in the region, which may not be the closest hospital. In addition, the care and assessment that an EMS team provides a stroke victim shortens the time lag between the onset of stroke symptoms and the evaluation and treatment of the stroke.
When calling 911, it is important to:
- Provide the emergency dispatch operator with the location of the emergency.
- If calling from a cell phone, provide the operator with the wireless phone number so the emergency operator can call back in case the call gets disconnected.
- Remember that many emergency operators currently lack the technical capability to receive texts, photos, and videos.
- Learn and use the state’s designated number for highway accidents or other non-life-threatening incidents.
FCC RULES FOR 911 CALLS
The FCC’s basic 911 rules require wireless service providers to transmit all 911 calls to a Public Safety Answering Point (PSAP) regardless of whether the caller subscribes to the provider’s service or not. Phase II E911 rules require wireless service providers to provide the latitude and longitude of callers to PSAPs. This information must be accurate to within 50 to 300 meters depending on the type of location technology used.
FCC rules also require Commercial Mobile Radio Service providers and providers of interconnected text messaging services to be capable of supporting text-to-911 service.
Rules also require providers offering voice service to be capable of transmitting 911 calls from individuals with speech or hearing disabilities via a text telephone (TTY) device other than mobile radio handsets. Rules have been amended to transition from TTY technology to RTT (real-time text), which allows the use of texting to communicate during a phone call (FCC, 2025a, 2025b).
ANSWERING PATIENT QUESTIONS
Q: What first aid should I give someone with a stroke?
A: Make sure the person is in a safe place, then call 911. Calling for assistance is the most critical first aid. The 911 operator will give you further advice about first aid.
Q: What should I do if I think I may be having a stroke myself?
A: A stroke is an emergency like a heart attack. Call 911 immediately, or get someone to call for you. Don’t wait for the symptoms to go away, and don’t worry that you may be mistaken. Paramedics would much rather come and reassure you than see you experience the consequences of an untreated stroke.
Q: If I’m close to a hospital, shouldn’t I drive myself rather than waste time calling 911?
A: Strokes can disrupt your ability to drive, so do not drive anywhere if you think you are having a stroke. It’s also better medically for you to wait for an EMS team, so don’t let someone else drive you to a hospital if it is possible to get trained professionals to take you.
Strokes need immediate treatment, but they must be treated properly. The EMS team that comes when you call 911 knows the best first aid to administer. They know which treatments to start on the way to the hospital, they know which hospital can give you the best stroke treatments, and they will call ahead so that the hospital will be prepared to speed you past the front desk and into a treatment room.
The Role of Emergency Response
EMS DISPATCHERS (PUBLIC SAFETY TELECOMMUNICATORS)
The role of EMS dispatchers (911 operators) also involves the first two links in the stroke chain of survival:
- Detection: Identifying a possible stroke
- Dispatch: Responding with speed to bring EMS to the patient
Dispatchers play a key role in the diagnosis of stroke. EMS dispatchers are the first medical contact the patient has. Their job is to interrogate the caller about the presence or absence of priority symptoms. EMS dispatchers have these responsibilities:
- Identifying the presenting problem
- Choosing, notifying, and sending the team of responders that is appropriate for each emergency
- Advising the callers on possible first aid for the patient
- Getting critical background information about the patient
(Wenstrup et al., 2024)
Identifying the Problem
Without ever seeing the patient, dispatchers are tasked with identifying the complaint, triaging the patient’s severity, and providing prearrival instructions to callers.
Once the nature and location of the emergency have been confirmed, the dispatcher’s responsibility turns to identifying the chief complaint, age, level of consciousness, and breathing status of the patient.
Stroke is difficult to identify over the phone, as callers often use vague terms to describe symptoms. Despite the challenges, however, EMS dispatchers are able to correctly identify strokes with surprising accuracy. The dispatcher will interrogate callers for time of symptom onset, rule out common stroke mimics (e.g., hypoglycemia), gather important previous medical history (e.g., prior strokes), and discover pertinent medications (e.g., antiplatelet agents or anticoagulants), thereby helping responders make improved triage and transport decisions.
With a few key questions, EMS dispatchers can respond by alerting an EMS team and shorten time-critical response. Using a stroke diagnostic tool, such as BE FAST (see below), the dispatcher will ask the patient (or ask the caller to ask the patient) to:
- Smile to check for facial drooping
- Raise both arms to check for weakness or paralysis on either side
- Repeat a simple phrase such as “the early bird catches the worm” to hear if speech is unusual
Patients are scored based on their response. If the score is high, it is more likely the person is having a stroke (CDC, 2024c; NINDS, 2025; Wenstrup et al., 2024).
The patient’s last known well (LKW) time must be established, with the goal of determining time of symptom onset. This information becomes critical due to time constraints around treatment. It is best obtained from the patient, if possible, but family, friends, and bystanders may have information to contribute. LKW is the time when the person was last known to be at baseline. It should not be interpreted as the time the patient was found with symptoms, as the onset of brain ischemia may have started before symptoms were recognized (ASA, n.d.-b).
BE FAST STROKE ASSESSMENT TOOL
The mnemonic BE FAST is an easy way for EMS dispatchers to remember the sudden signs of stroke. The following information can be elicited by either the patient or someone other than the patient.
| Initial | Stands for… | Description |
|---|---|---|
| (Cleveland Clinic, 2025) | ||
| B | Balance | Is there sudden trouble with balance or coordination? |
| E | Eyes | Is there suddenly blurred vision, double vision, or vision loss in one or both eyes without pain? |
| F | Face drooping | Does one side of the face droop or is it numb? |
| A | Arm weakness or numbness | When the person is asked to raise both arms, does one drift downward? |
| S | Speech difficulty | Ask the person to repeat a simple sentence like “The sky is blue.” Is speech suddenly slurred, garbled, nonsensical? Is the person unable to speak, or hard to understand? |
| T | Time to call 911 | If the person shows any of these symptoms, even if the symptoms go away, 911 should be called and the person should get to the hospital immediately. |
(See also “Cincinnati Prehospital Stroke Scale” later in this course.)
ANSWERING PATIENT QUESTIONS
Q: How can I tell if someone is having a stroke?
A: Strokes come on suddenly. Sometimes there is a severe headache, but many times there is no pain at all. When someone has a stroke, they are suddenly not able to do something they could do before. Classic stroke symptoms are:
- A sudden weakness of the face, arm, or leg, often on just one side of your body
- A sudden numbness of the face, arm, or leg, often on just one side of your body
- Sudden confusion, trouble speaking, or difficulty understanding things
- Sudden trouble seeing with one eye or with both eyes
- Sudden trouble walking, dizziness, or loss of balance or coordination
- A sudden severe headache that can’t be explained
A person having a stroke may show one or more of these signs. Any of the above symptoms signals an emergency, so call 911 just as you would if you saw a car accident or if a person was choking, had sudden chest pain, or became unconscious or unresponsive. You don’t have to be certain that the person is actually having a stroke.
Prehospital Triage Factors
EMS dispatchers decide what type of response is appropriate for each emergency. They choose:
- The skill level and equipment of the EMS response team: basic life support (BLS) or advanced life support (ALS)
- The type of vehicle to send
- The initial speed requirement (e.g., sirens, flashing lights, etc.)
Prehospital triage factors for acute stroke includes:
- Symptom onset
- Patient stability
- Distance to stroke-capable facility
- Run times
- Stroke designation tiers
- Comprehensive stroke center (CSC)
- Thrombectomy-capable stroke center (TSC)
- Primary stroke center (PSC)
- Acute stroke-ready hospital (ASRH)
- Availability of services
- Mobile stroke unit (MSU)
- Ground transport
- Air transport
- Public versus private EMS
- Patient preference
Acute strokes are given a priority dispatch requiring the same level of emergency treatment as heart attacks and trauma. When patients having a stroke are more than one hour’s travel time by ambulance from a hospital that is equipped to treat acute strokes, then air transport should be considered (ASA, n.d.-b).
Advising on Possible First Aid
From the time the call is dispatched to the time the first unit arrives on scene, the EMS dispatcher plays an important role in providing prearrival instructions (PAI). These are a set of medically approved, standardized, and protocolized instructions given to a layperson by the dispatcher.
The dispatcher offers prearrival instructions, which can include:
- If the caller is the patient, instruct them to lie down.
- If the person is unconscious, provide instructions on airway control.
- Keep the person calm and reassure them that help is on the way.
- Do not allow the person to move around.
- If the person is having difficulty breathing, keep the neck straight and remove pillows.
- Do not give the person anything to eat or drink.
- Gather the person’s medications (if any).
- Unlock the doors to allow EMS quick entry.
- If anything changes or the person’s condition worsens, call back immediately.
(ADH, n.d.)
Collecting Critical Information
When an EMS operator suspects that a call concerns an individual experiencing a stroke, the operator also begins collecting critical background information. Dispatchers make a special effort to get an estimate of the elapsed time since any potential stroke symptoms first appeared and to collect as much relevant data as possible, including:
- Past medical or surgical history
- Past history of a stroke
- Recent trauma or injury
- History of diabetes
- Recent seizure activity
- Recent severe headache
- Time the person was last known well (LKW), without any symptoms of stroke
- Medications the person is currently taking
(ADH, n.d.)
EMS RESPONDERS
The links in the stroke chain of survival that EMS responders are concerned with include:
- 1. Detection: Rapid EMS confirmation of a possible stroke
- 3. Delivery: Rapid management and transport
- 4. Door: Appropriate triage to a stroke center or high-acuity area facility
EMS best practice states that “time loss is brain loss,” and most patients who call EMS with symptoms of stroke are those who are within three hours of symptom onset. Stroke should be a priority dispatch with prompt EMS response (Jauch, 2024).
Confirming a Possible Stroke
A prehospital stroke assessment is completed using an assessment tool. The tool most commonly used is the Cincinnati Prehospital Stroke Scale (CPSS) (see below), a simple three-item scale based on the National Institutes of Health Stroke Scale and designed specifically for use by EMS. Another tool, the Los Angeles Prehospital Stroke Screen (LAPSS), comprises multiple elements, including the history, blood glucose, and specific physical findings (ASA, 2022).
CINCINNATI PREHOSPITAL STROKE SCALE
In the CPSS, the patient is asked to perform three actions. An abnormal response to any of the three indicates that it is likely that the patient is having or has recently had a stroke. The actions and the range of stroke and nonstroke responses are:
- “Can you smile and show me your teeth?”
- Stroke likely = one side of the face does not move at all
- Stroke less likely = both sides of the face move equally
- “Please hold both arms out in front of you and close your eyes.”
- Stroke likely = one arm drifts more or one arm does not move
- Stroke less likely = both arms move together or not at all
- “Please repeat this sentence: ‘You can’t teach an old dog new tricks.’”
- Stroke likely = no speech, incorrect words, or slurring
- Stroke less likely = correct words are repeated without slurring
Individuals with one of these three findings as a new event have a 72% probability of an ischemic stroke. If all three findings are present, the probability of an acute stroke is more than 85%.
(ASA, 2022)
Determining Stroke Severity
When a potential stroke has been confirmed, a stroke severity tool is utilized to differentiate a patient with large vessel occlusion from one without. This distinction is critical for EMS when determining the best destination hospital. Such assessment tools include:
- RACE (Rapid Arterial Occlusion Evaluation Scale)
- FAST-ED (Field Assessment Stroke Triage for Emergency Destination)
First responders and emergency personnel can also access a mobile application (app) to assess the severity of the stroke using one of several stroke scales. These scales measure certain physical indicators, which may include the ability to squeeze and release a hand, control eye movement, make facial expressions, feel a pinprick, and more. Based on results from the stroke scale, the app recommends the type of facility where a stroke patient can receive appropriate treatment (SNIS, 2025).
Collecting Critical Background Information
Regardless of the information provided to the responders that has been collected by the dispatcher, EMS responders attempt to collect other essential information about the patient. The history is direct and focused to prevent delaying transport. A baseline neurologic assessment and a medication list with a focus on anticoagulation is obtained, and it is determined if the patient has significant prestroke disability or any comorbid conditions that may impact treatment decisions (e.g., recent surgery).
It is critically important to determine when the patient was last known well (LKW), since time is important in determining treatment. A patient who woke up with new symptoms should be considered LKW at the last time he or she was seen awake, even if that was the evening prior.
Because time is of the essence, responders also gather telephone numbers of relatives and witnesses. If knowledgeable acquaintances are available, they are asked to meet responders at the receiving hospital, or, if necessary, to travel with responders. For emergency treatments, it is helpful if next of kin are immediately available for consent (ASA, 2022).
CASE
Marcella has just finished her training to become an EMS first responder. She performed well in all the training classes, but she is still quite nervous about her first call as a full-fledged EMS professional. Within the first half hour of her first shift, Marcella hears the call from the dispatcher about a likely stroke victim. Rushing to the scene, Marcella and her team are greeted at the door by the patient’s daughter, who is frantic with worry.
The patient is an 86-year-old African American woman sitting on the sofa. Marcella does an initial visual assessment and notices that the woman’s face appears to be sagging on the right side. While another team member is getting the woman’s vital signs, Marcella asks the woman to “smile and show me your teeth.” The woman’s face clearly shows asymmetry. Then Marcella asks the woman to stretch out her arms as far apart as she can. The woman tries, but Marcella notices that her left arm is drifting down. More certain that the team is dealing with a stroke victim, Marcella asks the woman to repeat the sentence “You can’t teach an old dog new tricks.”
When the woman slurs her words, Marcella tells the other team members that the assessment indicates the patient is experiencing a stroke. While the patient is being prepared for transport, critical background information is obtained and a stroke severity assessment is completed by one of the team using the tool FAST-ED. The team is able to quickly transport the patient, whose vital signs remain stable, in under 10 minutes to the nearby stroke center.
Later that evening, while reflecting on her first day as an EMS professional, Marcella realizes the importance of her stroke training. Within 30 minutes of the onset of symptoms, the woman was examined by stroke specialists, and she now has a good prognosis for eventual recovery.
Transport and Delivery
One of the most important components of stroke care, advance notification to the receiving hospital, is provided by EMS as soon as possible in the case of a potential stroke patient. This prenotification allows the receiving institution to activate local protocols, ready necessary medications, prepare and hold the brain-imaging facilities, and prepare to assess the patient upon arrival (ASA, 2022, n.d.-b).
Additional Care En Route
Instructions for care en route can include:
- Assess and reassess ABCs. Do not treat hypertension unless directed by medical command.
- Perform cardiac monitoring. Do not delay transport to obtain a 12-lead ECG.
- Provide oxygen at 4 liters/min per nasal cannula to maintain oxygen saturation of 94% to 99%. Routine oxygen administration is not indicated.
- Perform blood glucose assessment. Treat if less than 60 mg/dL. Do not treat with oral medication. Maintain strict “nothing by mouth” (NPO).
- Establish IV access, at least one in each antecubital fossa. Do not administer excess fluid or glucose.
(ASA, 2022; HCPS, 2025)
CASE
Recently trained as an EMS provider, John takes a call from the dispatcher about an 83-year-old female patient with a possible stroke. On arrival, after taking the patient’s vital signs, John notes that the patient has a blood pressure of 200/90 mmHg, a respiration rate of 28 breaths/minute, and a blue tinge around her mouth. John’s supervisor instructs him to place an oxygen mask on the patient, start an IV line, and continue monitoring the patient’s blood pressure.
When John asks about the potential dangers of the patient’s high blood pressure, the supervisor tells him that during an acute stroke, the current recommendations are to avoid attempting to control blood pressure until the patient can be fully evaluated by medical personnel. John continues to monitor the patient’s blood pressure, which remains the same, and her other vital signs. After five minutes on oxygen, John notices the patient’s color and respiration rate normalizing. Another five minutes later, the EMS team and the patient arrive at the hospital, where the stroke team takes over the patient’s care.
EMERGENCY DEPARTMENT STROKE EVALUATION AND MANAGEMENT
EMS delivery ideally involves transporting the patient to the nearest facility with appropriate stroke resources. Acute stroke treatment protocols involve specialized knowledge and practical experience. However, the facilities, equipment, and personnel for acute stroke management are expensive and are not available at most hospitals.
Emergency department care addresses these links in the stroke chain of survival:
- 5. Data: Obtaining laboratory results; performing physical and neurologic exams and brain imaging
- 6. Decision: Determining appropriate treatment
- 7. Drug: Administering drug therapy if appropriate
Types of Stroke Care Facilities
The objective of stroke centers is to improve the quality and organization of acute stroke care. Certifying organizations reward designated facilities for following evidence-based guidelines and having the ability to provide basic and advanced levels of care. Some of these organizations include The Joint Commission (TJC) and the Healthcare Facilities Accreditation Program (HFAP), with TJC being the older of the two. Each agency has different outcome measures, including healthcare team experience, patient volume, research, and survey frequency.
The Joint Commission classifies hospitals into four categories based upon the level of care they are able to provide for stroke patients:
- Acute stroke-ready hospital
- Primary stroke center
- Thrombectomy-capable stroke center
- Comprehensive stroke center
ACUTE STROKE-READY HOSPITAL (ASRH)
Acute stroke-ready hospitals tend to be smaller hospitals located in rural and suburban areas. An acute stroke-ready hospital differs from a non–stroke center in that they have around-the-clock access to stroke expertise (either by telephone or in person) and the ability to administer IV fibrinolytics prior to transferring a patient for more advanced care.
TJC certification for these facilities requires that they provide the following:
- A medical program director with sufficient knowledge of cerebrovascular disease
- Stroke protocols and an acute stroke team available 24 hours/7 days per week
- Initial assessment performed by an emergency department physician, nurse practitioner, or physician assistant
- CT, MRI (if used), and laboratory capability 24/7
- Access to a neurologist 24/7 in person or by telemedicine
- Neurosurgical and neurointerventional transfer services include a written protocol with communication and feedback from the receiving facility
- IV fibrinolytic administration capability
- Telemedicine available within 20 minutes
- Required ongoing education and training for all staff who care for stroke patients
- Education on stroke symptom recognition and activation of the stroke alert process for all organization staff
- Collaborative relationship with local EMS, provides educational opportunities to prehospital personnel
- Use of three inpatient and two outpatient acute stroke-ready measures to evaluate clinical performance
Following initial certification, recertification is required every two years following an on-site review (TJC, 2025a, 2025b).
PRIMARY STROKE CENTER (PSC)
To be certified as a primary stroke center, an emergency department (or its hospital) must meet the following criteria:
- Acute stroke team available 24/7
- A stroke unit or designated beds for the acute care of stroke patients
- CT, MRI, laboratory, CT angiography, MRA (magnetic resonance angiography) availability 24/7; at least one modality for cardiac imaging available to all patients admitted for stroke
- Neurologist available 24/7 in person or via telemedicine
- Operating room (OR) availability 24/7 in a PSC that provides neurosurgical services
- Neurointerventional transfer services include a written protocol with communication and feedback from the receiving facility
- Telemedicine available
- Treatment with IV fibrinolytics and medical management of stroke
- Required ongoing education and training for all staff who care for stroke patients
- Education on stroke symptom recognition and activation of the stroke alert process for all organization staff
- Provision of education to prehospital personnel and a minimum of two stroke education activities per year to the public
- Use of eight standardized measures to evaluate clinical performance
Following initial certification, recertification is required every two years following an on-site review (TJC, 2025a, 2025b).
TELESTROKE CONSULTATION
Ideally, the treatment of all acute strokes is provided in PSCs or higher-level facilities, but many areas of the country are far from such centers. One way to extend the range of acute stroke treatment, especially the administration of fibrinolytic agents, into areas far from stroke specialists is by using video teleconsultation, or telestroke.
Telestroke (telemedicine) is a two-way videoconference between distant stroke-care specialists (neurologists) and local emergency medicine doctors to recommend diagnosis and treatment that can be given in the local community. This avoids the need for transfer to another medical center, thereby reducing the delay between recognition of stroke and appropriate treatment.
As in a direct on-site consultation, physicians and patients communicate using digital video cameras, internet telecommunications, robotic telepresence, smartphones, tablets, and other technology.
In telestroke, many people work together as a team, including a program manager, a clinical coordinator, vascular neurologists, neurosurgeons, and radiologists at the distant site and emergency medicine physicians and other staff at the originating site. Radiology technicians, informational technology staff, researchers, nurses, nurse practitioners, and other staff also are important members of the team (Mayo Clinic, 2023).
THROMBECTOMY-CAPABLE STROKE CENTER (TSC)
A thrombectomy-capable stroke center is a facility that has performed mechanical thrombectomy (MT) and postprocedure care for at least 15 patients with ischemic stroke in the past 12 months or at least 30 patients over the past 24 months. The requirements for this certification include:
- A program medical director with a neurology background and ability to provide clinical and administrative guidance to program
- A dedicated neuro-intensive care unit or designated intensive care beds for complex stroke patients available 24/7; on-site critical care coverage and acute stroke team available 24/7
- Catheter angiography, computed tomography/computed tomography angiography (CT/CTA), MRI (including DWI [diffusion-weighted imagery]), labs available 24/7; carotid duplex ultrasound and TEE (transesophageal echocardiogram) available as indicated
- A neurologist accessible 24/7 in person or via telemedicine and written call schedule for attending physicians available 24/7
- OR available 24/7
- Physician privileged to perform MT available on-site within 30 minutes; endovascular licensed practitioners available on-site; written neurointerventional plan and call schedule readily available
- Treatment capability that includes IV fibrinolytics, endovascular therapy
- Required ongoing education and training for all staff who care for stroke patients
- Education on stroke symptom recognition and activation of the stroke alert process for all organization staff
- Provision of educational opportunities to prehospital personnel, EMS records of care
- Having standardized measures for clinical performance: eight inpatient stroke (STK) measures and five ischemic comprehensive stroke (CSTK) measures, for a total of 13
(TJC, 2025a, 2025b)
COMPREHENSIVE STROKE CENTER (CSC)
Comprehensive stroke center certification is available only in Joint Commission–accredited acute care hospitals. Organizations seeking CSC certification must meet all of the following eligibility requirements:
- Program medical director with extensive expertise available 24/7
- Dedicated neuro-intensive care unit beds for complex stroke patients and neuro-intensivist coverage 24/7
- Catheter angiography, CT/CTA, MRI, labs, MRA (including DWI) 24/7; carotid duplex, TEE, TTE (transthoracic echocardiogram), TCD as indicated
- Neurologist who meets emergent needs of multiple complex stroke patients; written call schedule for attending physicians providing availability 24/7
- Physician privileged to perform MT available on-site within 30 minutes; endovascular licensed practitioners available on-site; written neurointerventional plan and call schedule readily available
- Neurosurgical services available 24/7
- Treatment: IV fibrinolytics; endovascular therapy; treatment of aneurysms using an FDA-approved device; carotid artery procedures, neurosurgical services as indicated by patient need
- Required ongoing education and training for all staff who care for stroke patients
- Education on stroke symptom recognition and activation of the stroke alert process for all organization staff
- Sponsor a minimum of two public education opportunities annually; present a minimum of two educational courses annually for internal staff or individuals external to the CSC (e.g., referring hospitals)
- Participate in patient-centered stroke research approved by the institutional review board
- Use of eight core inpatient stroke measures and 10 comprehensive stroke measures, for a total of 18, to evaluate clinical performance
(TJC, 2025a, 2025b)
MOBILE STROKE UNIT (MSU)
Mobile stroke units are based on the theory that bringing stroke treatment to patients could reduce prehospital delays, improve thrombolysis times, and subsequently improve patient accounts. MSUs have been shown to administer fibrinolytics 38.5 minutes faster than traditional EMS units that transport stroke patients to emergency departments. The percentage of patients treated with fibrinolytics within 60 minutes of the last time they were well was only 3% via traditional EMS transport but 33% via MSUs (Bradley, 2023).
An MSU is an ambulance equipped with all the traditional medications, tools, and resources used to treat stroke patients in a hospital setting. Most importantly, MSUs house portable CT scanners capable of eight-slice image acquisition that is comparable to in-hospital CT image quality. MSUs are equipped with lead-reinforced walls and floors, lead vests and personal radiation detectors are worn by staff, and protocols are in place to protect staff members and the public from radiation.
The team can vary per MSU program but always includes EMS personnel, an appropriately credentialed clinician responsible for tissue plasminogen activator (tPA) treatment decisions, and a technologist to operate the CT scanner. Others may include a vascular neurologist either on board or via telemedicine and a registered nurse with specialized stroke training (Bradley, 2023).
STROKE PROTOCOLS
When a potential stroke patient enters any ED, staff must begin a protocol that can lead directly to the administration of a fibrinolytic drug at the present hospital or at a stroke center. The main goals are rapid access to thrombolysis for ischemic stroke patients and stabilization and rapid admission to a stroke unit for all stroke patients.
Upon arrival, a typical protocol to accomplish the goal of rapid treatment includes:
- Immediate emergency department triage to high-acuity area
- Prompt emergency department evaluation
- Stroke team mobilization
- Lab studies and brain imaging
- Diagnosis and determination of most appropriate therapy
- Administration of drugs/treatment/interventions
- Timely admission to stroke unit, ICU, or transfer
(Physiopedia, 2025c)
Triage
Time to treatment is critical. Therefore, patients with suspected acute stroke are assigned the same high priority as patients with acute myocardial infarction or serious trauma, regardless of the severity of their neurologic deficits.
About one half of all stroke patients will not enter the emergency department by ambulance. The ED registration staff must be trained to recognize signs of possible stroke. The front desk nurse should have a written stroke-recognition checklist. This will ensure that any triage nurse can quickly channel potential stroke victims into the ED’s stroke protocol.
IDENTIFICATION OF A POTENTIAL STROKE VICTIM
The Emergency Severity Index (ESI) is a five-level emergency department triage algorithm that provides clinically relevant stratification of patients into five groups, from Level 1 (most urgent) to Level 5 (least urgent), on the basis of acuity and resource needs, including labs, ECG, X-rays, CT, MRI, ultrasound-angiography, IV hydration, IV or IM medications, specialty consultation, and simple and complex procedures (ENA, 2023).
A five-level ESI triage algorithm. (Source: Adapted from Gilboy et al., 2011.)
This algorithm triages patients based on the severity of symptoms.
- Level 1 represents a patient who has no pulse, may be intubated or unable to breathe on their own, and may be unresponsive to noxious stimuli (P/U on APVU [alert, voice, pain, unresponsive] scale) or to verbal commands. Immediate lifesaving intervention such as resuscitation is required.
- Level 2 (where stroke patients should be placed) is a high-risk, emergency situation. The recommendation here is to imagine the hospital had “one last bed.” The stroke patient should get priority. This is true whether or not the patient is confused, lethargic, or disoriented and whether or not the patient is in pain.
- Levels 3, 4, and 5 are least urgent and would not apply to a stroke patient. They are dependent upon the number of resources required to provide appropriate care.
(ENA, 2023)
TARGET: STROKE
Target: Stroke, an initiative by the American Heart Association/American Stroke Association, is a national quality-improvement initiative that focuses on reducing the door-to-needle (DTN) times for patients being treated with tPA (tissue plasminogen activator) at hospitals. Since the benefits of tPA in ischemic stroke patients are time dependent, current guidelines recommend a DTN time of 60 minutes or less, meaning that the patient should receive the medication within 60 minutes of arriving at the emergency department.
Research indicates that less than 30% of stroke patients are treated within this crucial window. Additional significant disparities exist in the timely treatment of patients who are female, older, or African American.
Door-to-needle is a term used in the treatment of patients with tPA and is not to be confused with the term last known well (LKW), which is an assessment term that quantifies the time when the patient was last feeling well (or normal) prior to the onset of stroke symptoms.
The recommended time targets for key steps in the management of acute stroke using the Target: Stroke DTN are as follows:
- From the door to a physician: 10 minutes or less
- From the door to the stroke team: 15 minutes or less
- From the door to CT or MRI initiation: 25 minutes or less
- From the door to CT or MRI interpretation by a specialist: 45 minutes or less
- From the door to tPA treatment (door-to-needle time): 60 minutes or less
(AHA, 2025b; AHA, 2025c)
TIME SHEET DOCUMENTATION DURING TRIAGE
For patients with an acute onset of neurologic signs, triage nurses complete the following:
- A stroke recognition checklist
- A time sheet documenting:
- Time of onset of symptoms, or time when the patient was LKW
- Time of the patient’s arrival in the ED
- Time goal for the initial provider’s assessment (i.e., 10 minutes or less after the patient’s arrival at the ED)
- Time goal for stroke team’s or neurology specialty practitioner’s initial assessment (i.e., 15 minutes or less after the patient’s arrival)
- Time goal for a completed CT/MRI scan (i.e., 25 minutes or less after the patient’s arrival)
- Time goal for interpretation of neuroimaging scan (i.e., 45 minutes or less after the patient’s arrival)
- Time windows for the recombinant tissue plasminogen activator (rtPA) treatment of eligible patients
- ED goal (e.g., within 60 minutes of the patient’s arrival)
- 3-hour time window after onset of symptoms
- 4.5-hour time window in well-screened patients who are at low risk for bleeding
- 4.5- to 24-hour time window, evaluate candidate for mechanical thrombectomy
- Door to admission time of 3 hours or less after arrival for all patients
(ACLS, 2024; Filho & Samuels, 2025)
The time sheet then follows the patient to keep providers, nurses, and technicians on schedule.
ROLE OF THE EMERGENCY DEPARTMENT NURSE
The emergency department nurse who initially evaluates the patient:
- Notifies CT to anticipate an emergent CT scan
- Within 10 minutes of arrival, assesses vitals and provides oxygen if patient is hypoxemic
- Obtains IV access (if not done by EMS)
- Checks glucose (unless EMS glucose value is already known)
- Activates the stroke team
- Anticipates orders for:
- CT without contrast or MRI to rule out ICH or nonstroke lesions
- CT with contrast head/neck
- Laboratory tests for cardiac-specific troponin and chemistry panel
- Coagulation studies and deep vein thrombosis prophylaxis
- Arterial blood gas analysis to assess for hypoxemia
- 12-lead EKG to rule out myocardial infarction (MI) and atrial fibrillation
- Electroencephalogram (EEG) to rule out ongoing seizures
- Chest X-ray if clinically indicated
(ACLS, 2024; Tadi et al., 2023)
MOBILIZATION OF THE HOSPITAL’S STROKE TEAM
When a potential stroke patient has been identified, a stroke page is initiated from the incoming EMS vehicle or from the ED triage nurse. The stroke team responds to the page within 5 minutes and reviews the patient’s history, establishes a timeline of symptom onset, and performs a neurologic examination using either the NIH Stroke Scale or the Canadian Neurological Scale.
If the patient is determined to have had an ischemic stroke, fibrinolytics and/or thrombectomy will be considered. If CT or MRI subsequently shows intracranial hemorrhage (subarachnoid or intracerebral), immediate neurology and neurosurgery consults will be obtained (ACLS, 2024).
CASE
Eleanor, a 62-year-old African American female patient, arrives to the emergency department accompanied by her daughter. Eleanor presents with sudden onset of left-eye blindness beginning 30 to 45 minutes ago while she was at home reading a magazine. Her daughter called 911 for immediate transport. Eleanor says it was as if “someone had dropped a gray curtain over my left eye” but that her vision is improving.
The nurse in the ED, Joan, asks the patient if she has had a headache, weakness, dizziness, tingling, fatigue, or slurred speech in the past. Beyond occasional headaches, Eleanor denies any of these symptoms and adds that this blindness has never happened to her before. Eleanor’s health history reveals that she has well-controlled type 2 diabetes and hypertension, with untreated hyperlipidemia that was recently diagnosed.
Eleanor’s medications include metformin (Glucophage), 1,000 mg, by mouth twice daily; lisinopril (Zestril), 5 mg, by mouth daily; and hydrochlorothiazide (Esidrix), 25 mg, by mouth daily. Eleanor was previously on estrogen replacement therapy for eight years post hysterectomy. Her pertinent family history includes a mother who had a cerebrovascular event at age 82 years.
Based on Eleanor’s symptoms, medical history, and family history, the nurse immediately consults with the ED physician and alerts the stroke team. The nurse also reassures Eleanor and her daughter that they were right to call 911.
Stabilization of Comorbid Medical Problems
Within 10 minutes of arrival, a general examination is done to identify other potential causes of the patient’s symptoms and coexisting comorbidities or issues that may impact the management of a stroke.
IDENTIFY AND TREAT MEDICAL PROBLEMS
A quick but thorough examination is done to assess for circulation, airway, breathing, and vital signs and to medically stabilize any problems the patient may have in addition to the stroke.
- For oxygen saturation <94%, give O2 via nasal cannula at 2–3 L/min. Supplemental oxygen is not recommended in nonhypoxic patients with acute ischemic stroke (AIS).
- Hypoperfusion and hypovolemia should be corrected.
- Patients with elevated blood pressure (BP) who are eligible for fibrinolytic therapy should have BP carefully lowered to <185 mmHg systolic and <110 mmHg diastolic before IV fibrinolytic therapy is begun.
- Sources of elevated temperature >100.4 °F should be identified and treated. Antipyretic medications should be administered if indicated.
- Treat hyperglycemia to achieve blood glucose levels in the range of 140–180 mg/dL.
- Treat hypoglycemia (<60 mg/dL) in all patients with AIS.
- Establish IV access if not yet done. Patients eligible for rtPA therapy will need a minimum of two IV sites, one for IV fluids and/or IV medications and one dedicated to rtPA administration.
- Establish continuing cardiac monitoring.
- If the patient is alcoholic or malnourished, thiamine should be given.
(ACLS, 2024; Hoh et al., 2022; Powers et al., 2019)
Stroke Diagnostic Studies
A blood glucose laboratory test must be administered in all patients. Coagulation studies (international normalized ratio [INR], activated partial thromboplastin time [aPTT], and platelet count) may also be required if there is suspicion of coagulopathy. Because of the very low risk of unsuspected abnormal findings, fibrinolytic treatment should not be delayed while waiting for testing if there is no reason to suspect abnormal results (Powers et al., 2019).
Additional laboratory tests are tailored to the individual patient and may include the following:
- Cardiac biomarkers
- Toxicology screen
- Fasting lipid profile
- Erythrocyte sedimentation rate (ESR)
- Pregnancy test
- Antinuclear antibody (ANA)
- Rheumatoid factor
- Homocysteine level
- Rapid plasma reagent (RPR)
A urine pregnancy test should be obtained for all women of childbearing age with stroke symptoms. The safety of the fibrinolytic agent recombinant tissue-type plasminogen activator (rtPA) in pregnancy has not been studied in humans (Jauch, 2024).
Medical History
The medical history should include the patient’s chief complaint and the history of the present illness. The most important piece of historical data, however, is the time of symptom onset or last known well (LKW). The history should include all symptoms the patient has experienced, as well as the time and sequence of each of them. Obtaining this history may require interviewing a family member and/or a witness.
A medical history for stroke patients includes a review of systems, eliciting the following information:
- Hypertension
- Diabetes mellitus
- Tobacco use
- High cholesterol
- History of coronary artery disease, coronary artery bypass, or atrial fibrillation
In younger patients, elicit a history of:
- Recent trauma
- Coagulopathies
- Illicit drug use (especially cocaine)
- Migraines
- Oral contraceptive use
Nausea, vomiting, headache, and a sudden change in the patient’s level of consciousness helps distinguish ischemic from hemorrhagic stroke (Jauch, 2024).
Patient Examination
Patient examination includes a focused physical examination, a neurologic examination, and a formal stroke assessment.
FOCUSED PHYSICAL EXAMINATION (WITH ECG)
The purpose of the focused general physical is to:
- Detect extracranial causes of stroke symptoms
- Distinguish stroke from stroke mimics
- Determine and document the degree of neurologic deficit using the NIH Stroke Scale in order to determine severity and possible location of the stroke and for future comparison
- Localize the lesion
- Identify comorbidities
- Identify conditions that can influence treatment decisions, such as recent surgery or trauma, active infection, or active bleeding
The physical examination must include all major organ systems, including a careful head and neck exam for signs of trauma, infection, and meningeal irritation. Vital signs can point to impending clinical deterioration and may assist in narrowing the differential diagnosis.
A search for the cardiovascular causes of stroke requires examination of the following:
- Ocular fundi for retinopathy, embolic, or hemorrhage
- Heart for arrhythmias, such as atrial fibrillation, gallop, or murmur
- Peripheral vascular including palpation of carotid, radial, and femoral pulses and auscultation for carotid bruit
- Unequal pulses or blood pressures in the extremities, which may indicate the presence of an aortic dissection
(Jauch, 2024)
NEUROLOGIC EXAMINATION
A brief but accurate neurologic exam should be done, with the goals of:
- Confirming the presence of stroke syndrome
- Distinguishing stroke from stroke mimics
- Establishing a neurologic baseline to assess improvement or deterioration of condition
- Establishing stroke severity to assist in prognosis and therapeutic selection
The essential components of the neurologic examination include:
- Cranial nerves
- Motor function
- Sensory function
- Cerebellar function
- Gait
- Expressive and receptive language capabilities
- Mental status and level of consciousness
The skull and spine should be examined for signs of meningismus (neck stiffness, headache, and other symptoms suggestive of meningeal irritation) (Jauch, 2024).
FORMAL STROKE ASSESSMENT
American Heart Association/American Stroke Association (AHA/ASA) guidelines recommend all potential stroke victims be assessed using the NIH Stroke Scale (NIHSS). This is a measure of the severity of neurologic deficits and can be used to objectively monitor the improvement or deterioration of the stroke. The NIHSS is designed to be simple, valid, and reliable and can be administered consistently by physicians, nurses, or therapists.
Standardized stroke assessment tools do not replace a neurologic exam. Instead, the stroke scale is an efficient way to objectively determine the severity and possible location of the stroke. NIHSS scores are helpful in identifying patients who would likely benefit from fibrinolytic therapy and those at greater risk of hemorrhagic complications of fibrinolytic use.
The NIHSS focuses on six major areas of the neurologic examination: These include:
- Level of consciousness
- Visual function
- Motor function
- Cerebellar function
- Sensation and extinction (formerly known as neglect)
- Language
The NIHSS is a 42-point scale. Patients with minor strokes usually have a score of <5. An NIHSS score >10 correlates with an 80% likelihood of proximal vessel occlusions.
| Instructions | Scale | Score |
|---|---|---|
| (Jauch, 2024; Physiopedia, 2025b) | ||
| Level of consciousness (LOC) observed | Alert | 0 |
| Drowsy | 1 | |
| Stuporous | 2 | |
| Comatose-unresponsive | 3 | |
| Orientation questions: What month is it? What is your age? | Answers both correctly | 0 |
| Answers one correctly | 1 | |
| Answers both incorrectly | 2 | |
| Response to commands: Open and close eyes. Grip and release nonparetic hand. | Performs both tasks correctly | 0 |
| Performs one task correctly | 1 | |
| Performs neither | 2 | |
| Best gaze: “Follow my finger with your eyes.” | Normal horizontal movements | 0 |
| Partial gaze palsy | 1 | |
| Forced deviation | 2 | |
| Visual fields | No visual field deficit | 0 |
| Partial hemianopia | 1 | |
| Complete hemianopia | 2 | |
| Bilateral hemianopia | 3 | |
| Facial palsy: Show teeth. Raise eyebrows. Squeeze eyes shut. | Normal, symmetrical | 0 |
| Minor facial weakness | 1 | |
| Partial facial weakness | 2 | |
| Complete unilateral palsy | 3 | |
| Motor arms: Extend arms and hold 10 seconds, preferably with palm facing up (each arm tested and scored separately). | No drift | 0 |
| Drift before 10 seconds | 1 | |
| Falls before 10 seconds | 2 | |
| Falls, no effort against gravity | 3 | |
| No movement | 4 | |
| Motor legs: In supine position, raise leg 30 degrees (each leg tested and scored separately). | No drift | 0 |
| Drift before 5 seconds | 1 | |
| Falls before 5 seconds | 2 | |
| Falls, no effort against gravity | 3 | |
| No movement | 4 | |
| Cerebellar testing: Limb ataxia (finger-nose-finger and heel-shin tests on both sides). | Absent | 0 |
| Ataxia in one limb | 1 | |
| Ataxia in two limbs | 2 | |
| Sensory: Pinprick to face, arm, leg. | No sensory loss | 0 |
| Mild sensory loss | 1 | |
| Severe sensory loss | 2 | |
| Sensory: Extinction. Double simultaneous test. | No neglect | 0 |
| Partial neglect (1 sensory modality lost) | 1 | |
| Complete neglect (2 modalities lost) | 2 | |
| Best language: Ask the patient to name items and describe pictures. | No aphasia | 0 |
| Mild to moderate aphasia | 1 | |
| Severe aphasia | 2 | |
| Mute, global aphasia | 3 | |
| Dysarthria: Assess speech clarity to “mama, baseball, huckleberry, tip-top, fifty-fifty.” | Normal articulation | 0 |
| Mild to moderate dysarthria | 1 | |
| Severe dysarthria with near to unintelligible or worse | 2 | |
| Total score: | _____ | |
| Score interpretation | No stroke | 0 |
| Minor stroke | 1–4 | |
| Moderate stroke | 5–15 | |
| Moderate/severe stroke | 15–20 | |
| Severe (major) stroke | 21–42 | |
For hemorrhagic strokes, the Glasgow Coma Scale (GCS) neurologic assessment tool is used to describe the general level of consciousness in patients with traumatic brain injury or suspected hemorrhagic stroke. Like the NIHSS, the GCS is not a diagnostic tool, and it does not replace the neurologic exam.
The GCS is the gold standard used to objectively describe the extent of impaired consciousness in all types of acute medical and trauma patients. The scale assesses patients according to three aspects of responsiveness: eye opening, motor response, and verbal response. Reporting each of these separately provides a clear, communicable picture of a patient’s state.
| Neurologic Aspect | Scale | Score |
|---|---|---|
| (Gaines, 2023; Jain et al., 2025) | ||
| Eye opening | Spontaneously | 4 |
| To sound | 3 | |
| To pain | 2 | |
| No eye opening | 1 | |
| Motor response | Obeys command | 6 |
| Localizes pain | 5 | |
| Withdraws from pain | 4 | |
| Abnormal flexion (decorticate) to pain | 3 | |
| Abnormal extension (decerebrate) to pain | 2 | |
| No motor response | 1 | |
| Verbal response | Oriented and converses | 5 |
| Disoriented and confused | 4 | |
| Inappropriate words | 3 | |
| Incomprehensible sounds | 2 | |
| No verbal response | 1 | |
| Total score: | ____ | |
| Score interpretation | Minor brain injury | 13–15 |
| Moderate brain injury | 9–12 | |
| Severe brain injury (comatose) | 3–8 | |
The highest score (15) indicates that the patient is fully conscious, and the lowest possible score (3) indicates the patient is comatose or deceased (Gaines, 2023).
NEUROLOGIC ASSESSMENT OF REFLEXES AND MENINGES
Deep tendon reflexes and pathologic reflexes help differentiate upper motor neuron (UMN) from lower motor neuron (LMN) lesions. Testing of reflexes includes:
- Biceps reflex
- Brachioradialis reflex
- Triceps reflex
- Patellar/knee jerk reflex
- Achilles tendon reflex
- Jendrassik maneuver
- Babinski sign
- Hoffman’s sign
- Chaddock’s sign
- Oppenheim’s sign
Meningeal signs indicate irritation of the meninges that is caused by subarachnoid hemorrhage. Assessment of the meninges includes:
- Assessment of nuchal rigidity
- Kernig sign
- Brudzinki sign
- Amos sign
(Goldemund, 2025)
Hypothesis and Diagnosis for Stroke Type and Etiology
As information accumulates, the stroke team builds evidence for the diagnosis of “stroke” or “nonstroke.” For likely strokes, the team will also be weighing the evidence for and against intracranial bleeding.
FORMING THE HYPOTHESIS
Hypothesis generation begins as soon as the first information about the patient becomes available, and as information is gathered, the clinician proceeds systematically from the more general to the more specific.
After a history is obtained, the clinician plans the physical examination to look for additional findings to help confirm or refute the preliminary diagnoses. Overall, the process of diagnosis should be logical, systematic, and sequential.
When physical examination is being done, it is important to consider the differential diagnoses that can mimic stroke. It has been reported that 19% of patients diagnosed with acute ischemic stroke (AIS) by neurologists before cranial imaging actually had noncerebrovascular causes for their symptoms.
COMMON STROKE MIMICS
The most frequent stroke mimics include:
- Seizure
- Systemic infection
- Brain tumor
- Toxic-metabolic disorders such as hyponatremia and hypoglycemia
- Positional vertigo
- Conversion disorder (a psychiatric disorder in which the patient develops paralysis, numbness, blindness, deafness, or seizures, with no underlying neurologic pathology)
(Jauch, 2024)
After completing the initial assessment, the goal of subsequent evaluation is to determine the underlying pathophysiology of the stroke in order to guide therapy. After hemorrhagic stroke has been ruled out, the next stage of evaluation is focused on distinguishing between embolic and thrombotic stroke.
Features of the clinical history can be helpful in the determination of type of stroke, including:
- Clinical course. The pace and course of signs and symptoms and their clearing are the most important historical information for differentiating stroke subtypes.
- Ecology. The known demographic and historical features (including age, sex, and race) provide probabilities of having one or more of the stroke subtypes.
- Previous transient ischemic attack (TIA). A history of TIA, especially more than one, in the same territory strongly favors presence of thrombosis; attacks in more than one vascular territory suggest brain embolism from the heart or aorta.
- Activity at onset or just before the stroke. Hemorrhages can be precipitated by sexual activity or other physical activity, while thrombotic strokes are unusual under these circumstances. Sudden coughing and sneezing can precipitate brain embolism. Getting up during the night to urinate appears to promote brain embolism.
- Associated symptoms. The presence of certain associated symptoms can be suggestive of a stroke subtype. Examples include fever suggestive of embolic stroke due to endocarditis; infections that predispose to thrombosis; and accompanying symptoms such as headache, vomiting, seizures, and decreased level of consciousness.
(Caplan, 2025b)
CRANIAL IMAGING TO CONFIRM DIAGNOSIS
Because time is of primary importance, there should be a standing order for a cranial scan for all potential stroke patients. There should also be a plan for getting the scan read quickly. Cranial imaging should be completed within 25 minutes of the patient’s arrival at the ED, and the interpretation by the radiologist on call should be available within 20 minutes of the scan’s completion.
Imaging Studies
Imaging is done to exclude hemorrhage, assess degree of brain injury, and identify the lesion responsible for the ischemic deficit.
Noncontrast computed tomography (NCCT) of the head is the main diagnostic study used to determine stroke. It is the most rapid and cost-effective strategy available. Its main limitation, however, is limited sensitivity in the acute setting. The goals of CT in the acute setting are to exclude intracranial hemorrhage, look for early features of ischemic stroke, and exclude other intracranial pathologies that may mimic a stroke.
Multimodal CT techniques, including CT perfusion imaging and CT angiography, make CT capable of addressing all acute imaging needs:
- Ruling out thrombectomy candidates
- Ruling out hemorrhage
- Identifying large vessel occlusion
- Detecting infarct core and penumbra
- Assessing collateral flow
CT perfusion has become a critical tool in the selection of patients for fibrinolytic treatment as well as increasing the accurate diagnosis of ischemic stroke by nonexpert readers fourfold compared to routine noncontrast CT. It allows both the core of the infarct and the surrounding penumbra that can potentially be salvaged to be identified.
CT angiography may identify thrombus within an intracranial vessel and may guide intra-arterial thrombolysis or clot retrieval. It may also establish stroke etiology and evaluate carotid and vertebral arteries in the neck.
Multiphase or delayed CT angiography is showing benefit, either replacing CT perfusion or as an additional fourth step in the stroke CT protocol, as it guides patient selection for endovascular therapy by assessing collateral blood flow in the ischemic and infarct tissue.
MRI with magnetic resonance angiography (MRA) has been a major advance in the neuroimaging of stroke. MRI not only provides great structural detail but also can demonstrate early cerebral edema. In addition, MRI has proven to be sensitive for detection of acute intracranial hemorrhage. However, MRI is not as available as CT scanning in emergencies, many patients have contraindications to MRI imaging (e.g., pacemakers, implants), and interpretation of MRI scans may be more difficult.
Diffusion-weighted imaging (DWI) is highly sensitive to early cellular edema, which correlates well with the presence of cerebral ischemia. DWI shows far greater contrast and is superior at highlighting tissue injury within minutes of a cerebral infarct. It provides information on the viability of brain tissue, showing image contrast that is dependent on the molecular motion of water, which may be substantially altered by disease.
Transcranial doppler ultrasound (TCD) is useful for evaluating more proximal vascular anatomy—including the middle cerebral, intracranial carotid, and vertebrobasilar artery—through the infratemporal fossa. It has been utilized for the diagnosis of intracranial vessel occlusion, as well as for the differentiation between ischemic and hemorrhagic stroke, in the context of a negative CT and a clinically suspicious patient presentation.
The use of single-photon CT (SPECT) scanning in stroke is still experimental and available only at select institutions. Theoretically, it can define areas of altered regional blood flow.
(Jauch, 2024; Sharma, 2025a, 2025c)
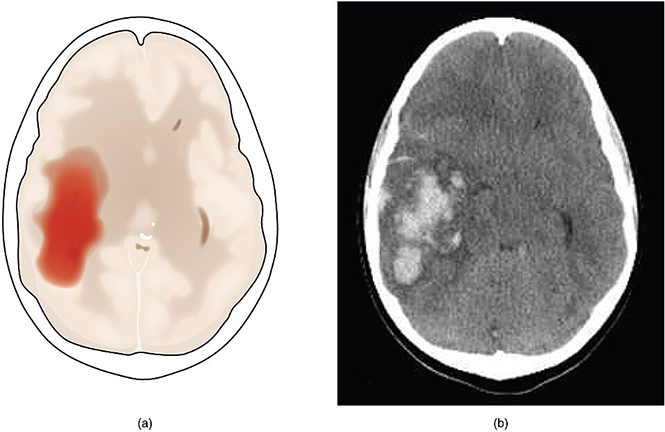
CT scan showing a hemorrhagic stroke (shaded area on the left). (Source: OpenStax College. CC BY 3.0.)
LUMBAR PUNCTURE (LP)
A lumbar puncture is done following a negative noncontrast CT if the history and symptoms contribute to increased clinical suspicion for subarachnoid hemorrhage. A lumbar puncture can be performed up to 12 hours from onset of symptoms.
The lumbar puncture involves the collection of at least four tubes of cerebrospinal fluid (CSF) to detect xanthochromia, a pink or yellow coloration of the CSF caused by the breakdown of red blood cells and subsequent release of heme pigments, including bilirubin. This is sometimes the only sign of an acute subarachnoid hemorrhage. Xanthochromia is typically present in the CSF within 6 to 12 hours after the onset of symptoms (Dugas et al., 2024).
OTHER DIAGNOSTIC PROCEDURES
Once a determination of the cause of a stroke is made, other diagnostic procedures may be required to aid in decision-making for treatment.
Carotid ultrasound is a two-step procedure that uses sound waves to create detailed images of the buildup of plaque and spectral analysis to measure blood flow velocity in the carotid arteries. It is done if the patient has a TIA or a medical condition that increases the risk of stroke.
Cerebral angiogram uses a catheter under X-ray imaging guidance and injection of contrast material to examine blood vessels in the brain for abnormalities such as aneurysms, plaque buildup, or thrombosis. The procedure produces very detailed, clear, and accurate images of blood vessels in the brain (Mayo Clinic, 2025).
Transesophageal echocardiogram is usually recommended when a very specific part of the heart requires imaging with greater resolution. It allows for better visualization and definition of structures within the heart and can be especially helpful in identifying thrombi (Stanford Medicine, 2025).
EARLY TREATMENT AND ACUTE STROKE MANAGEMENT
Early treatment with drug administration, if appropriate, is the next link in the chain of survival. If the patient is not a candidate for drug therapy, endovascular therapy may be the treatment of choice.
Ischemic Stroke Treatment
For ischemic strokes, the goal is to preserve tissue in the penumbra, where perfusion, although decreased, remains sufficient to prevent further infarction. Fibrinolytic therapy is the recommended treatment for select ischemic stroke patients.
FIBRINOLYTIC THERAPY
Fibrinolysis is the natural degradation of a blood clot’s fibrin network that results in the release of cellular components into the bloodstream. Fibrinolysis is required for the restoration of blood flow and is regulated by plasminogen activators that convert plasminogen into plasmin. Fibrinolysis can occur naturally in the body or via medications. The use of medications to dissolve blood clots is thrombolysis, also referred to as fibrinolytic therapy or fibrinolytic therapy. The chief benefit of fibrinolytic therapy is improved final functional outcome, and the chief risk is major early intracerebral hemorrhage, which occurs in about 10% of patients (Saver, 2024).
Tissue plasminogen activator (tPA) drugs catalyze the conversion of plasminogen to plasmin, the primary enzyme involved in dissolving blood clots associated with myocardial infarction and stroke. Examples of these drugs currently used to manage stroke include alteplase and tenecteplase. Alteplase or tenecteplase is given intravenously within 3 hours (and for some patients up to 4.5 hours) of stroke onset to break apart a clot blocking blood low within the brain.
Recombinant tissue plasminogen activator (rtPA) is a type of medication that dissolves blood clots associated with stroke. Intravenous alteplase, an rtPA medication sold under the brand name Activase, has been the primary fibrinolysis treatment for acute ischemic stroke for nearly three decades, but the more recent plasminogen activator drug tenecteplase (TNK), sold under the brand name TNKase, has been shown to have advantages over alteplase. Tenecteplase is the first medication in 30 years to be approved by the FDA for the treatment of acute ischemic stroke in adults.
Tenecteplase has been shown to have a longer half-life and lower clearance, with more fibrin specificity, less platelet aggregation, and possibly a lower rate of intracranial hemorrhage than alteplase. Because it is easier to administer (a single bolus), it reduces the potential for medication errors, dose interruptions, and time delays. Tenecteplase can improve recanalization and reperfusion without increasing the risk of hemorrhage and may lyse large vessel clots more effectively, making it a desirable alternative to alteplase (Genentech, 2025; Hagag, 2025; Wang et al., 2024).
| Alteplase | Tenecteplase |
|---|---|
| (Genentech, 2025; Paz et al., 2024) | |
| Recombinant tissue plasminogen activator that converts plasminogen to plasmin | Genetically bioengineered mutant recombinant tissue plasminogen activator that has a greater specificity for fibrin |
| Trade names: Activase, Actilyse | Trade names: TNKase, Metalyse |
| Regular dose: 0.9 mg/kg | Regular dose: 0.25 mg/kg |
| Maximum dose: 90 mg | Maximum dose: 50 mg |
| Powder that is reconstituted with sterile water | Powder that is reconstituted with sterile water |
| Given as an intravenous bolus followed by an infusion over one hour | Given as a single intravenous bolus over five seconds |
| Low vascular reperfusion | High vascular reperfusion |
| Good 90-day functional outcome | Better 90-day functional outcome |
| Good early major neurologic improvement | Significant early major neurologic improvement |
| Less fibrin specific | More fibrin specific |
| 5-minute half-life | 20-minute half-life |
| Inactivated by alteplase inhibitors | Not inactivated by alteplase inhibitors |
| Increased risk of intracranial hemorrhage | Reduced risk of intracranial hemorrhage |
| Side effects: bleeding, allergic reactions, fever, nausea | Side effects: bleeding, allergic reactions, fever, nausea |
| Expensive | Less expensive |
Eligibility for Fibrinolytic Therapy
The following table indicates inclusion criteria guidelines for administration of fibrinolytic therapy in patients whose onset of symptoms is known to be under 3 hours.
| (Saver, 2024) | |
| By stroke status: |
|
|---|---|
| By blood vessel status: |
|
| By thrombotic status: |
|
| Other criteria: |
|
| Understanding of risks/benefits: |
|
Eligibility criteria for fibrinolytic therapy at 3 to 4.5 hours from onset are similar but more stringent, with any one of the following being an additional exclusion criterion:
- Over 80 years of age
- Use of oral anticoagulants, regardless of the INR
- Baseline NIHSS score >25
- Combination of both previous ischemic stroke and diabetes mellitus
- Imaging evidence of ischemic damage to more than one third of the middle cerebral artery (MCA) territory
(Oliveira-Filho & Samuels, 2025; Saver, 2024)
Administration of Fibrinolytic Therapy
The protocol for administering fibrinolytic therapy should be written and approved by members of the stroke team and should be reviewed prior to administration. Treating an ischemic stroke with fibrinolytic medications must be done promptly. Therefore, stroke EDs need electronic standing orders for the drugs and an established procedure for quickly dispensing the drugs from the pharmacy at any hour.
INFORMED CONSENT OR PRESUMED CONSENT?
The 2018 AHA/ASA guidelines state: “The benefit of IV alteplase is well established for adult patients with disabling stroke symptoms regardless of age and stroke severity. Because of the proven benefit and the need to expedite treatment, when a patient cannot provide consent (e.g., aphasia, confusion) and a legally authorized representative is not immediately available to provide proxy consent, it is justified to proceed with IV thrombolysis in an otherwise eligible adult patient with a disabling AIS” (Powers et al., 2019).
The alteplase dosage is calculated based on the patient’s weight, with a maximum total of 90 mg over 60 minutes. Ten percent of the dose is given as an IV bolus over one minute, followed by an IV infusion of the remainder of the dose over one hour. It may be administered intravenously or intra-arterially (an off-label route).
Prior to rtPA administration, a registered nurse ensures that:
- Blood pressure has been carefully lowered to maintain systolic BP <185 mmHg and diastolic BP <110 mmHg before initiating fibrinolytic therapy
- Due to an increased risk of intracranial bleeding, INR, aPTT, and blood glucose have been completed
- For patients who are hemodynamically unstable, any prior anticoagulant therapy is discontinued before and during the fibrinolytic infusion to minimize risk of bleeding
- Vital signs and neurologic assessment have been completed
- CT scan has been completed and interpreted
- Inclusion/exclusion criteria have been met and stroke scale is completed
- Continuous ECG and SpO2 monitoring are in place
- Appropriate lab studies are completed
- Patient’s identity has been verified according to institutional protocol
- rtPA dose is verified to the order with a second RN or physician
- All procedures that might cause bleeding (indwelling urinary catheters, nasogastric tubes) are completed
- At least two large-bore IVs are in place
Initiating the infusion, the nurse makes certain that the rtPA is infused with no other drugs.
During rtPA administration, the nurse:
- Maintains the patient on strict bed rest during treatment
- Completes a neurologic assessment and takes vital signs every 15 minutes during infusion for 2 hours, then every 30 minutes for 6 hours, then hourly until 24 hours after treatment
- Increases frequency of BP monitoring if systolic BP >180 mmHg or if diastolic BP >105 mmHg; administers antihypertensive as needed to maintain these levels
- Monitors the patient and notifies the physician for adverse allergic reactions
- Discontinues infusion and obtains an emergency CT scan and appropriate laboratory work if the following signs of intracranial bleeding occur:
- Acute hypertension
- Severe headache
- Nausea and/or vomiting
- Worsening neurologic exam
- Avoids invasive procedures and IM injections, and performs venipunctures carefully and only as required, avoiding internal jugular and subclavian venous punctures
- Frequently assesses all punctures for bleeding; if bleeding occurs, stops the infusion immediately
- If extravasation occurs, stops the infusion and applies local therapy
Post administration, the nurse:
- Checks vital signs and neurologic status:
- Every 15 minutes for 2 hours, then
- Every 30 minutes for 6 hours, then
- Every 60 minutes until 24 hours after rtPA treatment
- Maintains BP <180/105 mmHg for at least 24 hours post treatment
- Withholds antiplatelet or anticoagulant therapy and invasive procedures for 24 hours following treatment
- Monitors for serious adverse events, such as bleeding and angioedema:
- Concomitant use of angiotensin-converting enzyme (ACE) inhibitors may increase the risk of orolingual angioedema.
- Concomitant use of anticoagulants and drugs that inhibit platelet function increase the risk of bleeding.
- Delays insertion of nasogastric tubes, indwelling catheters, or intra-arterial pressure catheters if patient can be managed without them
- Obtains follow-up CT or MRI scan 24 hours after treatment and before starting anticoagulants or antiplatelet agents
(LNC, 2024)
ADVERSE REACTIONS TO rtPA TREATMENT
- Bleeding (most common)
- Orolingual angioedema
- Arrhythmias
- Hypotension
- Edema
- Cholesterol embolization
- Venous thrombosis
- Re-embolization of deep venous thrombi (DVT) in patients with pulmonary embolism
- Nausea
- Vomiting
- Hypersensitivity reactions
(LNC, 2024)
Management of bleeding within 24 hours after administration of alteplase requires the nurse to:
- Stop alteplase infusion
- Obtain complete blood count (CBC), prothrombin time (PT) test (INR), aPTT, fibrinogen level, type, and crossmatch
- Obtain emergent nonenhanced head CT
- Per order, administer cryoprecipitate (includes factor VIII): 10 U infused over 10–30 minutes (onset in 1 hour, peaks in 12 hours); administer additional dose for fibrinogen level <150 mg/dL
- Per order, administer tranexamic acid 1 g (or 10 to 15 mg/kg) once; administers at a rate not to exceed 100 mg/minute (over 10 to 20 minutes) OR ε-aminocaproic acid 4–5 g over 1 hour, followed by 1 g per hour IV for 8 hours or until bleeding is controlled
- Obtain hematology and neurosurgery consult
- Manage BP, intracranial pressure (ICP), cerebral perfusion pressure (CPP), mean arterial pressure (MAP), temperature, and glucose
Management of orolingual angioedema requires the nurse to:
- Maintain airway:
- Intubation may not be needed if edema is limited to anterior tongue and lips.
- Edema involving larynx, palate, floor of mouth, oropharynx with rapid progression (within 30 minutes) poses higher risk of respiratory compromise requiring intubation.
- Awake fiberoptic intubation is preferred.
- As ordered, perform the following:
- Discontinue IV alteplase infusion and hold ACE inhibitors
- Administer IV methylprednisolone 125 mg
- Administer IV diphenhydramine 50 mg
- Administer ranitidine 50 mg IV or famotidine 20 mg IV
- If there is an increase in angioedema, administer epinephrine (0.1%) 0.3 mL subcutaneously or by nebulizer 0.5 mL
- Administer icatibant (selective bradykinin B2 receptor antagonist), 3 mL (30 mg) subcutaneously in abdomen. Additional 30-mg doses may be given at 6-hour intervals, not to exceed three injections in 24 hours.
(Powers et al., 2019)
Like alteplase, tenecteplase dosage is also calculated based on the patient’s weight, with a maximum dose of 50 mg. It is given as a single intravenous bolus over five seconds.
Tenecteplase administration and monitoring requires the nurse to:
- Push medication IV over five seconds
- Not combine medication with any dextrose-containing solutions
- Flush with normal saline before and after tenecteplase administration
- Check vital signs and neurologic status:
- Every 15 minutes for 2 hours, then
- Every 30 minutes for 6 hours, then
- Every 60 minutes until 24 hours after treatment
- Maintain BP <180/105 mmHg for at least 24 hours post treatment
- Withhold antiplatelet or anticoagulant therapy and invasive procedures for 24 hours post treatment
- Monitor for serious adverse events, such as bleeding and angioedema:
- Concomitant use of angiotensin-converting enzyme (ACE) inhibitors may increase the risk of orolingual angioedema.
- Concomitant use of anticoagulants and drugs that inhibit platelet function increase the risk of bleeding.
- Obtain follow-up head CT if neuro status declines
(NDHHS, n.d.)
INTRA-ARTERIAL FIBRINOLYTIC ADMINISTRATION
Clinical trials and registry data have proven the efficacy of administering alteplase or tenecteplase intra-arterially (IA) within 4.5 hours of symptom onset plus mechanical thrombectomy within 6 hours of symptom onset. This is now the standard of care to treat strokes caused by large vessel occlusion in patients meeting eligibility criteria.
Compared with intravenous therapy, IA therapy offers several advantages, including a higher concentration of lytic agent delivered to the clot target, lower systemic exposure to the drug, and higher recanalization rates. Disadvantages include additional time required to initiate therapy, availability only at specialized centers, and mechanical manipulation within potentially injured vessels (Saver, 2024).
Complications
Although reports indicate that rtPA and TNK may have similar rates of complications, it is not known how the unique pharmacology of tenecteplase interacts with the microenvironment of the ischemic brain. The difference in medication delivery between alteplase (IV infusion over 60 minutes) and tenecteplase (IV bolus over five seconds) makes it challenging to draw conclusive evidence regarding the similarities and differences in the drugs’ complications.
Symptomatic intracranial hemorrhage (ICH) after IV rtPA for ischemic stroke occurs in roughly 6% of patients. Approximately half of symptomatic intracranial hemorrhages occur by 10 hours after treatment, with the rest occurring by 36 hours. Intracranial hemorrhage occurring after 36 hours is not likely due to rtPA.
ICH may be signaled by acute hypertension, headache, neurologic deterioration, and nausea or vomiting. If ICH is suspected, an emergent head CT scan and labs, including PT, aPTT, platelet count, and fibrinogen, are done. If ICH is present on CT scan, lab results are evaluated and, if necessary, six to eight units of cryoprecipitate containing fibrinogen and factor VIII, six to eight units of platelets, and/or fresh frozen plasma are administered. Use of recombinant factor VII may also be considered but carries a risk of inducing thrombotic events.
Certain patients are more susceptible to ICH following fibrinolytic medication administration. These include patients with:
- Older age
- Higher baseline glucose
- Greater stroke severity
- Prior hypertension
- Congestive heart failure
- Renal impairment
- Hyperglycemia and diabetes mellitus
- Ischemic heart disease
- Atrial fibrillation
- Baseline antiplatelet use
- Smoking
- Higher baseline neutrophil count and neutrophil-to-lymphocyte ratio
(Rose et al., 2023; Saver, 2024)
ANSWERING PATIENT QUESTIONS
Q: What are clot-dissolving or clot-busting drugs?
A: Clot-dissolving drugs are enzymes that break the bonds holding clots together. Clot-dissolving drugs have been used for a long time to treat blood clots elsewhere in the body.
The drug alteplase has been approved by the U.S. Food and Drug Administration for dissolving blood clots in the brain. Alteplase is usually injected in a vein, where it is carried by the bloodstream to the clot in order to break up the threads of protein that hold the clot together. Not all strokes can be treated with alteplase, and alteplase can sometimes cause bleeding in the brain. Nonetheless, when an experienced physician recommends using alteplase for a person who has just had a stroke, the benefits outweigh the risks.
More recently, tenecteplase has been approved by the U.S. Food and Drug Administration as a newer “clot-busting” drug for the treatment of acute ischemic stroke in adults. The medication is administered via a quick intravenous injection over five seconds to dissolve clots. Tenecteplase is thought to yield better clinical outcomes compared with alteplase.
TREATMENT WITH OTHER ANTITHROMBOTIC DRUGS
Aspirin is regarded as the best-known antiplatelet medication. For those treated with alteplase or tenecteplase, aspirin administration is commonly delayed until 24 hours later. Aspirin, however, is not recommended as a substitute for acute stroke treatment in patients who are otherwise eligible for fibrinolytic therapy or mechanical thrombectomy.
In patients presenting with noncardioembolic ischemic stroke (NIHSS score ≤3) who did not receive IV alteplase, treatment with dual antiplatelet therapy (aspirin and clopidogrel) started within 24 hours after symptom onset and continued for 21 days is effective in reducing recurrent ischemic stroke for a period of up to 90 days from symptom onset (Powers et al., 2019).
Anticoagulant treatment is seldom given for treatment of acute ischemic stroke because of the risk of excessive bleeding. Full-dose anticoagulant therapy with heparin or low-molecular-weight heparin is used by some clinicians for certain types of strokes, such as those caused by cardioembolism or dissection.
Low-dose anticoagulant therapy with heparin or low-molecular-weight heparin is sometimes used for people with ischemic stroke who are paralyzed from the stroke in order to help prevent thrombi from forming in the legs or other veins, which could cause pulmonary embolism (Caplan, 2025a).
ENDOVASCULAR TREATMENT
Mechanical Thrombectomy
Thrombectomy is a mechanical interventional procedure by which a blood clot is removed under image guidance using endovascular devices. The use of mechanical thrombectomy is considered the standard of care for a large vessel occlusion. Early intra-arterial treatment with mechanical thrombectomy is safe and effective for reducing disability and is superior to standard treatment with intravenous thrombolysis alone for ischemic stroke caused by a documented large artery occlusion in the proximal anterior circulation.
Thrombectomy utilizes various techniques, most commonly stent retrieval, direct aspiration, or a combination of both. The primary purpose of mechanical thrombectomy in ischemic stroke is to rescue the ischemic penumbra.
AHA/ASA guidelines for the early management of AIS recommend the following criteria for endovascular mechanical thrombectomy therapy:
- Prestroke modified Rankin scale <2
- Alberta Stroke Program Early CT Score (ASPECTS) of ≥6 within 6 hours of symptom onset
- NIH stroke scale score ≥6
- Start of procedure within 6 hours of symptom onset
- Causative occlusion of the internal carotid artery or the proximal middle cerebral artery
- Age 18 years or older
ASSESSMENT TOOLS FOR MEETING ENDOVASCULAR TREATMENT CRITERIA
Modified Rankin Scale (mRS). The mRS assesses disability in patients who have had a stroke and is compared over time to check for recovery and degree of continued disability. The mRS correlates with physiologic indicators such as stroke type, lesion size, and neurologic impairment. A score of 0–4 indicates no disability to moderate disability; a score of 5 indicates disability requiring constant care for all needs; and a score of 6 indicates death (Swieten, 2024).
Alberta Stroke Program Early CT Score (ASPECTS). ASPECTS is a 10-point quantitative CT scan score that uses a reproducible grading system to assess early ischemic changes on pretreatment CT studies in patients with middle cerebral artery stroke. A normal CT scan receives 10 points, and a score of 0 indicates diffuse involvement through the middle cerebral artery territory. An ASPECTS score of ≤7 is associated with negative functional outcome, determined by mRS (Barber, 2024).
Mechanical thrombectomy has been shown to improve clinical outcomes versus standard care alone in select patients with large vessel ischemic stroke presenting up to 24 hours after the start of symptoms. Current indications for its use have extended the time of thrombectomy to 24 hours post symptom onset.
Contraindications for its use include:
- Intracranial hemorrhage
- Large infarct core with minimal penumbra
- Small vessel occlusion
- Coagulopathies that cannot be corrected
- Elevated blood pressure (systolic >185 mmHg or diastolic >110 mmHg) that cannot be corrected
(Mathews & De Jesus, 2023; Oliveira-Filho & Samuels, 2025)
Both second-generation stent retrievers and catheter aspiration devices can be used for mechanical thrombectomy. The choice depends on local expertise and availability. In some cases, the stent retriever and aspiration techniques may be used in combination. Catheterization is performed via a femoral artery puncture under general anesthesia or conscious sedation. The catheter is guided to the internal carotid artery and from there to the site of the occlusion. The tiny netlike stent retriever is then inserted into the catheter and guided to the occlusion. The stent is then pushed directly through the clot, after which it expands to the size of the artery wall. At this point, the retriever has captured the clot, and it is removed as the device is pulled back.
Mechanical thrombectomy devices can remove a clot in a matter of minutes, whereas pharmaceutical fibrinolytics, even those delivered intra-arterially, may take as long as two hours to dissolve a thrombus. AHA/ASA guidelines state that patients eligible for IV alteplase should receive it even if mechanical thrombectomy is being considered, and that patients under consideration for the procedure should not wait for observation to assess for clinical response following the IV (Powers et al., 2019).
Symptomatic intracerebral hemorrhage is a potential adverse event of mechanical thrombectomy, resulting from instrument manipulation. Despite this complication, the risk of symptomatic intracerebral bleeding is not significantly different from the medical standard of care. Complications can include:
- Dislodging of embolic material distal to the occlusion
- Stenosis at the thrombectomy site
- Vessel perforation and dissection
- Groin and retroperitoneal hematomas at the puncture site
- Reocclusion secondary to a high platelet count on admission, preexisting stenosis, or embolic material around the thrombectomy site
Although these acute complications are not common, they indicate a poor prognosis (Mathews & De Jesus, 2023).
TREATMENT IN CEREBRAL INFARCTION (TICI) SCALES
The thrombolysis in cerebral infarction (TICI) scale is a radiologic prognosis tool for determining the response of fibrinolytic therapy for ischemic stroke. The original TICI scale was based on the angiographic appearances of the treated occluded vessel and its distal branches.
| Grade | Perfusion |
|---|---|
| (Sharma, 2025e) | |
| 0 | No perfusion |
| 1 | Penetration with minimal perfusion |
| 2 | Partial perfusion |
| 2A | Partial filling (less than two thirds) of the entire vascular territory is visualized |
| 2B | Complete filling of all of the expected vascular territory is visualized but the filling is slower than normal |
| 3 | Complete perfusion |
In 2013, a modified TICI scale (mTICI) was implemented to better reflect the increased use of endovascular therapies in stroke management.
| Grade | Perfusion |
|---|---|
| (Sharma, 2025d) | |
| 0 | No perfusion |
| 1 | Antegrade reperfusion past the initial occlusion, but limited distal branch filling with little or slow distal reperfusion |
| 2A | Antegrade reperfusion of less than half of the occluded target artery previously ischemic territory (e.g., in one major division of the middle cerebral artery (MCA) and its territory) |
| 2B | Antegrade reperfusion of more than half of the previously occluded target artery ischemic territory (e.g., in two major divisions of the MCA and their territories) |
| 2C | Near complete perfusion except for slow flow or distal emboli in a few distal cortical vessels (not part of the original mTICI score but added later and now widely accepted as being part of the scoring) |
| 3 | Complete antegrade reperfusion of the previously occluded target artery ischemic territory, with absence of visualized occlusion in all distal branches |
In 2019, the expanded treatment in cerebral infarction (eTICI) scale was modified from the mTICI and TICI scales. The modified Rankin scale (mRS) shift at 90 days was used to determine a significant difference in outcomes for patients with partial recanalization after endovascular clot retrieval between those with reperfusion of 50%–66%, 67%–89%, and 90%–99% in addition to those previously defined by mTICI.
| Grade | Perfusion |
|---|---|
| (Sharma, 2025b) | |
| 0 | No perfusion noted (0% reperfusion) |
| 1 | Reduction in thrombus but without any resultant filling of distal arterial branches |
| 2A | Reperfusion of 1%–49% of the territory |
| 2B50 | Reperfusion of 50%–66% of the territory |
| 2B67 | Reperfusion of 67%–89% of the territory |
| 2C | Extensive reperfusion of 90%–99% of the territory |
| 3 | Complete antegrade reperfusion of the previously occluded target artery ischemic territory, with absence of visualized occlusion in all distal branches |
Emergency CEA/Carotid Angioplasty
A carotid endarterectomy (CEA) is a surgical procedure done to open and clean a carotid artery. AHA/ASA 2019 guideline recommendations state that the usefulness of emergent or urgent CEA/carotid angioplasty and stenting when clinical indicators or brain imaging suggests a small infarct core with large territory at risk, compromised by inadequate flow from a critical carotid stenosis or occlusion, or in the case of acute neurologic deficit after CEA, in which acute thrombosis of the surgical site is suspected, is not well established.
In patients with unstable neurologic status (e.g., stroke in evolution), the efficacy of emergency or urgent CEA/angioplasty and stenting is not well established (Powers et al., 2019).
BLOOD PRESSURE CONTROL IN ISCHEMIC STROKE
Treating hypertension in the acute setting is controversial, since both high and low blood pressures are associated with poor outcomes. The rationale for lowering blood pressure is to reduce cerebral edema and limit hemorrhagic transformation. However, rapid lowering of blood pressure worsens cerebral hypoperfusion, exacerbates stroke symptoms, expands ischemic core, increases the size of infarction, and is not associated with greater therapeutic benefit (Desai, 2024).
AHA/ASA guidelines indicate that in patients who do not qualify for either intravenous alteplase or mechanical thrombectomy, blood pressure should not be lowered unless it exceeds 220/120 mmHg. However, patients with AIS presenting with symptoms of other severe acute comorbidities, (e.g., acute coronary event, acute heart failure, aortic dissection, preeclampsia/eclampsia) may require an emergency blood pressure reduction. Blood pressure reduction should be individualized since an exaggerated drop can result in complications, including stroke progression or acute kidney injury. To date, no blood pressure reduction strategy has been shown to be superior.
Patients who are eligible for treatment with IV thrombolysis should have their blood pressure carefully lowered so it is <185/110 mmHg and with a blood pressure goal <180/105 mmHg for the first 24 hours following fibrinolytic administration.
In patients undergoing mechanical thrombectomy and who have not received IV thrombolysis, it is recommended that blood pressure be maintained at ≤185/110 mmHg before the procedure and ≤180/105 mmHg during and for the 14 hours post procedure, regardless of whether the recanalization has been achieved.
In patients with blood pressure ≥220/120 mmHg who did not receive IV alteplase or mechanical thrombectomy and have no comorbid condition requiring urgent antihypertensive treatment, the benefit of initiating or reinitiating treatment of hypertension within the first 48 to 72 hours is uncertain. It might be reasonable to lower blood pressure by 15% during the first 24 hours after stroke onset.
Low blood pressure may be triggered by conditions such as hypovolemia, cardiac arrhythmia, or blood loss due to periprocedural hemorrhage during mechanical thrombectomy. Guidelines recommend hypotension and hypovolemia be corrected to maintain a systemic perfusion level necessary to support organ function. It is not indicated at what blood pressure level should be initiated, how long it should be maintained, or which blood pressure level should be a treatment goal (Aziz & Mistry, 2022; Desai, 2024; Powers et al., 2019).
SUPPORTIVE CARE
Meticulous attention to the care of the stroke patient during treatment for ischemic stroke can prevent further neurologic injury and minimize common complications, optimizing the chance of functional recovery. Supportive care for patients during treatment for ischemic stroke includes:
- Bed rest for the first 12 to 24 hours
- Routine placement on aspiration, deep venous thrombosis, fall, and seizure precautions
- Head of bed lowered if perfusion limitation is suspected but raised in those suspected of having cerebral edema or elevated intracranial pressure
- Airway and ventilation maintenance
- Continuous hemodynamic monitoring
- Strict glucose control regardless of whether the patient has a known history of diabetes
- Close monitoring for signs of neurologic worsening
- Nothing by mouth (NPO) until dysphagia screening is completed within 4 to 24 hours
- Minimizing skin friction and pressure
(Soetanto, 2025)
Intracranial Hemorrhagic (ICH) Stroke Treatment
Intracranial hemorrhage includes four broad types of hemorrhage, including:
- Epidural: Bleeding between the skull and the dura mater
- Subdural: Bleeding between the dura mater and the arachnoid membrane
- Subarachnoid: Bleeding between the arachnoid membrane and pia mater
- Intracerebral or intraparenchymal: Bleeding within the brain tissue
Each type involves different causes and variable clinical findings, prognosis, and outcomes. Most ICHs are due to head injury. Others may be the result of an aneurysm or an arteriovenous malformation.
Although intracerebral hemorrhage is the second most common cause of stroke, it accounts for an inordinate amount of morbidity and mortality. Bleeding occurs within the brain quickly, without warning signs, and severely enough to result in coma or death.
Management and treatment depend on whether the stroke is within the brain (intracerebral) or on the surface between the brain and skull (subarachnoid). The goal is to stop the bleeding, repair the cause, relieve symptoms, and prevent complications. Treatment may be a combination of surgery and medications as well as:
- Advanced trauma life support
- Control of bleeding
- Seizure control
- Blood pressure control
- Intracranial pressure control
(Rordorf & McDonald, 2025a; Rordorf &McDonald, 2025b; Tenny et al., 2024)
ADVANCED TRAUMA LIFE SUPPORT MEASURES
Management begins with ensuring adequate airway, breathing, and circulation; the acquisition of an emergent CT scan; and rapid stabilization of vital signs. Intubation should be considered if the patient has a deteriorating Glasgow coma, a Glasgow coma score of ≤8, or worsening neurologic status. Patients with elevated intracranial pressure should be intubated and hyperventilated (Tenny et al., 2024).
CONTROL OF BLEEDING
Anticoagulation-associated intracranial hemorrhage has a high morbidity and mortality rate. Patients on warfarin have an increased incidence of hemorrhagic stroke. The necessity of reversing anticoagulation is a medical emergency, and reversals must be accomplished as quickly as possible to prevent further expansion of the hematoma. All anticoagulant and antiplatelet drugs should be stopped immediately, and medication-specific reversal agents should be administered.
- Warfarin. Four-factor prothrombin complete concentrate (4F-PCC) or, if unavailable, three-factor prothrombin plex with recombinant activated factor VII or fresh frozen plasma may be given. IV vitamin K should also be given to sustain the short-acting effect of 4F-PCC or FFP.
- Direct oral anticoagulants. Two specific reversal agents for direct oral anticoagulants have FDA approval: idarucizumab for dabigatran, and andexanet alfa for apixaban, edoxaban, and rivaroxaban.
- Heparin and low-molecular-weight heparin. Reversal agents include protamine sulfate for heparin and andexanet alfa for low-molecular-weight heparin.
Patients with severe coagulation factor deficiency or severe thrombocytopenia should receive appropriate factor replacement or platelet transfusion (Freeman & Weitz, 2025; Rordorf & McDonald, 2025a).
SEIZURE CONTROL
For those with acute ICH who have a seizure, immediate intravenous antiseizure medication should be initiated to reduce risk of recurrent seizure. These drugs, however, have not been shown to improve functional outcome or reduce the rate of poststroke epilepsy. There are many antiseizure medications, which may include:
- Brivaracetam
- Cannabidiol
- Carbamazepine
- Gabapentin
- Phenobarbital
- Phenytoin
- Valproate
For patients who have an early seizure (<14 days from onset), continue treatment for several days and then wean when clinically stable if seizures do not recur. For patients who have later seizures (>14 days from onset), continue with long-term seizure therapy. For patients with acute ICH who do not have a seizure, antiseizure medication prophylaxis should not be administered (Rordorf & McDonald, 2025a).
BLOOD PRESSURE CONTROL
Elevated blood pressure is common in patients with acute ICH. Uncontrolled elevations in blood pressure and blood pressure variability are risk factors for hemorrhage expansion and poor outcome. Lowering blood pressure has been shown to reduce the risk of recurrent stroke by up to 30%.
In patients with spontaneous ICH requiring acute BP lowering, titrate to ensure continuous smooth and sustained control of BP. Avoiding peaks and large variability can be beneficial for functional outcomes. Initiating treatment within two hours of ICH onset and reaching target BP can be beneficial and also improve functional outcomes. In patients with moderate to severe ICH with systolic BP (SBP) of 150 to 220 mmHg, acute lowering of SBP to a target of 140 mmHg with the goal of maintaining in the range of 130 to 150 mmHg is safe and may be reasonable for improving functional outcomes
For patients with large or severe ICH or those requiring surgical decompression, the safety and efficacy of intensive BP lowering are not well established.
In patients with ICH of mild to moderate severity presenting with SBP >150 mmHg, acute lowering of SBP to <130 mmHg is potentially harmful (Greenberg et al., 2022).
For systolic BP >220 mmHg, rapidly lower to <220 mmHg. For SBP of 150 to 220 mmHg, lower to a target of 130 mmHg. Recommended medications include:
- Nicardipine for patients with a systolic BP ≥160 mmHg
- Labetalol for patients with systolic BP <160 mmHg
Other intravenous medications that may be used include:
- Clevidipine
- Esmolol
- Enalaprilat
- Fenoldopam
Nitrates and nitroprusside are typically avoided because they may increase intracranial pressure (Goldemund, 2025; Rordorf & McDonald, 2025a).
INTRACRANIAL PRESSURE CONTROL
Acute ICH may lead to increased intracranial pressure due to several mechanisms, including:
- Mass effect of the initial hematoma
- Expansion or rebleeding of the ICH
- Cerebral edema surrounding the hemorrhage
- Hydrocephalus from ventricular outflow obstruction
To improve jugular venous outflow and lower intracranial pressure, the head of the bed should be elevated to 30 degrees, and the head should be maintained at midline and not turned to either side. The patient may require analgesia (morphine or alfentanil [Afentna]) and sedation (propofol) to assist with this recommendation (Kitagawa, 2022).
Mannitol 20% is given at a dose of 1.0 to 1.5 g/kg. Corticosteroids should not be administered for treatment of elevated ICP.
Hyperventilation after intubation and sedation to a PCO2 of 28 to 32 mmHg will be necessary if the ICP increases further.
Recommendations are to monitor ICP with a parenchymal or ventricular catheter for all patients with a Glasgow coma scale <8 or those with evidence of transtentorial herniation or hydrocephalus. Transtentorial herniation is life-threatening, involving the movement of brain tissue from one intracranial compartment to another. It is a time-critical pathology that may be reversed with emergent surgical intervention and medical management.
The ventricular catheter has the advantage of draining of CSF in the event of hydrocephalus. The aim of management is to maintain cerebral perfusion pressure of 50 to 70 mmHg (Unnithan et al., 2023).
SURGICAL INTERVENTIONS
Despite much progress in the acute management of ICH, the ideal surgical management is still to be determined. Minimally invasive surgery, however, does seem to be more effective than conservative treatment in patients with ICH in reducing both morbidity and mortality.
Indications for surgical intervention depend on the ICH size and location, the time since onset, and the clinical status of the patient. Patients presenting acutely with a cerebellar hemorrhage greater than 3 cm in diameter and brainstem compression will most likely require urgent surgical decompression.
Decompression surgery relieves pressure on the brain, allowing pooled blood to be removed and repair to be done to damaged blood vessels, thereby reducing secondary damage to the brain resulting from increased intracranial pressure. There are four surgical methods to accomplish this:
- Craniotomy with open surgery is performed when a hematoma is very large or when it is compressing the brainstem. It involves removal of a portion of the skull to drain the hematoma and repair blood vessels.
- Simple aspiration involves drilling a small hole in the skull and draining the hematoma using a tube or catheter. This is a relatively noninvasive procedure but does not always allow for complete drainage of the hematoma.
- Endoscopic evacuation is similar to simple aspiration in that it involves drilling a hole in the skull, but instead of using traditional surgical instruments, the hematoma can be reached and drained using a tiny camera-guided instrument.
- Stereotactic aspiration uses CT scanning to locate the hematoma and a specially developed suction tool to drain it. The patient is immobilized in a stereotactic head frame that allows for a greater degree of precision and accuracy than otherwise would be possible.
(Fischer & Das, 2023; Stieg, 2024)
TREATMENT OF ANEURYSMS
There are two types of treatment for aneurysm: surgical clipping and endovascular coiling.
Surgical clipping is a procedure done to close off an aneurysm to prevent rebleeding. A portion of the skull is removed to access the aneurysm, and a metal clip is placed on the neck of the aneurysm to stop blood flow to it. This clamp can keep the aneurysm from bursting, or it can keep an aneurysm that has recently hemorrhaged from bleeding again. Following clamping, the skull portion is replaced.
Endovascular coiling is a less-invasive procedure than clipping and has been used increasingly in recent years with excellent success. During this procedure, a tiny detachable coil is advanced through an artery in the groin into the aneurysm, filling it with the coil. A blood clot forms within the coil, blocking blood flow to the aneurysm and preventing it from rupturing again. Endovascular treatment may be preferred over surgical clipping when:
- An aneurysm is in a difficult location to access surgically
- The aneurysm is small necked and located in the posterior fossa
- The patient is an older adult
- The patient has a poor clinical grade
(Brown, 2024)
ARTERIOVENOUS MALFORMATION (AVM) TREATMENT
An AVM causes blood to flow directly from the arteries to the veins without supplying blood to vital tissues, resulting in brain tissue that starts to die off. Three surgical options are used: conventional surgery, endovascular embolization, and radiosurgery. Endovascular embolization and radiosurgery are less invasive than conventional surgery and offer safer treatment options for AVMs located deep inside the brain. An AVM grading system can help estimate the risk of surgery based on the size of the AVM, location in the brain, surrounding tissue involvement, and any leakage.
Surgical Resection
If an AVM has bled and/or is in an area that can be easily accessed, then conventional brain surgery is recommended. AVMs may be approached via a craniotomy over the cerebral convexity, via the base of the skull, or via the ventricular system. Arterial feeders are isolated and ligated, and the tangle of abnormal blood vessels is resected. Postsurgical angiography is performed to ensure that no residual AVM remains. There have, however, been instances of reappearance of AVMs years following a negative postresection angiogram (Mount Sinai, 2025; NINDS, 2024a).
Endovascular Embolization
This procedure involves the insertion of a long, thin catheter through a groin artery then threaded through blood vessels to the brain using X-ray imaging. The catheter is positioned into one of the arteries feeding the AVM, and a substance is injected, such as fast-drying glue-like materials, fibered titanium coils, and tiny balloons that will travel through blood vessels and create an artificial blood clot in the center of an AVM. This is repeated for each vessel that feeds the AVM. This procedure is less invasive than surgical resection and is frequently used prior to it to make the procedure safer by reducing the size of the AVM or the likelihood of bleeding. Endovascular embolization by itself does not typically eliminate the AVM, and therefore is almost always used as a preliminary step in preparation for either microsurgical resection or stereotactic radiotherapy (Mount Sinai, 2025; NINDS, 2024a).
Stereotactic Radiosurgery (SRS)
SRS is an even less invasive therapeutic approach often used to treat a small AVM that has not ruptured but is in an area difficult to reach by regular surgery. This treatment uses proton beam, linear accelerator, or gamma knife methods to precisely deliver a high dose of radiation to destroy the AVM. SRS directs many highly targeted radiation beams at the AVM to damage the blood vessels and cause scarring. Over the next several months, the irradiated vessels gradually degenerate and eventually close, leading to resolution of the AVM (Mount Sinai, 2025; NINDS, 2024a).
SUBARACHNOID HEMORRHAGE (SAH) TREATMENT
Treatments for SAH include surgical intervention with clipping or endovascular coiling. Subarachnoid hemorrhage has a poor prognosis due to the wide array of associated complications that can develop. Only about one third of patients with SAH have a “good” result after treatment (Singer et al., 2025).
Delayed Cerebral Ischemia (DCI)
Delayed cerebral ischemia continues to be a major complication and source of morbidity following SAH. Current agreement is that the origin and development of DCI is a multifactorial and complex process that includes vasospasm but also neuroinflammation as well as microthrombosis, disturbance in neuronal electrophysiology, and breakdown of the blood-brain barrier (Thilak et al., 2025).
Induced hypertension has long been the choice of therapy for cerebral vasospasm and DCI, and was originally part of the triple-H therapy (hypertension, hypervolemia, and hemodilution). This therapy, however, has been found to lack a clinical effect on patient outcomes and remains controversial.
The only treatment shown to prevent DCI is the oral vasodilator nimodipine. This is the only FDA-approved drug shown to reduce ischemic neurologic deficit from DCI, and it is the only recommendation in current clinical guidelines. Even with nimodipine, however, DCI remains a common complication (Sadan & Akbik, 2022).
Hydrocephalus
Hydrocephalus can lead to cognitive decline and neurologic deterioration, even when the primary cause of SAH has been treated successfully. Hydrocephalus results from a blood clot from the SAH becoming lodged in one of the CSF drainage sites. CSF is produced in the ventricles of the brain and travels out through small openings know as foramina. If these openings are clogged, the CSF is still produced but has nowhere to go. This allows for pressure buildup that spreads to the brain and skull.
Acute hydrocephalus can be managed with a closed external ventricular drainage (EVD) system for a few days until the CSF dynamics stabilize. When the patient becomes continuously dependent on EVD, the decision to convert to a permanent CSF diversion is required, with a ventriculoperitoneal shunt as the first treatment of choice.
The prolonged use of an EVD may increase the risks of meningitis and/or ventriculitis, which can impact outcome, and also increases the risk of the patient becoming shunt dependent (Bertuccio et al., 2023).
SUPPORTIVE CARE
Supportive preventive measures should be taken for all patients, including:
- Keeping the head of the bed elevated >30 degrees
- Providing sedation for comfort
- Administering antipyretics for temperature >100.4 °F
(Rordorf & McDonald, 2025a)
Patients being treated for hemorrhagic stroke require:
- Constant hemodynamic monitoring in an ICU
- Frequent neurologic assessment
- Bed rest with sedation and head of bed elevated to 30 degrees; strict bed rest until etiology of hemorrhage is determined
- Cautious use of sedatives and analgesics due to potential to mask neurologic findings
- Pain control for headache
- Temperature control (elevated temperature can increase the degree of ischemic damage; maintain normal temperature using acetaminophen PO/PR; consider cooling devices)
- Maintaining hydration with intravenous IV normal saline
- Maintaining NPO status until swallowing function is evaluated
- Avoiding hypoglycemia
- Correcting hyperglycemia to 140 to 180 mg/dL
- Correcting metabolic acidosis
- Continuous ICP monitoring using direct measurement
- Hypertension treatment with IV medication
(Oliveira-Filho & Mullen, 2025; Rordorf & McDonald, 2025a)
PREVENTING AND MANAGING COMPLICATIONS POST 24 HOURS
After the patient’s stroke has stabilized for 12 to 24 hours, collaborative care shifts from preserving life to preventing complications, reducing disability, and attaining optimal functioning. This most often requires intensive care in a unit staffed by ICU nurses who are trained to recognize and manage intracranial complications.
Postprocedural Care
For patients who have had surgery, complications may develop within 24 to 48 hours of catheter sheath removal. The site should be monitored for:
- Arterial spasm
- Pain
- Swelling
- Bruising
- Erythema
- Groin hematoma/excessive bleeding
- Pulsatile mass
- Drainage from the puncture site
- Retroperitoneal bleed
- Pseudo-aneurysm or arteriovenous fistula
- Arterial occlusion
- Distal pulse assessment every 15 minutes for 1 hour, every 30 minutes for 1 hour, every 1 hour for 4 hours
(Hill et al., 2022)
Deterioration of Neurologic Functioning
Early neurologic deterioration following an acute stroke is associated with poor outcomes. The key to managing complications in the stroke ICU is recognizing them quickly. The deterioration in a patient’s neurologic status is always a signal to search quickly for a complication.
Therefore, a core responsibility for stroke nurses is the monitoring and management of neurologic status. This is often very frequent in hyperacute stroke care, where observations may be required every 15 minutes for the first 2 hours, every 30 minutes for the next 2 hours, and every 60 minutes until 24 hours have passed. Assessment includes:
- Level of consciousness (e.g., Glasgow coma score)
- NIHSS
- Pupil responses
- Motor function
- Vital signs
Neurologic deterioration is defined as an increase in the NIHSS score by ≥4 points and a decrease of ≥2 points on the Glasgow coma score. Patients are also assessed every 15 minutes for the first 2 hours for signs of change, which include nausea, vomiting, headache, and seizure (Babkair et al, 2023; Tumber, 2024).
ISCHEMIC BRAIN SWELLING
Injured brain tissue swells from edema, and sufficient swelling will push the brain against the skull or nondispensable edges of the dura. In these situations, the brainstem is often squeezed, and the patient will show signs of cerebral herniation. Cerebral herniation should be suspected when new neurologic signs include:
- Abnormal breathing pattern
- Unintended muscle contractions
- Cranial nerve problems, especially asymmetry or loss or reduction of pupillary responses
- Peripheral motor deficits
- Headache
- Irregular respirations
- Neck pain or stiffness
- Nausea and vomiting
- Increasing sleepiness
- Loss of consciousness
Brain herniation is a life-threatening emergency, and ventriculostomy or decompression craniectomy may be a necessary treatment option for many patients (Ishida, 2025; Nehring et al., 2023).
INCREASED INTRACRANIAL PRESSURE
Both ischemic and hemorrhagic strokes sometimes increase intracranial pressure indirectly as a result of brain edema. Hemorrhagic strokes can also increase intracranial pressure directly by adding extravascular blood to the restricted intracranial space.
Clinically, elevated ICP presents with:
- Headache
- Vomiting
- Decreased level of consciousness
- Papilledema (optic disc swelling)
- Periorbital bruising
- Cushing’s triad (bradycardia, irregular respirations, hypertension)
Many believe Cushing’s triad is related to brainstem compression and is an especially ominous sign requiring urgent intervention.
Patients with signs of increased ICP or herniation should be intubated and hyperventilated. But excessive hyperventilation is to be avoided, as it may potentiate vasospasm and ischemia. Other interventions for increased ICP include:
- Osmotic agents (e.g., mannitol), which can decrease ICP dramatically
- Although controversial, intravenous steroids (e.g., dexamethasone)
- Diuretics (carbonic anhydrase inhibitors) such as acetazolamide
Patients must remain on strict bed rest, with head of the bed elevated at 30 degrees to ensure optimal venous drainage. Barbiturates can be considered in cases where sedation and usual methods of treatment are not successful in reducing the ICP (Pinto & Adeyinka, 2025).
INTRACRANIAL REBLEEDING
Another cause of deteriorating neurologic functioning in the stroke ICU is additional intracranial bleeding. This problem can be recognized using brain imaging, usually CT scan.
- Ischemic strokes. Symptomatic intracerebral hemorrhage can be a catastrophic complication of acute ischemic stroke, with poorer clinical outcomes at three months. Hemorrhage transformation represents the conversion of an ischemic infarction into an area of hemorrhage. Hemorrhagic transformation of an ischemic infarct usually occurs within 7 to 14 days postictus. Ischemic tissues have a natural tendency to bleed, as acute cerebral ischemia leads to the death of capillary cells, which causes vascular permeability and extravasation of blood in the brain parenchyma. Vasospasm can also lead to hemorrhagic transformation, and intravenous thrombolysis has been shown to increase the risk of mild to severe intracranial hemorrhage (Charbonnier et al., 2021; Zubair & Sheth, 2023).
- Intracerebral hemorrhages. Over 70% of intracerebral hemorrhages expand in the first 24 hours due to continued or repeat bleeding, and the risk of recurrent intracerebral hemorrhage remains elevated for 12 months at a minimum. One major factor in the rebleed is the use of anticoagulant medication. For rebleed prevention, blood pressure management for a target BP of 130/80 mmHg is recommended when the patient is medically and neurologically stable (Grainger et al., 2024; Rajashekar & Liang, 2023; Silva et al., 2024).
- Subarachnoid hemorrhages. The most dreaded early complication of SAH is rebleeding, the greatest risk being within the first 72 hours after ictus, and it is associated with a 60% case-fatality rate. Measures to prevent rebleeding include bed rest in a quiet, darkened room; analgesia; and sedation. Stool softeners are given to prevent the Valsalva maneuver, with resultant peaks in SBP and ICP. The only effective treatment is prevention by obliterating the ruptured aneurysm via clipping surgically or occluding endovascularly with a coil (Calviere et al., 2022).
Pulmonary Complications
PNEUMONIA
Pneumonia develops in 3% to 10% of patients with acute stroke and is associated with a higher mortality and poorer long-term outcomes.
Aspiration is the cause of about 60% of poststroke pneumonia. Aspiration pneumonia following stroke is usually due to stroke-related dysphagia or to a decreased level of consciousness that results in compromised cough reflex and glottic closure.
Measures to prevent aspiration include keeping the patient NPO initially and subsequently modifying the diet for those who have a persistent dysphagia.
The patient is screened on admission for swallowing problems using a formal screening protocol by a trained healthcare professional. Patients who fail the swallowing screening are referred to a speech pathologist for a comprehensive assessment. Additional preventive measures include patient mobilization when neurologically stable and good pulmonary care (Ishida, 2025).
COMPLICATIONS RELATED TO INTUBATION AND MECHANICAL VENTILATION
For intubated patients, risk reduction measures include daily assessment for potential extubation, minimizing sedation, suctioning of secretions, elevating the head of the bed when possible, and maintaining ventilator circuits. Avoid prophylactic antibiotics and agents that suppress gastric secretions. Additional preventive measures include patient mobilization when neurologically stable and good pulmonary care (Ishida, 2025).
ABNORMAL BREATHING PATTERNS
Stroke may disrupt breathing either by causing a disturbance of central rhythm generation, by interrupting the descending respiratory pathways leading to a reduced respiratory drive, or by causing bulbar weakness leading to aspiration.
Abnormal breathing patterns are a common consequence of stroke, with an incidence of over 60%. Among the recognized abnormal respiratory patterns are Cheyne-Stokes breathing, periodic breathing, apneustic breathing, central sleep apnea, ataxic breathing, and failure of automatic breathing:
- Cheyne-Stokes breathing involves a period of rapid and shallow breathing followed by slow, heavier breathing along with periods of apnea.
- Apneustic breathing is characterized by regular deep inspirations with an inspiratory pause followed by inadequate expiration. This respiratory pattern carriers a poor prognosis.
- Ataxic breathing is a pattern characterized by complete irregularity of breathing, with irregular pauses and increasing periods of apnea.
Close observation of the stroke patient for these potential disturbances and implementation of prophylactic measures can prevent significant morbidity and mortality (Patrizz et al., 2023).
OXYGEN DESATURATION
Stroke patients may exhibit a decline of more than 50% in respiratory function. Mild hypoxia is common and may lead to supplementary brain damage as a result of reduced blood supply. The underlying condition causing hypoxia must be treated to manage and improve patient outcome.
Body position may have a significant impact on oxygenation. A semi-sitting position is better at improving ventilation-perfusion matching. This position affects oxygenation and arterial blood gas parameters by raising SpO2 and PaO2 and decreasing PaCO2.
The semi-Fowler position maximizes lung volumes, flow rate, and capacities; decreases abdominal contents’ pressure on the diaphragm; and increases respiratory system compliance, resulting in increased oxygenation and decreased PaCO2.
Patients with very low oxygen levels must be treated with oxygen, and so it is important to monitor oxygen levels to maintain oxygen saturation >95%. However, there is clear and unambiguous evidence that stroke patients do not need routine oxygen and that it does not improve recovery from stroke (Emfietzoglou, 2025; Holland, 2024; Patrizz et al., 2023).
NEUROGENIC PULMONARY EDEMA (NPE)
Neurogenic pulmonary edema is rare and most often develops abruptly and progresses quickly after a neurologic insult. NPE is an increase in interstitial and alveolar fluid that can occur following stroke, particularly subarachnoid hemorrhage, and can be fatal. The patient presents with dyspnea, tachycardia, tachypnea, cyanosis, pink frothy sputum, crackles, and rales on auscultation. Blood tests reveal hypoxemia. Aspiration pneumonia, ventilator-associated pneumonia, and ventilation-induced lung injury have similar symptoms and must be ruled out.
General supportive care for NPE includes supplemental oxygen to correct hypoxemia and mechanical ventilation if necessary. With the use of low inflation volumes, positive end-expiratory pressure (PEEP) is added to prevent compression atelectasis (Al-Dhahir et al., 2025).
Cardiovascular Complications
CARDIAC PROBLEMS
Cardiac complications include arrhythmias, myocardial infarction, congestive heart failure, and neurogenic cardiac injury. MI occurs in approximately 1% to 2% of patients with acute stroke during initial hospitalization and is associated with poor outcomes.
Elevation of troponin and other cardiac enzymes after acute stroke may be related to stroke-induced elevation of circulating epinephrine and activation of the sympathoadrenal system contributing to myocardial damage.
Neurogenic cardiac damage may be due to underlying coronary disease, but this may not be the only mechanism, as it can occur in patients with subarachnoid hemorrhage, who are often young and do not have underlying heart disease.
Cardiac arrhythmias in the first 72 hours following an admission for acute stroke occur in approximately 25% of patients, atrial fibrillation being the most common. Cardiac events and cardiac death after acute stroke may be caused by acute myocardial infarction, heart failure, ventricular arrhythmias such as ventricular tachycardia or fibrillation, and cardiac arrest.
Other causes include focal atrial tachycardia, undetermined supraventricular tachycardia, ventricular ectopy, nonsustained ventricular tachycardia, and atrial flutter. Bradyarrhythmias have been found to be caused by atrial fibrillation.
Cardiac myofibrillar degeneration (myocytolysis) in the area of cardiac nerves has been found on autopsy in patients who died from acute stroke. The lesion differs from necrosis due to coronary disease because it is visible within minutes and the cells die in a hypercontracted state.
Initial ECG monitoring is undertaken for all patients with stroke. The duration and mode of monitoring is generally recommended for at least the first 24 hours, but for patients with embolic stroke of uncertain source, longer-term ECG monitoring (external or implantable) is used (Carrarini et al., 2022; Ishida, 2025).
HYPERTENSION
Elevated BP during the acute phase of intracranial hemorrhage (ICH) has been found to be associated with hematoma expansion, perihematomal edema, rebleeding, neurologic deterioration, and death. The first step in managing ICH is to reduce blood pressure as quickly as possible. IV antihypertensive drugs are standard treatment in this instance, while medications may also be prescribed to counteract any blood thinners the patient may be taking.
The consensus is that for ICH patients with systolic BP of 150 to 220 mmHg and without contraindications, acute lowering of systolic BP to 140 mmHg is considered safe and may improve functional outcomes.
The majority of patients with ischemic stroke have hypertension, and lowering BP may be critical in preventing recurrent stroke. However, if BP is lowered rapidly in the acute phase, adverse renal events may occur. Studies of antihypertensive treatment have shown evidence that in the acute ischemic stroke setting, antihypertensive treatment does not change cerebral perfusion. It is recommended that moderate hypertension in most patients who are not candidates for fibrinolytic therapy be permitted, and most patients will experience spontaneous reduction in BP over the first 24 hours without treatment. Guidelines state that there is no one ideal blood pressure reading for patients with ischemic stroke. Guidelines also recommend carefully lowering blood pressure in patients who are otherwise eligible for treatment with fibrinolysis.
In some instances, part of the treatment goals for ischemic stroke is to enhance blood flow through a blocked vessel that may partially reopen, allowing some blood to flow through. One strategy to accomplish this is to increase blood pressure to push the blood through those narrower vessels. If a patient is on antihypertensives, they are stopped and blood pressure is allowed to rise. This is referred to as permissive hypertension (Bath et al., 2022; Davenport, 2023; Moawad, 2024).
VENOUS THROMBOEMBOLISM (VTE)
Venous thromboembolism includes deep vein thrombosis (DVT) and pulmonary embolism (PE). DVT may develop as early as 24 to 48 hours after stroke onset and has a peak incidence between two and seven days. Pulmonary embolism, often unassociated with clinically recognized DVT, accounts for up to 25% of early deaths after stroke and is the most common cause of death at its peak occurrence about two to four weeks after stroke onset. Patients with hemiparesis are predisposed to DVT development. Additional risk factors include advanced age, high stroke severity, and immobility.
VTE prophylaxis is indicated for all patients with acute stroke and restricted mobility using thigh-length intermittent pneumatic compression (IPC), starting on admission, for patients within 72 hours of acute stroke. IPC is contraindicated in those with evidence of leg ischemia due to peripheral vascular disease, leg ulcerations, dermatitis, severe leg edema, or confirmed DVT.
Additionally, pharmacologic VTE prophylaxis is recommended for select patients within 48 hours of acute ischemic stroke onset who have restricted mobility. This may include subcutaneous low-molecular-weight heparin (LMWH) once daily or subcutaneous low-dose unfractionated heparin two to three times daily. This recommendation applies only to patients for whom the benefit of anticoagulation is assessed to outweigh the risk of bleeding.
IPC is the mainstay for prevention of venous thromboembolism in patients with acute intracerebral hemorrhage (ICH) and should be started on admission. Once bleeding has stopped, low-dose LMWH or unfractionated heparin after one to four days of onset for patients with lack of mobility should be started. Risk of hematoma expansion may weigh against the use of anticoagulation.
For patients with subarachnoid hemorrhage and decreased mobility, IPC is started on admission and prior to aneurysm treatment. LMWH or unfractionated heparin can be added once the aneurysm is secured for those who continue to have restricted mobility.
Once patients become fully ambulatory, mechanical and pharmacologic interventions are generally discontinued (Ishida, 2024, 2025).
Other Common Complications
Other complications that occur frequently in ICU stroke patients include hyperthermia, hyperglycemia, dysphagia, and infections.
HYPERTHERMIA
Fever is independently associated with poor outcomes. All stroke patients should have their temperature monitored at least four times a day for 72 hours. Sources of hyperthermia (temperature >100.4 °F) are identified and treated with antipyretic medications such as acetaminophen. Elevated temperature in the first 24 hours of being admitted to ICU has been associated with an increased risk of in-hospital death.
The benefit of hypothermia is not well established, and it should only be offered in the context of ongoing clinical trials. Studies suggest that induction of hypothermia is associated with an increased risk of infection, including pneumonia (Oliveira-Filho & Mullen, 2025; Powers et al., 2019).
HYPERGLYCEMIA AND HYPOGLYCEMIA
Hyperglycemia is believed to decrease reperfusion. Persistent hyperglycemia during the first 24 hours after onset of AIS is associated with worse outcomes and an increase in 30-day mortality rate, and it is an independent risk factor for greater infarct growth and hemorrhagic stroke conversion. Considering available evidence, it is reasonable to maintain blood glucose between 140 and 180 mg/dL during hospitalization with subcutaneous or IV insulin when needed, being aware that IV insulin increases risk of hypoglycemia. Studies have found that aggressive methods for reducing high blood sugar are not more effective than standard lower-risk treatment.
Hypoglycemic patients with glucose <60 mg/dL should be treated to achieve normoglycemia with IV dextrose 50% to reach a level ≥80 mg/dL. The brain requires glucose for metabolism, and the metabolic requirement of the brain is high; therefore, episodes of hypoglycemia can decrease repair of the brain (Anadani et al., 2022; Lui et al., 2025).
DYSPHAGIA
Poststroke dysphagia is a very common complication and is the major risk factor for aspiration pneumonia. Independent predictors of dysphagia include male gender, age older than 70, disabling stroke, impaired pharyngeal response, incomplete oral clearance, and palatal weakness or asymmetry. Dysphagia will usually improve spontaneously with return of safe swallowing function by two weeks in about 90% of patients.
Patients with acute stroke should have their swallowing screened within four hours of arrival at the hospital and before being given any oral food, fluid, or medication. Personnel specifically trained in swallowing screening using a validated tool should perform the screening to observe how well swallowing muscles move and to help determine which nerves, muscles, and reflexes are impaired.
The water-swallowing test (WST) is a bedside screening tool used to assess for aspiration in clinical practice, but it is limited in its accuracy. Different food textures and viscosities might be used according to the levels of dysphagia, which cannot be demonstrated by WST. A more ideal bedside screening tool is the volume-viscosity swallow test (V-VST), which uses boluses of different volumes and viscosities of food. It has an advantage by indicating the appropriate diet for stroke patients in order to minimize the risk of complications.
Patients who fail the swallowing screening are referred to a speech pathologist for a comprehensive assessment, which may include instrumental examination. Video fluoroscopy (VFS) with modified barium swallow may also be performed once the patient is stable in order to assess severity of dysfunction and risk of aspiration. Intravenous hydration with normal saline is administered to maintain volume status.
Patients with acute stroke who cannot take food and fluids orally due to persistent dysphagia, altered mental status, and/or mechanical ventilation should receive nutrition and hydration via nasogastric nasoduodenal, or percutaneous endoscopic gastrostomy tube feedings while undergoing efforts to restore swallowing (Ishida, 2025).
URINARY TRACT INFECTION
Urinary tract infection is a common problem following stroke and occurs in approximately 11% to 15% of patients who are followed for up to three months. It is a serious complication that impairs stroke recovery, is associated with poorer neurologic outcomes and longer hospital stays, and can be life-threatening in about 1% of cases. UTI is also a common complication in patients who have been followed for up for 30 months.
The main risk factors for UTI are:
- Female sex
- Older age
- High modified Rankin scale score (a measure of poststroke disability)
- Postvoid residual volume >100 mL
It is a common practice to place an indwelling catheter in patients with stroke due to incontinence, immobility, or convenience. This is an important risk factor for infection, and the duration of catheterization is directly related to the risk. Therefore, an indwelling catheter should be avoided whenever possible. The use of external catheter systems or intermittent catheterization are alternatives associated with a lower risk of urinary tract infections (Ishida, 2025).
GASTROINTESTINAL COMPLICATIONS
Gastrointestinal (GI) hemorrhage is one of the more common complications and has both serious and nonserious manifestations in about 1.1% to 3% of stroke patients. These patients have worse outcomes, including a higher rate of dependency and mortality. Overt GI bleeding may be severe or even life-threatening. Occult bleeding is generally less serious.
Risk factors for GI hemorrhage include:
- Older age
- Severe stroke
- Posterior circulation stroke
- Prestroke dependence
- History of peptic ulcer disease or cancer predating stroke
Stress ulcer prophylaxis with proton pump inhibitors or H2 antagonists is effective for reducing overt GI bleeding but may increase the risk of nosocomial pneumonia. Therefore, prophylaxis is not routinely used for patients with acute stroke but is reserved for select patients who require intensive care management or are otherwise at high risk.
Evidence suggests that enteral nutrition alone may reduce the risk of overt GI bleeding due to ulceration and that stress ulcer prophylaxis may be ineffective or harmful among patients who are receiving enteral nutrition (Ishida, 2025).
DELIRIUM
Delirium is a common poststroke complication associated with poor outcomes and increased mortality. Up to 25% of stroke patients experience delirium in the acute phase of stroke, and up to two thirds of these patients experience delirium within 24 hours of admission. Stroke patients with delirium have been found to have more severe stroke-related disabilities on admission and do not show improvement during their stay in a stroke unit. This may likely be due to disturbances in cerebral neurotransmitters and cerebral networking, with more severe strokes leading to a high risk of delirium.
Prevention of delirium and minimizing its negative consequences should be a priority in stroke care. However, there is little evidence about the efficacy of delirium prevention intervention in stroke patients, and not all interventions can easily be applied to this patient population. Surveys have shown that less than half of clinicians have standardized delirium management and less than one third are using a valid assessment frequently. A standardized delirium management includes both pharmacologic and nonpharmacologic measures. Delirium management can include:
- Delirium screening three times within 24 hours using one of the following:
- Nursing Delirium Screening Scale (Nu-DESC) with DSM-V criteria for validation
- 4 Assessment Test for Delirium (4AT)
- Confusion Assessment Method ICU (CAM-ICU)
- Interprofessional evaluation of possible underlying causes and treatment using checklists
- Nonpharmacologic interventions such as reorientation, education, and mobilization
- In case of persisting symptoms, specific pharmacologic intervention as an option
(Mukminin et al., 2025; Nydahl et al., 2022)
NURSING CARE BEYOND 24 HOURS
After the first 24 hours, the nursing staff can begin to focus on other patient management issues that may arise. During this same period, acute rehabilitation is started, usually within 24 to 48 hours, and continues from stroke onset to four days following ischemic stroke and from onset to seven days following hemorrhagic stroke. Following this acute stage, the patient is transferred to a setting that provides comprehensive long-term stroke rehabilitation.
During the postacute phase, the following assessments are completed:
- Mental status (memory, attention span, perception, orientation, affect, speech/language)
- Sensation and perception (patients usually have decreased awareness of pain and temperature)
- Motor control (extremities, swallowing ability)
- Nutritional and hydration status
- Skin integrity
- Activity tolerance
- Bowel and bladder function
- Function in daily activities
The major nursing care planning goals for patient can include:
- Improved mobility
- Avoidance of shoulder pain
- Maximize functional independence in self-care
- Sensory and perceptual deprivation relief
- Prevention of aspiration
- Bowel and bladder continence
- Skin integrity maintenance
- Improved thought processes
- Improved communication
- Improved sexual function
- Absence of complications
Nursing interventions during this stage include:
- Improving mobility and preventing deformities
- Ambulation
- Preventing shoulder pain
- Enhancing self-care
- Managing sensory-perceptual problems
- Assisting with nutrition
- Attaining bowel and bladder control
- Improving thought processes
- Improving communication
- Maintaining skin integrity
- Improving family coping
- Helping the patient cope with sexual dysfunction
(Belleza, 2024; Travers & Bazaar, 2024)
(See also “Rehabilitation in the Acute Stroke Setting” later in this course for occupational therapy, physical therapy, and speech-language therapy interventions.)
Impaired Physical Mobility
Impaired physical mobility may be related to hemiparesis, loss of balance and coordination, spasticity, or brain injury. Nursing interventions for improving mobility and preventing deformities include:
- Positioning to prevent contractures, including using measures to relieve pressure, assist in maintaining good body alignment, and prevent compressive neuropathies
- Applying a splint at night to prevent flexion of an affected extremity
- Preventing adduction of an affected shoulder using a pillow placed in the axilla
- Elevating an affected arm to prevent edema and fibrosis
- Positioning fingers so that they are barely flexed by placing the hand in slight supination (when upper extremity spasticity is noted, avoiding use of a hand roll and using a dorsal wrist splint)
- Changing position every two hours; placing patient in a prone position for 15 to 30 minutes several times a day to maintain hip extension
- Collaborating with physical and occupational therapy to establish an exercise program and to receive instructions for correctly performing active and passive range-of-motion exercises
- Providing full range of motion four or five times daily to maintain joint mobility, regain motor control, prevent contractures in the paralyzed extremity, prevent further deterioration of the neuromuscular system, and enhance circulation; if tightness occurs in any area, performing range-of-motion exercises more frequently
- Encouraging exercising to prevent venous stasis
- During exercise, observing for signs of pulmonary embolus or excessive cardiac workload (e.g., shortness of breath, chest pain, cyanosis, and increasing pulse rate)
- Supervising and supporting the patient during exercises; planning frequent short periods of exercise; encouraging patient to exercise unaffected side at intervals during the day
- Teaching patient to maintain balance in sitting position and standing and walking as soon as standing balance is achieved
(Belleza, 2024)
Acute Pain
Patients may experience pain related to hemiplegia and disuse. Interventions include:
- Using proper patient movements and positioning; placing flaccid arm on a table or pillows when the patient is seated; use of sling when ambulating
- Never lifting the patient by the flaccid shoulder or pulling the affected arm or shoulder
- Elevating arm and hand to prevent edema
- Administering analgesic agents as indicated
(Belleza, 2024)
Self-Care Deficits
Following a stroke with functional deficits, the patient requires assistance in managing self-care, which includes bathing, hygiene, toileting, dressing, grooming, and feeding. The nursing care plan to enhance self-care may include:
- Collaborating with the interdisciplinary team, including occupational therapy
- Encouraging personal hygiene activities as soon as the patient can sit up; selecting activities that can be done with one hand
- Working with the patient to set realistic goals and add a new task daily
- Encouraging the patient to carry out all self-care activities on the unaffected side
- Ensuring that the patient does not neglect the affected side, providing assistive devices as necessary
- To help improve morale, making sure the patient is fully dressed during ambulatory activities
- Assisting with dressing activities using clothing with hook-and-loop (Velcro) closures; putting on the garment on the affected side first
- Keeping the environment uncluttered and organized
- Providing emotional support, encouragement, and positive feedback for accomplishments and efforts
(Belleza, 2024)
Disturbed Sensory Perception
Sensory perception disturbance includes kinesthetic, tactile, or visual problems related to altered sensory perception, transmission, and/or integration. To help manage sensory perceptual difficulties, nursing care includes:
- Approaching the patient with decreased field of vision on the side where visual perception is intact; placing all visual stimuli on this side
- Teaching the patient to turn and look in the direction of the defective visual field to compensate for the loss
- Making eye contact with the patient and drawing attention to the affected side
- Increasing natural or artificial lighting in the room; providing eyeglasses to improve vision
- Reminding the patient with hemianopsia of the other side of the body and placing extremities so the patient can see them
(Belleza, 2024)
Impaired Swallowing (Dysphagia) and Nutrition
In order to maintain nutrition and hydration and avoid aspiration for patients with dysphagia, the nursing care plan includes:
- Observing the patient for paroxysms of coughing, food dribbling out of or pooling in one side of the mouth, food retained in the mouth, or nasal regurgitation when swallowing liquids
- Starting enteral diet within seven days of admission following acute stroke (early feeding has been found to reduce the risk of death)
- Consulting with the speech-language therapist to evaluate gag reflexes and to assist in teaching alternate swallowing techniques:
- Advising the patient to take small boluses of food
- Informing the patient of foods that are easier to swallow
- Providing thickened liquids or pureed diet as indicated
- Having suction equipment available at the bedside, especially during early feeding attempts
- For patients with dysphagia, using nasogastric tubes for feeding in the early phase of stroke (first 7 days) and percutaneous gastrostomy tube if unable to swallow safely for longer than 2 to 3 weeks
- Preparing for tube feedings by elevating the head of the bed and checking tube placement; administering the feeding slowly; ensuring the cuff of tracheostomy tube is inflated (if applicable); monitoring and reporting excessive retained or residual feeding
- Having the patient sit upright, preferably in a chair, while eating and drinking, maintaining upright position for 45 to 60 minutes after eating
- Advancing the diet as tolerated
- Considering nutritional supplements for those who are or are at risk for malnourishment
- Implementing oral hygiene protocols to reduce risk of pneumonia
(Belleza, 2024; LNC, 2025; Powers et al., 2019)
Alteration in Bowel and Bladder Elimination
It is common for patients to have problems controlling their bladder and/or bowels following a stroke. Urinary incontinence is more common than fecal incontinence. Around one half of stroke patients will have some form of incontinence, and for many, it is temporary. Only around 15% of stroke patients will still have continence issues a year after stroke.
When a person experiences a stroke, brain damage can occur near the micturition center of the brain, and bladder function can be impacted. Loss of bowel control can also occur post stroke. Most stroke patients have motor impairments that can interfere with the ability to reach a restroom in time, leading to functional incontinence. Poststroke incontinence symptoms can be improved and can be cured; however, it takes time (Bladder & Bowel Community, 2024).
Urinary problems may include:
- Urgency
- Frequency
- Nocturnal incontinence
- Functional incontinence
- Reflex incontinence
- Overflow incontinence
Bowel problems can include:
- Fecal incontinence
- Constipation
- Constipation with overflow
- Fecal impaction
The nursing care plan for prevention or improving bowel and bladder problems includes:
- Collaborating with physical and occupational therapy
- Performing intermittent sterile catheterization during periods of loss of sphincter control
- Analyzing the patient’s voiding pattern and offering urinal, bedpan, or bedside commode on patient’s voiding schedule
- Assisting male patients to an upright posture for voiding
- Establishing a regular time (e.g., after breakfast) for toileting
- Initiating a bladder and bowel training program
- Performing a bedside bladder ultrasound after voiding to check for residual early in the program
- Providing high-fiber diet and maintaining fluid intake of 2,000 to 3,000 mL per day unless contraindicated
- Requesting and administering stool softeners or laxatives
- Administering enemas if needed
(Belleza, 2024; RNpedia, 2025)
Risk for Injury
A very common complication of acute stroke is injury, in particular those resulting from falls. The decline of neuromotor performance caused by the stroke contributes to the majority of falls in stroke survivors. Other injuries can also occur as a result of loss of visual field, changes in depth perception, presence of diplopia, or other visual impairments, as well as impaired sensory awareness, including position of body parts and joint sense. Patients may be inattentive to body parts and segments of the environment, and may lack recognition of familiar objects/persons.
Interventions include:
- Implementing a falls prevention protocol
- Encouraging patients with nondominant (right) hemisphere injury to slow down and check each step or task as it is completed
- Reminding patients who have a dominant (left) hemisphere injury to scan the environment
- Encouraging making a conscious effort to scan the rest of the environment by turning head from side to side
- Giving short, simple messages and questions and step-by-step directions
- Keeping the environment simple to reduce sensory overload and enable concentration on visual cues; removing distracting stimuli
- Monitoring the environment for safety hazards
- Teaching patients to concentrate on body parts (using a mirror to help patients adjust to the misconception that a body part is not part of their body)
- Providing patients with wheelchair seat belts or supportive harnesses, if clinically indicated
- Placing items the patient uses within easy reach, such as call light, water, urinal, or ambulatory devices
- Responding to a call light as soon as possible
- Encouraging patients to wear their eyeglasses and hearing aids and ensuring that these items are within reach and accessible
(Belleza, 2024)
Risk for Impaired Skin Integrity
Stroke patients are at risk for skin breakdown as a result of the inability to feel or move extremities, incontinence, inability to communicate needs, pain, discomfort, and decreased nutritional status. Nursing interventions include:
- Performing regular skin assessment with emphasis on bony areas and dependent body parts during hospitalization and inpatient rehabilitation using an objective scale such as the Braden Scale or Norton Scale
- Providing skin hygiene measures, such as using emollients for dry skin and keeping the skin clean and dry
- Maintaining nutrition and hydration
- Turning and repositioning every two hours; positioning patient on affected side for only 30 minutes at a time
- Encouraging the use of lifting devices to move or reposition the patient in bed
- Minimizing skin friction and providing pressure relief via early mobility, using specialized mattresses, wheelchair cushions, and seating until sufficient mobility returns
(Wayne, 2024)
Impaired Communication (Aphasia/Dysphasia)
Speech problems following stroke sometimes resolve within hours or days. Some problems, however, are more permanent and require speech and language therapy to improve communication. There are four broad categories of communication problems:
- Expressive (Broca’s) aphasia: knowing what one wants to say but unable to find the words
- Receptive (Wernicke’s) aphasia: hearing what is said or reading but unable to make sense of the words
- Anomic or amnesia aphasia: having difficulty using the right names for objects, people, places, or events
- Global aphasia: inability to speak, understand speech, read, or write
(ASA, 2024)
Nursing interventions for patients experiencing impaired communication may include:
- Collaborating with speech-language therapy, along with active patient participation, to establish goals
- Reinforcing the individually tailored program
- Making the milieu conducive to communication
- Remaining sensitive to the patient’s reactions and needs
- Responding to the patient in an appropriate manner and treating the patient as an adult
- Providing emotional support and understanding to allay anxiety
- Avoiding completing the patient’s sentences
- Being consistent in daily routines; providing a written schedule, checklists, or other means to help with memory and concentration (e.g., communication board)
- When speaking to the patient, speaking slowly, giving one instruction at a time, and allowing the patient time to process
- Talking to aphasic patients while performing care activities to provide social contact
(Belleza, 2024)
Emotional Changes
Emotional changes are common after a stroke and can impact rehabilitation outcome. Stroke patients may experience feelings of irritability, anger, forgetfulness, carelessness, or confusion. These emotional responses, however, tend to improve over time.
Patients may also experience pseudobulbar affect (PBA), in which there is a disconnect between the frontal lobe (which controls emotions) and the cerebellum and brain stem (where reflexes are mediated). The effects are uncontrollable and occur without an emotional trigger. These individuals have involuntary bouts of crying, laughter, or anger that may be out of proportion to the situation. They may also have inappropriate emotional responses, such as laughing in sad or somber occasions. They may also rapidly switch between laughing and crying.
Stroke patients often experience anger that is directed toward hospital staff as well as family members. This anger may be the result of damage to the brain, the loss of ability to communicate, or inability to make choices about their daily activities. The only way for a patient to exert control may be to refuse to do tasks or to be involved in treatment.
Stroke patients can easily become irritated, frustrated, and angry and may use language that they did not use prior to the stroke. This may occur when the person is attempting to accomplish something that formerly was easy and has become difficult post stroke.
Nurses are among the members of the rehab team who address the psychosocial needs of patients by providing support and guidance to improve coping. Occupational therapists as well are responsible for promoting coping and adjustment to the consequences of stroke.
Interventions can include:
- Speaking in a calm and gentle tone using a nonthreatening approach
- Recognizing triggers and striving to avoid them whenever possible
- Offering distractions such as engaging in a different task or exercise
- Using redirection and diversion to help alleviate stimulation and discomfort
- Scheduling rest periods in between activities
- Placing the patient in a calm, low-stimulus environment with low lighting and few individuals
- Avoiding pressuring or requiring the patient to make a decision
It is important to explore the patient’s previous methods of dealing and coping with life’s problems and the presence and quality of their support systems in order to build on past successes and to mobilize resources (ASA, 2025b; Curran, 2025).
DEPRESSION AND ANXIETY
Two of the most common emotional/psychological problems that can result from a stroke are depression and anxiety. Depression affects one third to two thirds of stroke survivors, and anxiety affects about 20%.
Many patients may experience bouts of crying, feel hopeless, and withdraw from social activities. Others may experience general feelings of fear and anxiety, which may result in acute anxiety attacks.
Depression following stroke may make rehabilitation more challenging. Risks for the development of poststroke depression (PSD) include physical disability, severity of stroke, prestroke depression, and cognitive impairment. Clues for PSD can be subtle, such as declining to participate in therapy.
In cases of suspected H2 antagonist–related depression, interventions may include:
- Assessing for history of depression
- Performing an early depression screen
- Providing interventions to enhance rehabilitation
- In the absence of contraindications, administering antidepressants as ordered, including monitoring the patient closely for effectiveness and side effects
(Comer & Jones, 2024; Green et al., 2021)
When working with patients experiencing anxiety, it is important to remain calm and in control in order to work effectively with the patient. Interventions for anxiety include:
- Maintaining a calm, nonthreatening manner
- Establishing trust by listening, showing warmth, answering questions directly, offering unconditional acceptance, being available, avoiding asking the patient to make choices
- Remaining with the patient at times when levels of anxiety are high, reassuring the patient of their safety and security
- Moving the patient to a quiet area with minimal stimuli
- Providing reassurance and comfort measures
- Administering antidepressants and anxiolytics as ordered and monitoring for effectiveness and side effects
- Observing for increasing anxiety
(Vera, 2025)
SELF-MANAGEMENT, FAMILY SUPPORT, AND EDUCATION
Self-management for survivors of stroke seeks to optimize independence in the posthospital environment by offering support and education to both patients and caregivers on the skills of decision-making and problem-solving, as well as establishing goals for stroke prevention and recovery. Teaching should begin in the acute setting.
Examples of self-management interventions include:
- Providing the family with information about the expected outcome of the stroke and counseling them to avoid doing things for the patient that the patient can do for themself
- Encouraging family to support patient and give positive reinforcement
- Enhancing self-efficacy with activities of daily living
- Teaching problem-solving skills and strategies
- Engaging patients in occupational therapy and other rehabilitation programs
- Teaching stress management techniques and maintenance of personal health for family coping
- Incorporating information for survivors of stroke and their family caregivers on provisions for stroke care after discharge
- Developing attainable goals for the patient at home by involving the full healthcare team, patient, and family
(Belleza, 2024; Green et al., 2021; Vera, 2025)
REHABILITATION IN THE ACUTE STROKE SETTING
The primary goals of rehabilitation in the acute setting involve:
- Prevention of medical complications
- Prevention of deconditioning and contractures
- Training of new skills
- Optimizing poststroke rehabilitation:
- Early assessment with standardized evaluations and validated assessment tools
- Early employment of evidence-based interventions relevant to individual patient needs
- Patient access to an experienced multidisciplinary rehabilitation team
- Ongoing medical management of risk factors and comorbidities
(Physiopedia, 2025e)
NEUROPLASTICITY AND STROKE REHABILITATION
The brain uses neural connections to send and retrieve information, and when a portion of these connections are damaged by a stroke, they can create new pathways through a process called neuroplasticity. Following a stroke, the plasticity process is initiated in an attempt to compensate for both the lesion itself and its remote effects. Stroke rehabilitation is based on the awareness that the brain has this intrinsic ability to reorganize its function and structure in response to injuries.
Neuroplasticity is experience and learning dependent. This means that if an individual repeatedly experiences or repeatedly practices something, the brain will reshape itself accordingly. It is necessary to take advantage of this neuroplasticity at every stage in the recovery process. Neuroplasticity is most receptive immediately following a stroke, and rehabilitation must begin as soon as possible to maximize recovery.
Enhancing neuroplasticity involves increasing brain-derived neurotrophic factor (BDNF), a protein that supports and encourages growth of new neurons and synapses critical for neuroplasticity. One way to boost BDNF is with aerobic exercises that increase the heart rate. Another way is by eating foods that contain omega-3 fatty acids, such as salmon or chia seeds (FlintRehab, 2023).
BRUNNSTROM 7 STAGES OF RECOVERY FROM STROKE
The Brunnstrom approach is a type of physical therapy treatment for patients following damage to the central nervous system based on the principle that movement recovery follows a specific sequence. Rehabilitation is tailored to the patient’s individual recovery stage. Recovery is not guaranteed to progress through all seven steps; it may slow or plateau at any of them.
Physical and Occupational Therapy Assessment Tools
Standardized evaluations and valid assessment tools are essential for evaluating patients following a stroke in order to develop a comprehensive treatment plan. Both physical and occupational therapists employ many of the assessment tools listed in the tables below (AHA, 2023).
| Tool | Assesses for … |
|---|---|
| Stroke Impact Scale (SIS) | Stroke recovery as measured in eight domains: strength, hand function, mobility, activities of daily living (ADLs), emotion, memory, communication, and social participation |
| Functional Independence Measure (FIM) | Physical and cognitive disability (to measure burden of care) |
| Alpha FIM Instrument | Motor and cognitive function |
| Modified Rankin Scale (mRS) | Rating global outcome |
| Barthel Index (BI) of Activities of Daily Living | Independence in self-care |
| 6-Minute Walk Test (6MWT) | Walking capacity and endurance |
| Functional Autonomy Measurement System (SMAF) | Functional independence |
| Activities of Daily Living (ADL) Index | Performance of basic ADLs and mobility tasks |
| Tool | Assesses for … |
|---|---|
| Chedoke-McMaster Stroke Assessment Scale (CMSA) | Physical impairment and disability |
| Fugl-Meyer Assessment (FMA) of Motor Recovery after Stroke | Motor function in upper and lower extremity, balance, sensation, range of motion, and pain |
| Rivermead Motor Assessment (RMA) | Gross function in leg, trunk, and arm movement |
| Stroke Rehabilitation Assessment of Movement (STREAM) | Voluntary movement of upper and lower limbs and basic mobility |
| Tool | Assesses for … |
|---|---|
| Berg Balance Scale (BBS) | Balance |
| Functional Ambulation Categories (FAC) | Rating ambulation status |
| Mini BEST Test | Balance control |
| Rivermead Mobility Index (RMI) | Performance of functional activities |
| Timed Up and Go (TUG) test | Mobility and balance |
| Functional Reach Test (FRT) | Maximum distance the patient can reach forward while standing in a fixed position |
| Tool | Assesses for … |
|---|---|
| Actional Research Arm Test (ARAT) | Grasp, rip, pinch, and gross movement |
| Box & Block Test (BBT) | Unilateral gross manual dexterity |
| Chedoke Arm and Hand Activity Inventory (CAHAI) | Arm and hand function while performing bilateral functional tasks |
| Nine Hole Peg Test (NHPT) | Manual dexterity |
| Wolf Motor Function Test (WMFT) | Upper extremity motor ability |
| Tool | Assesses for … |
|---|---|
| Behavioral Inattention Test (BIT) | Visual neglect |
| Line Bisection Test (LBT) | Unilateral spatial neglect |
| Motor-free Visual Perception Test (MPT) | Visual perception |
CMS INPATIENT REHABILITATION FACILITY-PATIENT ASSESSMENT INSTRUMENT (IRF-PAI)
The Center for Medicare and Medicaid Services (CMS) requires the IRF-PAI to be completed on admission and at discharge to collect patient assessment data for quality measure calculation and payment determination for all patients who receive services in an inpatient rehabilitation setting.
The IRF-PAI assesses:
- Hearing, speech, and vision
- Cognitive patterns
- Mood
- Functional abilities
- Bladder and bowel
- Health conditions
- Swallowing/nutritional status
- Skin conditions
- Medications
- Special treatments, procedures, and programs
(CMS, 2024)
Major Therapy Approaches
Several approaches can be used in the rehabilitation of patients following stroke, and it is not uncommon for elements of several different approaches to be used when treating a patient. Some common approaches include, but are not limited to:
- Traditional therapy: Employs straightforward training in range of motion, strengthening, mobilization, gait and balance, and compensatory techniques.
- Bobath concept: Involves neurodevelopmental training (NDT), which suppresses abnormal muscle patterns before normal patterns are introduced. Abnormal patterns are modified at proximal key points of control, such as neck, spine, shoulder, and pelvis.
- Brunnstrom movement therapy: Involves central facilitation and aims to enhance specific synergies through the use of cutaneous/proprioceptive stimuli.
- Proprioceptive neuromuscular facilitation (PNF): Aims to stimulate nerve/muscle/sensory receptors to evoke response through manual stimuli to increase ease of movement and promote function.
- Sensorimotor therapy (Rood approach): Uses cutaneous sensorimotor stimulation to modify muscle tone and voluntary activity.
- Motor relearning program (Carr approach): Uses cutaneous sensorimotor stimulation to modify muscle tone and voluntary activity.
- Constraint-induced movement therapy (CIMT): Used to improve and increase the use of the more affected extremity while restricting the use of the less affected arm.
- Functional electrical stimulation (FES): Used to improve strength in the upper and lower extremities; also assists in management of dependent peripheral edema and establishes early proprioceptive joint sense in sensory-compromised patients.
- Electromyographic biofeedback (EMG-BF): Attempts to modify autonomic functions, pain, and motor disturbances through acquired volitional control using auditory, visual, and sensory clues.
- Robotic devices: Help therapists ensure that exercises are performed properly. They collect performance information and objectively measure progress. Examples include:
- LokomatPro Gait Trainer
- Erigo Pro tilt table
- Andago, which bridges the gap between treadmill and free walking
- Diego, for arm and shoulder rehabilitation
- PABO, which provides interactive therapies for the whole body, hands, fingers, arms, and legs
(IdeasForOT, n.d.; Physiopedia, 2025a)
Physical Therapy for Acute Stroke Rehab
Physical therapy is one of the core professional disciplines involved in stroke rehabilitation. Rehabilitation therapy typically begins in the acute-care hospital once the patient’s condition has stabilized, often within 48 hours following the stroke. The main goal of physical therapy is to help mobilize the patient’s return to activities at home, at work, and in the community. Treatment plans focus on improving mobility, addressing pain, and providing guidance on ways to prevent complications that may occur after a stroke.
PHYSICAL THERAPY ASSESSMENT
Following an acute stroke, physical therapists begin assessment by obtaining:
- History of the present illness
- Past medical history
- Standardized review of systems
- Social history
- List of medications being taken
- Family history
- Prior activity level
- History of any recent alterations in function prior to stroke
- Mobility issues
- Personal care ability and use of aids/devices to assist
- Cognition
- Communication
- Swallowing
- Pain
- Risk for fatigue
- Perceptions of poststroke abilities
- Expectations of treatment
(Physiopedia, 2025d; Physiopedia, 2025e)
Physical examination includes objective testing of the following:
- Posture
- Passive range of motion
- Muscle strength
- Coordination
- Involuntary movements
- Muscle tone
- Deep tendon reflexes
- Sensation
- Functional activities
- Mobility, including bed mobility
- Transfers
- Sitting and standing balance
- Upper and lower limb function
- Stairs
- Gait
ACUTE CARE PHYSICAL THERAPY INTERVENTIONS
Early initiation of mobility-focused physical rehabilitation as soon as the patient is medically stabilized has been associated with decreased deconditioning, improved long-term functional outcomes, and decreased risk of hospital readmission.
In the acute care setting, evaluation and interventions provided by physical therapists may include any of the following, depending on a patient’s functional deficits and ongoing needs:
- Positioning/bed mobility. Physical therapists advise on safe and correct positioning of the patient in multiple positions, including supine, side lying (on both affected and unaffected sides), and sitting, in order to avoid injury and promote the patient’s ability to self-mobilize. Early bed mobility training may include teaching patients how to roll side to side, transition between supine and sitting, sit supported in bed, and sit supported out of bed (with or without back support). Proper positioning can help reduce muscle pain, spasms, slowness, or stiffness that can occur following a stroke.
- Range-of-motion (ROM) exercises. Both passive and active exercises may be initiated early and performed daily in order to promote and maintain joint mobility, protect compromised joints (such as a subluxed shoulder), prevent contractures, increase circulation to extremities, and decrease vascular complications of immobility. ROM exercises may be performed more frequently if patients have increased risk of joint contractures. Effective positioning strategies are important in maintaining soft tissue length and to encourage proper joint alignment. Patients may also be taught ROM self-activities.
- Managing spasticity. If spasticity is present, early mobilization and daily stretching may be employed to maintain length of spastic muscles and soft tissues and promote optimal positioning. Modalities may include application of cold or heat, massage, and electrical stimulation.
- Facilitating upright sitting. Sitting upright is an important way to build endurance, provide maximum stimulation, and give the patient a sense of normalcy during the acute care phase. Early training in sitting focuses on achieving a symmetrical posture with optimal spine and pelvic alignment.
- Exercises to improve respiratory and circulatory functions. If not medically contraindicated, exercises to optimize respiratory and circulatory function may be initiated during this phase. Exercises may include deep breathing and coughing; chest expansion exercises; ankle pumps; and active, active-assisted, or passive upper and/or lower extremity exercises.
- Decubiti (pressure injury) prevention measures. In order to prevent the complication of pressure injury/ulcers, physical therapists work with and make recommendations to the interdisciplinary team to ensure that patients are properly positioned and that pressure points are protected by appropriate padding, cushioning, and/or unweighting. PT may advise on the use of pressure-reducing devices such specialized beds/mattresses, foot/ankle positioners, or pressure-relieving wheelchair cushions (such as those with a gel or air-cell core). Improving a patient’s ability to independently mobilize is one of the more important physical therapy interventions with regard to preventing pressure injuries.
- Transfer techniques. Physical therapy interventions include teaching the patient how to safely transfer between multiple types of surfaces, including bed to/from chair and sitting to/from standing. Physical therapists also advise clinical staff on how to appropriately support/assist the patient during transfers (including demonstrating and performing correct transfer technique using assistive devices such as gait belts, assistive devices, or mechanical lifts) as well as recommend the appropriate level of assistance to be used.
- Balance improvement measures. As allowed by a patient’s functional mobility status, physical therapists may assist with early-stage balance training activities, including specific bed exercises (e.g., pelvic bridging), sitting on the edge of the bed (with or without external support), standing (with or without support), and progressing to ambulation as appropriate. Improving static and dynamic balance, along with improved ability to ambulate (or self-propel a wheelchair), can lead to greater independence and overall well-being as a patient prepares for discharge.
- Balance and walking speed. Backward walking training (BWT) has been shown to significantly improve motor functions, including the 10-meter walk test (10MWT), cadence, Berg Balance Scale (BBS), paretic step length, and stride length (Wen & Wang, 2022).
- Deconditioning prevention measures. To prevent deconditioning, physical therapists make recommendations for and encourage early bed mobility and as much out-of-bed time as medically appropriate and tolerated by the patient. Such activities may include side-to-side rolling, transitioning between supine and sitting, sitting upright in an appropriate chair, transferring between sitting and standing, and (when appropriate) ambulation with appropriate assistive devices or propelling a wheelchair.
- Assistive device training. Prior to discharge, physical therapists may recommend and/or train the patient in the use of appropriate assistive devices, such as:
- Wheelchairs
- Walkers (rolling, standard, hemi-, etc.)
- Canes (straight, quadripod, etc.)
- Orthoses (i.e., when foot drop is present)
- Any devices previously used by the patient (such as orthotics, prosthetics, etc.)
- Patient/family education. Physical therapists work with family members/caregivers in order to provide training in how to help with appropriate exercises for the patient as well as how to safely help the patient with functional mobility at home. They also provide education about the physical effects of the stroke and what continued rehabilitation may be able to accomplish for the patient.
- Discharge planning. Planning for discharge from inpatient rehabilitation begins on admission. Throughout the hospitalization, physical therapists continually reassess the patient’s functional mobility status in an effort to assist in determining the most appropriate setting for the next level of care. The physical therapist may make a home visit prior to discharge to determine the need for architectural and/or other safety modifications.
(APTA, 2025; Bruno-Petrina, 2024; NINDS, 2024b; Physiopedia, 2025e; Shahid et al., 2023)
Occupational Therapy for Acute Stroke Rehab
Occupational therapy plays a significant role in acute-care settings by facilitating early mobilization, improving function, preventing further decline, and coordinating care, including transition and discharge planning.
Treatment approaches are aimed to meet the ultimate goal of maximizing function and independence. They include:
- Rehabilitating and restoring function using physical, cognitive, perceptual, and functional activities
- Teaching restorative or compensatory techniques with or without the use of adaptive equipment, as appropriate
- Providing education on energy conservation techniques that address self-care, functional ability, or therapeutic exercise
- Recommending adaptive equipment and home modifications, if needed
(Stromsdorfer, 2025)
OCCUPATIONAL THERAPY ASSESSMENT
The occupational therapist (OT) assesses a person’s abilities, including level of functional independence, perceptual-cognitive skills, sensory-motor skills, communication skills, quality of life, and levels of anxiety and/or depression. Elements of an occupational therapy assessment include:
- Interviews with the patient and/or family to establish the patient’s prior life roles and the tasks and activities involved in those roles
- Analysis of prehospitalization roles and the patient’s likelihood of resuming them
- Observation of the patient’s abilities to perform personal self-care (e.g., showering, dressing, toileting, grooming, eating)
- Identifying what the patient needs and wants to do, including the supports and barriers
- Visual-perceptual screening for impairments that can interfere with the ability to organize, interpret, and give meaning to information that is seen, impacting the ability to learn; assessing visual fields, convergence, and oculomotor abilities
- Memory, cognition, and executive functioning screening to determine the impact of changes on abilities to resume daily functioning
- Sensory and motor assessments, with particular emphasis on upper limb and hand function, functional mobility, and transfers
Assessment tools may include:
- Katz Index of Independence in Activities Daily Living provides a measurement of ability to perform independent activities that are part of the daily routine.
- Executive Function Performance Test (EFPT) assesses how a patient completes four basic tasks.
- Sensory Processing Measure, second edition (SPM-2), provides a complete picture of sensory integration and processing in multiple environments and provides additional descriptive clinical information on processing vulnerability within each sensory system.
(Neurolutions, 2025)
ACUTE CARE OCCUPATIONAL THERAPY INTERVENTIONS
Occupational intervention in acute stroke care initially is directed at sensory-motor and perceptual-cognitive performance skills, as well as reeducation and training in the basic and instrumental activities of daily living (ADLs and IADLs). Later, interventions oriented more toward the social and labor integration of the person are considered.
Occupational therapy intervenes using specific procedures and activities to develop, maintain, improve, and/or recover the performance of the following functions and activities:
- Basic and instrumental ADLs
- Health management
- Rest and sleep
- Education
- Work
- Play
- Performance skills (motor, sensory-perceptual, emotional regulation, social, and communication)
(Steenblock, 2025; Vanderpool, 2025)
Occupational therapy interventions in the acute stage include:
- Positioning and seating: Correct positioning in good body alignment to reduce the risk of:
- Aspiration
- Shoulder pain
- Pressure areas
- Deep vein thrombosis and pulmonary embolism
- Contractures
- Chronic pain in affected joints
- Muscle spasticity
- Extremity swelling
- Upper limb positioning: Addressing upper limb positioning to prevent shoulder trauma, lessen pain, reduce swelling, and encourage independence in feeding and other self-care activities
- Mobilization techniques: Utilizing positioning, turning, and transferring techniques to assist with mobility, and employing neuromuscular reeducation, trunk stabilization, and balance activities to improve the patient’s ability to move in and out of bed and maintain an upright posture necessary to perform self-care
- Prevention of pressure injuries (decubiti): Utilizing methods to prevent pressure injuries, such as:
- Cushions and padding
- Barrier sprays
- Lubricants
- Special mattresses
- Protective dressings
- Splinting
- Use of positioning devices
- ADLs: Providing training in self-care activities (e.g., bathing, dressing) with adaptive or durable medical equipment and/or compensatory techniques if needed, such as linking behaviors that naturally go together, providing cueing, focusing on one task or step at a time and completing it before moving on to the next, substituting hook-and-loop fasteners (e.g., Velcro) for buttons on clothing, etc.
- Cognition and perception: Addressing cognitive and perceptual deficits, including compensatory techniques
- Assistive and adaptive devices and techniques: Providing training in the use of upper extremity adaptive devices (i.e., for eating, bathing, grooming, and transferring) and wheelchair management
- Management of shoulder pain: Strategies for protecting the joint and reducing pain, including:
- Use of a neurologic support (e.g., GivMohr sling) to promote proper upper extremity positioning and arm swing (which also prevents the limitations that may occur when using a traditional orthopedic sling)
- Use of support while in the wheelchair to manage the affected side to avoid pain and prevent subluxation
- Discharge planning: Making recommendations for ongoing rehab in settings appropriate to the level of the patient’s rehabilitation needs, including inpatient, outpatient, skilled nursing, home health, community, nontraditional, or other postacute settings, according to the patient’s ability level and stroke severity
Speech-Language Therapy for Acute Stroke Rehab
Speech-language therapy is another core element of stroke rehabilitation and has a key role in the identification, assessment, and management of potentially life-threatening eating, drinking, and swallowing problems (dysphagia) and the development of other means of communication. Recovery of language skills is usually a slow process, and although most people make significant progress, few people regain full preinjury communication levels.
SPEECH AND LANGUAGE SWALLOWING ASSESSMENT
All stroke patients are screened at bedside for dysphagia before being given food or fluids. Tools used by speech-language therapists to assess swallowing include:
- Mann Assessment of Swallowing Ability (MASA). This tool was first introduced to identify dysphagia in acute-stage stroke patients. It is also used as a screening tool in any dependent older adult.
- Acute Stroke Dysphagia Screen. This is an easily administered and reliable tool used to detect both dysphagia and aspiration risk in acute stroke patients.
- Victorian Dysphagia Screening Model ASSIST Tool. This tool is applied by professionals who have completed an approved training in dysphagia screening and is recommended for use in the presence of persistent acute stroke symptoms.
- Swallowing Ability and Function Evaluation. This test evaluates swallowing; before it is administered, a baseline evaluation of cognition and proper motor function are obtained.
(Tiwana & Bordoni, 2023)
Patients with suspected dysphagia may require additional instrumental assessment to examine the impact of swallowing anatomy and physiology on clinical presentation. Patients may also require further assessment or reassessment depending on changes in functional or medical status, including the video fluoroscopic swallowing study (VFSS) or the flexible endoscopic evaluation of swallowing (FEES), sometimes called fiber-optic endoscopic evaluation of swallowing.
Difficulty swallowing may be caused by delayed swallowing reflexes, inefficient use of the tongue, and inability to detect food lodged in the cheeks after swallowing.
DYSPHAGIA INTERVENTIONS
A speech-language pathologist can work with an individual to devise strategies to overcome or minimize this deficit once the reason has been determined. A simple change of body position during eating can make a significant difference. A food’s texture can be altered to make swallowing easier; for example, thin liquids can be thickened to prevent choking. A change in eating habits, such as taking small bites and chewing slowly, may also help alleviate dysphagia. Other interventions may include:
- Utilizing sensory stimulation for heightened sensory input
- Utilizing therapeutic maneuvers (e.g., Mendelsohn maneuver, supraglottic swallow)
- Utilizing exercise programs (tongue resistance, ROM, tongue base, chewing)
(ASHA, 2025; Neurolutions, 2025)
SPEECH-LANGUAGE INTERVENTIONS
Strokes can result in difficulties with a patient’s ability to communicate ideas, needs, and feelings, which may include:
- Apraxia: difficulty or inability to move the mouth and tongue to speak
- Aphasia: impaired language, affecting production or comprehension of speech and ability to read or write
- Dysarthria: impaired intelligibility of speech as a result of weakness, paralysis, or incoordination of speech musculature
- Cognitive deficits: problems with attention, memory, perception, insight and judgment, organization, processing speed, problem-solving, reasoning, and executive functioning
Speech-language therapy aims to improve the ability to communicate by restoring as much language as possible, teaching the individual how to make up for lost language skills and finding other methods of communicating. Studies have found that therapy is most effective when it begins soon after the brain injury.
Specific exercises, guidance, explanations, techniques, or psychological support can all be used to treat dysarthria, depending on the individual. Some interventions are:
- Breathing exercises, to enhance breath support and control
- Nonspeech motions, to improve oral muscular strength, speed, and accuracy
- Text-to-speech tools as well as phonetic symbols, to summon out letters or words
- Instruction and education about dysarthria
- Collaboration with communication partners
- Involvement in communication support groups
For those with cognitive communication deficits, interventions may include:
- Using exercises or software to retrain discrete cognitive processes such as attention
- Using internal memory strategies or spaced retrieval training to solidify memories
- Completing practice tasks that are difficult in order to build independence
- Using external strategies for improving memory (e.g., memory books, smartphone apps)
- Education for patient and family
(SpeechTherapy.org, 2025; TactusTherapy, 2025)
DISCHARGE FROM THE HOSPITAL
As the time of discharge approaches, a patient’s limitations are assessed formally by specialists—including physical therapists, occupational therapists, speech-language pathologists, psychologists, and nutritionists. These professionals then make recommendations that can be taken into account before physicians begin discharging the patient. Nurses on the stroke team also initiate the patient’s transition into the appropriate supervised rehabilitation programs.
Preventing Secondary Stroke
The risk for a secondary stroke is as high as 23% within the first year (Johns Hopkins Medicine, 2025). Controlling risk factors for stroke is critical in the prevention of a secondary stroke. Risk factor control is affected by patient-, provider-, and system-level factors.
Studies show that stroke survivors who participate in rehabilitation programs that include exercise combined with education and counseling show improvements in fitness, cholesterol levels, and body weight, as well as a decrease in secondary stroke.
Stroke patients are encouraged to take medications, such as anticoagulants, antihypertensives, and statins, according to their healthcare professional’s instructions. Lifestyle habits are assessed, and shared decision-making between the healthcare professional and the patient addresses the patient’s wishes, goals, concerns, and circumstances. Formal programs in which physicians and other healthcare professionals help patients change their routines and behavior can help stroke survivors make, and keep, needed lifestyle changes (Bushnell et al., 2024).
The mnemonic ABCDE describes important elements in preventing secondary stroke (see table below).
| Element | Examples | |
|---|---|---|
| (Bushnell et al., 2024; Silver, 2024) | ||
| A | Antiaggregants |
|
| Anticoagulants |
|
|
| B | Blood pressure-lowering medications |
|
| C | Cholesterol-lowering medications |
|
| Cessation of smoking |
|
|
| Carotid revascularization | n/a | |
| D | Diet |
|
| Diabetes control | n/a | |
| E | Exercise |
|
Patient and Family Education and Support
Following stroke, patients and families are typically faced with multiple life changes and challenges as the patient transitions through the stages of recovery. Both the patient and family should be assessed, educated, and prepared for transitions between care stages and settings and screened for level of coping, risk for depression, and other physical and psychological issues.
Education and support for patient, families, and caregivers may include:
- Written discharge instructions and recommendations that identify collaborative action plans, follow-up care, and goals
- Accurate and up-to-date information about the next care setting, what can be expected, and how to prepare
- Access to restorative care and active rehabilitation to improve and/or maintain function based on the individualized care plan
- Access to a designated contact person in the hospital or community for continuity of care and queries
- Ongoing access to and advice from health and social service organizations appropriate to needs and stages of transition and recovery
- Links to and information about local community agencies, such as stroke survivor groups, peer survivor visiting programs, meal provider agencies, and other services and agencies
- Shared decision-making/participation regarding transitions between stages of care
- Counseling, preparation, and ongoing assessment for adjustment to:
- Change of living setting
- Change in physical needs and increased dependency
- Change in social roles and leisure activities
- Impact on other family members (e.g., spouse/partner, children)
- Loss of home environment
- Potential resource issues
- Advance care planning, palliative care, and end-of-life care, as applicable
(ASA, n.d.-a; Canadian Stroke Best Practices, 2019; MediGence, 2025)
CONCLUSION
Strokes, often called cerebrovascular accidents (CVAs), result from limitations or interruptions in cerebral perfusion. Stroke ranks fifth among all causes of death in the United States when considered apart from other cardiovascular diseases.
Most strokes result from blockages of an artery by a local blood clot or by an embolus from the heart or carotid artery. These strokes are called ischemic, and they are typically the product of years of atherosclerosis and hypertension. Strokes caused by intracranial bleeds are called hemorrhagic strokes, and they result from a ruptured cerebral artery or aneurysm. Hypertension is typically involved in generating a hemorrhagic stroke.
Symptomatically, all strokes appear as acute impairments in brain functioning. A person may suddenly have difficulty walking, seeing, speaking, or understanding. With severe hemorrhagic strokes, the person may lose consciousness. Most ischemic strokes are painless, although hemorrhagic strokes can produce severe headache.
An acute ischemic stroke is a medical emergency, requiring fast, organized care. There is a 4.5-hour interval after the onset of symptoms in which fibrinolytic therapy has a chance to reopen clogged cerebral arteries and save some of the underperfused brain tissue. Given this time constraint, EMS teams have the goal of getting potential stroke victims stabilized, evaluated, and to a stroke center in less than an hour.
The critical step in evaluating an acute stroke is making the distinction between ischemic and hemorrhagic strokes, and at this point, treatment paths for ischemic and hemorrhagic stroke patients diverge. For ischemic strokes, IV recombinant tissue plasminogen activator (rtPA) or tenecteplase (TNK) is administered. For hemorrhagic strokes due to a ruptured subarachnoid aneurysm, treatment by surgically clipping the aneurysm remnant or by endovascularly inserting a coil may be done.
Following initial evaluation and treatment, stroke patients are monitored in the ICU, and as soon as the patient is stabilized medically, usually within 24 to 48 hours, the rehabilitation team is consulted to assess rehabilitation needs, begin early rehabilitation efforts, and recommend the most appropriate poststroke setting. The goals of rehabilitation in the acute setting are to prevent, recognize, and manage comorbid medical conditions; to minimize impairments; and to maximize functional independence.
RESOURCES
National Institute of Neurologic Disorders and Stroke
Neurological flowsheet (Sutter Medical Center)
Stroke (Centers for Disease Control and Prevention)
What is a stroke? (NHLBI)
REFERENCES
Advanced Cardiac Life Support (ACLS) Certification Institute. (2024). Suspected stroke algorithm. https://resources.acls.com/free-resources/acls-algorithms/suspected-stroke
Al-Dhahir M, Hall W, Das J, & Sharma S. (2025). Neurogenic pulmonary edema. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK532984/
Alexandrov A & Krishnaiah B. (2025a). Intracerebral hemorrhage. Merck Manual. https://www.merckmanuals.com/professional/neurologic-disorders/stroke/intracerebral-hemorrhage
Alexandrov A & Krishnaiah B. (2025b). Subarachnoid hemorrhage. Merck Manual. https://www.merckmanuals.com/professional/neurologic-disorders/stroke/subarachnoid-hemorrhage?mredirectid=4209
American Heart Association (AHA). (2025a). Heart disease and stroke statistics 2025 update. https://www.heart.org/-/media/PHD-Files-2/Science-News/2/2025-Heart-and-Stroke-Stat-Update/2025-Heart-and-Stroke-Stats-Infographics.pdf?sc_lang=en
American Heart Association (AHA). (2025b). Phase III. Target: Stroke. Suggested time interval goals. https://www.heart.org/-/media/Files/Professional/Quality-Improvement/Target-Stroke/Target-Stroke-Phase-III/9-17-Update/DS14860-Time-Interval-One-Pager_v2.pdf?sc_lang=en
American Heart Association (AHA). (2025c). Target: Stroke—When seconds count. https://www.heart.org/en/professional/quality-improvement/target-stroke/learn-more-about-target-stroke
American Heart Association (AHA). (2025d). Women and risk of stroke infographic. https://www.goredforwomen.org/en/know-your-risk/risk-factors/risk-of-stroke-in-women-infographic
American Heart Association (AHA). (2024). Marijuana use linked to higher risk of heart attack and stroke. https://www.heart.org/en/news/2024/02/28/marijuana-use-linked-to-higher-risk-of-heart-attack-and-stroke
American Heart Association (AHA). (2023). Information on standardized assessment tools used in stroke rehabilitation. https://www.heart.org/-/media/Files/Affiliates/Regional-Pages/Montana/Handout-Standardized-Assessments-Used-in-Stroke-Rehab-2023-AAD.pdf?sc_lang=en
American Physical Therapy Association (APTA). (2025). Physical therapy guide to stroke. https://www.choosept.com/guide/physical-therapy-guide-stroke
American Speech-Language-Hearing Association (ASHA). (2025). Adult dysphagia. https://www.asha.org/practice-portal/clinical-topics/adult-dysphagia/
American Stroke Association (ASA). (n.d.-a). Caregiver guide to stroke. https://www.stroke.org/en/-/media/Stroke-Files/Caregiver-Support/Caregivers-Guide-to-Stroke/CaregiverGuideToStroke_2020.pdf?sc_lang=en
American Stroke Association (ASA). (n.d.-b). Recommendations for regional stroke destination plans in rural, suburban, and urban communities from the prehospital stroke system of care consensus conference: A consensus statement from the American Academy of Neurology, American Heart Association/American Stroke Association, American Society of Neuroradiology, National Association of EMS Physicians, National Association of State EMS Officials, Society of NeuroInterventional Surgery, and Society of Vascular and Interventional Neurology. https://www.stroke.org/en/-/media/Stroke-Files/EMS-Resources/Stroke-Destination-Change-032021/DS17296_Prehospital-SSOC-Statement-summary_Final.pdf?sc_lang=en
American Stroke Association (ASA). (2025a). Effects of stroke. https://www.stroke.org/en/about-stroke/effects-of-stroke
American Stroke Association (ASA). (2025b). Emotional and behavioral effects of stroke. https://www.stroke.org/en/about-stroke/effects-of-stroke/emotional-effects-of-stroke
American Stroke Association (ASA). (2025c). Hemorrhagic stroke. https://www.stroke.org/en/about-stroke/types-of-stroke/hemorrhagic-strokes-bleeds
American Stroke Association (ASA). (2025d). Stroke in children. https://www.stroke.org/en/about-stroke/stroke-in-children
American Stroke Association (ASA). (2025e). Stroke risk factors not within your control. https://www.stroke.org/en/about-stroke/stroke-risk-factors/stroke-risk-factors-not-within-your-control
American Stroke Association (ASA). (2025f). Types of stroke and treatment. https://www.stroke.org/en/about-stroke/types-of-stroke
American Stroke Association (ASA). (2024). Types of aphasia. https://www.stroke.org/en/about-stroke/effects-of-stroke/communication-and-aphasia/stroke-and-aphasia/types-of-aphasia
American Stroke Association (ASA). (2023a). Let’s talk about Black Americans and stroke. https://www.stroke.org/en/-/media/Stroke-Files/Lets-Talk-About-Stroke/Prevention/Lets-Talk-About-Black-Americans-and-Stroke.pdf?sc_lang=en
American Stroke Association (ASA). (2023b). Let’s talk about risk factors for stroke. https://www.stroke.org/en/help-and-support/resource-library/lets-talk-about-stroke/risk-factors
American Stroke Association (ASA). (2023c). Uncommon causes of stroke. https://www.strokeassociation.org/en/about-stroke/stroke-risk-factors/uncommon-causes-of-stroke
American Stroke Association (ASA). (2022). Stroke training for EMS professionals. https://www.stroke.org/en/-/media/Stroke-Files/EMS-Resources/Stroke-Training-for-EMS-Professionals.pdf?sc_lang=en
Anadani M, Nelson A, & Bruno A. (2022, January). Hyperglycemia management. Practical Neurology. https://practicalneurology.com/articles/2022-jan/hyperglycemia-management/pdf
Arkansas Department of Health (ADH). (n.d.). Best practices to improve coordinated stroke care for emergency medical service professional. https://healthy.arkansas.gov/wp-content/uploads/Arkansas_EMS_-_Stroke_Toolkit.pdf
Aziz Y & Mistry E. (2022, January). In-hospital blood pressure control after acute ischemic stroke. Practical Neurology. https://practicalneurology.com/articles/2022-jan/in-hospital-blood-pressure-control-after-acute-ischemic-stroke
Babkair L, Safhi R, Balshram R, Safhei R, Almahamdy A, Hakami F, & Alsaleh AM. (2023). Nursing care for stroke patients: Current practice and future needs. Nursing Reports, 13(3), 1236–50. https://pmc.ncbi.nlm.nih.gov/articles/PMC10535295/
Barber P. (2024). Alberta stroke program early CT score (ASPECTS). MDCalc. https://www.mdcalc.com/calc/3164/alberta-stroke-program-early-ct-score-aspects
Bath P, Song L, Silva G, Mistry E, Petersen N, Tsivgoulis G, Mazighi M, et al. (2022). Blood pressure management for ischemic stroke in the first 24 hours. Stroke, 53(4). https://doi.org/10.1161/STROKEAHA.121.03614
Belleza M. (2024). Cerebrovascular accident (stroke). Nurselabs. https://nurseslabs.com/cerebrovascular-accident-stroke/
Bertuccio A, Marasco S, Longhitano Y, Romenskaya T, Elia A, Mezzini G, Vitali M, et al. (2023). External ventricular drainage: A practical guide for neuro-anesthesiologists. Clinics and Practice, 13(1), 219–29. https://pubmed.ncbi.nlm.nih.gov/36826162/
Bladder & Bowel Community. (2024). CVA/stroke and incontinence. https://www.bladderandbowel.org/associated-illness/cva-stroke/
Bradley R. (2023). Mobile stroke units. American Nurse. https://www.myamericannurse.com/mobile-stroke-units/
Brain Aneurysm Foundation (BAF). (2025). Statistics and facts. https://www.bafound.org/statistics-and-facts/
Brown R. (2024). Stroke. Mayo Clinic.https://www.mayoclinic.org/diseases-conditions/stroke/diagnosis-treatment/drc-20350119
Bruno-Petrina A. (2024). Motor recovery in stroke. Medscape. https://emedicine.medscape.com/article/324386-overview#a2
Bushnell C, Kernan W, Sharrief A, Chaturvedi S, Cole J, Cornwell W, Cosby-Gaither C, et al. (2024). 2024 guideline for the primary prevention of stroke: A guideline from the American Heart Association/American Stroke Association. Stroke, 55(12). https://doi.org/10.1161/STR.000000000000047
Calviere L, Gathier C, Rafiq M, Koopman I, Rousseau V, Raposo N, Albucher A, et al. (2022). Rebleeding after aneurysmal subarachnoid hemorrhage in two centers using different blood pressure management strategies. Frontiers in Neurology, 13. https://pubmed.ncbi.nlm.nih.gov/35280266/
Canadian Stroke Best Practices. (2019). Section 1: Supporting people with stroke, their families and caregivers. https://www.strokebestpractices.ca/recommendations/managing-stroke-transitions-of-care/supporting-patients-families-and-caregivers-following-stroke
Caplan L. (2025a). Patient education: Ischemic stroke treatment (beyond the basics). UpToDate. https://www.uptodate.com/contents/ischemic-stroke-treatment-beyond-the-basics
Caplan L. (2025b). Overview of the evaluation of stroke. UpToDate. https://www.uptodate.com/contents/overview-of-the-evaluation-of-stroke
Caplan L. (2024). Stroke: Etiology, classification, and epidemiology. UpToDate. https://www.uptodate.com/contents/stroke-etiology-classification-and-epidemiology
Carrarini C, Di Stefano V, Russo M, Dono F, Di Pietro M, Furia N, Onofrj M, et al. (2022). ECG monitoring of post-stroke occurring arrhythmias: An observational study using 7-day Holter ECG. Scientific Reports, 12(1), 228. https://doi.org/10.1038/s41598-021-04285-6
Cedars Sinai. (2025). Ischemic stroke. https://www.cedars-sinai.org/health-library/diseases-and-conditions/i/ischemic-stroke.html
Center for Medicare & Medicaid Services (CMS). (2024). Inpatient rehabilitation facility—Patient assessment instrument. https://www.cms.gov/files/document/irf-pai-version-42-effective-10-01-24-final.pdf
Centers for Disease Control and Prevention (CDC). (2024a). About stroke. https://www.cdc.gov/stroke/about/
Centers for Disease Control and Prevention (CDC). (2024b). Risk factors for stroke. https://www.cdc.gov/stroke/risk-factors/index.html
Centers for Disease Control and Prevention (CDC). (2024c). Signs and symptoms of stroke. https://www.cdc.gov/stroke/signs-symptoms/index.html
Centers for Disease Control and Prevention (CDC). (2024d). Stroke facts. https://www.cdc.gov/stroke/data-research/facts-stats/index.html
Charbonnier G, Bonnet L, Biondi A, & Moulin T. (2021). Intracranial bleeding after reperfusion therapy in acute ischemic stroke. Frontiers in Neurology, 11. https://pmc.ncbi.nlm.nih.gov/articles/PMC7900408/
Children’s Hemiplegia and Stroke Association (CHASA). (2025). Pediatric stroke. https://chasa.org/medical/pediatric-stroke/
Cleveland Clinic. (2025). Stroke. https://my.clevelandclinic.org/health/diseases/5601-stroke
Cleveland Clinic. (2022). Vertebral artery dissection. https://my.clevelandclinic.org/health/diseases/23961-vertebral-artery-dissection
Cohen-Gadol A. (2024). Types of brain aneurysms. https://www.aaroncohen-gadol.com/en/patients/brain-aneurysm/types/types-of-aneurysms
Comer A & Jones A. (2024). Diagnosis and treatment of depression and anxiety in people who have experienced a stroke and their caregivers. Practical Neurology. https://practicalneurology.com/diseases-diagnoses/stroke/diagnosis-and-treatment-of-depression-and-anxiety-in-people-who-have-experienced-a-stroke-and-their-caregivers/32190/
Curran A. (2025). Labile emotional control: Nursing diagnosis & care plan. NurseStudy.net. https://nursestudy.net/labile-emotional-control-nursing-diagnosis/
Davenport S. (2023). How permissive hypertension for stroke works. MedicalNewsToday. https://www.medicalnewstoday.com/articles/permissive-hypertension-for-stroke
Desai S. (2024). Blood pressure in neurological emergencies: What does the evidence say? emDocs. https://www.emdocs.net/blood-pressure-management-in-neurologic-emergencies-what-does-the-evidence-say/
Dugas C, Jamal Z, & Bollu P. (2024). Xanthochromia. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK526048/
Emergency Nurses Association (ENA). (2023). Emergency severity index handbook (5th edition). https://californiaena.org/wp-content/uploads/2023/05/ESI-Handbook-5th-Edition-3-2023.pdf
Emfietzoglou M. (2025). High Fowler position: What is it, its uses, and how it helps breathing. Osmosis. https://www.osmosis.org/answers/high-fowler-position
Federal Communications Commission (FCC). (2025a). Enhanced 911—Wireless services. https://www.fcc.gov/general/enhanced-9-1-1-wireless-services
Federal Communications Commission (FCC). (2025b). PSAP text-to-911 readiness and certification registry. https://www.fcc.gov/general/psap-text-911-readiness-and-certification-form
Feigin V, Brainin M, Norrving B, Martins S, Pandian J, Lindsay P, Grupper M, & Rautalin I. (2025). World Stroke Organization: Global stroke fact sheet 2025. International Journal of Stroke, 20(2), 132–44. https://journals.sagepub.com/doi/10.1177/17474930241308142
Filho J & Samuels O. (2025). Approach to reperfusion therapy for acute ischemic stroke. UpToDate. https://www.uptodate.com/contents/approach-to-reperfusion-therapy-for-acute-ischemic-stroke
Fischer M & Das J. (2023). Cerebellar hematoma. StatPearls. https://www.ncbi.nlm.nih.gov/sites/books/NBK541076/
FlintRehab. (2025). ACA stroke: Symptoms, causes, & recovery from damage to the anterior cerebral artery. https://www.flintrehab.com/aca-stroke/
FlintRehab. (2023). Neuroplasticity after stroke: How the brain rewires itself to recover from injury. https://www.flintrehab.com/neuroplasticity-after-stroke/
Freeman W & Weitz J. (2025). Reversal of anticoagulation in intracranial hemorrhage. UpToDate. https://www.uptodate.com/contents/reversal-of-anticoagulation-in-intracranial-hemorrhage
Gaines K. (2023). Understanding and interpreting the Glasgow Coma Scale. Nurse.org. https://nurse.org/articles/glasgow-coma-scale/
Genentech. (2025). FDA approves Genentech’s TNKase in acute ischemic stroke in adults. https://www.gene.com/media/press-releases/15053/2025-03-03/fda-approves-genentechs-tnkase-in-acute-
Goldemund D. (2025). Basic neurological examination. https://www.stroke-manual.com/basic-neurological-examination/
Grainger B, McFadyen J, & Tran H. (2024). Between a rock and a hard place: Resumption of oral anticoagulant therapy after intracranial hemorrhage. Journal of Thrombosis and Haemostasis, 22(3), 594–603. https://www.jthjournal.org/article/S1538-7836(23)00790-0/fulltext
Green T, McNair N, Hinkle J, Middleton S, Miller E, Perrin S, Power M, Southerland A, & Summers D. (2021). Care of the patient with acute ischemic stroke (posthyperacute and prehospital discharge): Update to 2009 Comprehensive Nursing Care Scientific Statement. American Heart Association. https://www.heart.org/-/media/Files/Professional/Quality-improvement/Stroke-Month-2021/Nursing-Guides/Nursing-Care-of-the-Patient-With-Acute-Ischemic-StrokeMArch-2021.pdf
Hagag A, Kormod M, Ads M, El-Refaay M, Abozaid O, Ghanem O, Yasser M, et al. (2025). Tenecteplase versus alteplase in patients with acute ischemic stroke: An updated systemic review and meta-analysis. European Journal of Medical Research, 30, 726. https://pmc.ncbi.nlm.nih.gov/articles/PMC12333306/
Healthcare Provider Solutions (HCPS). (2025). Acute stroke. https://nhcps.com/lesson/acls-acute-stroke/
Hill M, Baumann J, & Newcommon N. (2022). Nursing care of the acute ischemic stroke endovascular thrombectomy patient. Stroke, 53(9), 2958–66. https://pubmed.ncbi.nlm.nih.gov/35722874/
Hoh B, Ko N, Amin-Hanjani S, Chou S, Cruz-Flores S, Dangayach N, Derdeyn C, et al. (2022). 2022 guideline for the management of patients with spontaneous intracerebral hemorrhage: A guideline from the American Heart Association/American Stroke Association. Stroke, 53(7). https://doi.org/10.1161/STR.00000000000004
Holland M. (2024). Oxygen levels: Vital minimums for stroke patient recovery. MedShun. https://medshun.com/article/what-is-the-minimum-oxygen-saturation-level-for-stroke-patients
IdeasForOT. (n.d.). A comparison of neurorehabilitation techniques used to treat the effects of cerebrovascular accidents. https://ideasforot.com/?page_id=251
Ikram A & Zafar A. (2023). Basilar artery infarct. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK551854/
Ishida K. (2025). Complications of stroke: An overview. UpToDate. https://www.uptodate.com/contents/complications-of-stroke-an-overview
Ishida K. (2024). Prevention and treatment of venous thromboembolism in patients with acute stroke. UpToDate. https://www.uptodate.com/contents/prevention-and-treatment-of-venous-thromboembolism-in-patients-with-acute-stroke
Jain S, Margetis K, & Iverson L. (2025). Glasgow coma scale. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK513298/
Jauch E. (2024). Ischemic stroke. Medscape. https://emedicine.medscape.com/article/1916852-overview#a4
Jichici D. (2024). Anterior circulation stroke. Medscape. https://emedicine.medscape.com/article/1159900-overview
Johns Hopkins Medicine. (2025). Three ways to avoid a second stroke. https://www.hopkinsmedicine.org/health/conditions-and-diseases/stroke/3-ways-to-avoid-a-second-stroke
Kesav P, Raj D, & John S. (2023). Cerebrovascular fibromuscular dysplasia—A practical review. Vascular Health and Risk Management, 28(19), 543–56. https://pmc.ncbi.nlm.nih.gov/articles/PMC10473246/
Kitagawa K. (2022). Blood pressure management for secondary stroke prevention. Hypertension Research, 45, 936–43. https://www.nature.com/articles/s41440-022-00908-1
Levine D, Duncan P, Nguyen-Huynh, & Ogedegbe O. (2020). Interventions targeting racial/ethnic disparities in stroke prevention and treatment. Stroke, 51(11), 3425–32. https://pubmed.ncbi.nlm.nih.gov/33104466/
Lippincott Nursing Center (LNC). (2025). Assessment and management of stroke. https://www.nursingcenter.com/clinical-resources/nursing-pocket-cards/assessment-and-management-of-stroke
Lippincott Nursing Center (LNC). (2024). Tissue plasminogen activator (tPA) for acute ischemic events. https://www.nursingcenter.com/clinical-resources/nursing-pocket-cards/alteplase-injection-for-acute-ischemic-stroke
Liuzzo D, Fell N, Heath G, Raghavan P, & Levine D. (2024). Behavioral risk profiles of stroke survivors among US adults: Geographic differences between stroke belt and non-stroke belt states. Preventing Chronic Disease, 21, 240113. http://dx.doi.org/10.5888/pcd21.240113
Lui F, Suheb M, & Patti L. (2025). Ischemic stroke. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK499997/
Madsen T, Ding L, Khoury J, Haverbusch M, Woo D, Ferioli S, La Rosa F, et al. (2024). Trends over time in stroke incidence by race in the greater Cincinnati Northern Kentucky stroke study. Neurology, 102(3). https://doi.org/10.1212/WNL.0000000000208077
Mathews S & De Jesus O. (2023). Thrombectomy. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK562154/
Mayo Clinic. (2025). Stroke. https://www.mayoclinic.org/diseases-conditions/stroke/diagnosis-treatment/drc-20350119
Mayo Clinic. (2023). Telestroke (stroke telemedicine). https://www.mayoclinic.org/tests-procedures/stroke-and-telemedicine/about/pac-20395081
MediGence. (2025). How family members can support a stroke patient’s recovery? https://medigence.com/blog/how-family-members-can-support-a-stroke-patients-recovery/
Moawad H. (2024). Hemorrhagic stroke: How to spot and prevent it. Very Well Health. https://www.verywellhealth.com/hemorrhagic-stroke-8647768
Morasch M. (2024). Vertebral artery revascularization. UpToDate. https://www.uptodate.com/contents/vertebral-artery-revascularization
Mount Sinai. (2025). Arteriovenous malformations of the brain. https://www.mountsinai.org/locations/cerebrovascular-center/conditions/vascular-malformations/brain
Mukminin M, Yeh T, Lin H, Rohmah I, & Chiu H. (2025). Global prevalence and risk factors of delirium among patients following acute stroke: a systematic review and meta-analysis. Journal of Stroke and Cerebrovascular Diseases, 34(2). https://doi.org/10.1016/j.jstrokecerebrovasdis.2024.108221
Murphy Z. (2025). Stroke syndromes: MCA, ACA, ICA, PCA, vertebrobasilar artery strokes: Pathophysiology. Ninja Nerd. https://www.ninjanerd.org/lecture/stroke-syndromes-mca-aca-ica-pca-vertebrobasilar-artery-strokes-pathophysiology/
National Health Care Provider Solutions (NHCPS). (2025). Acute stroke care guide. https://nhcps.com/lesson/acls-acute-stroke-care/
National Heart, Lung, and Blood Institute (NHLBI). (2023). Stroke causes and risk factors. https://www.nhlbi.nih.gov/health/stroke/causes
National Institute of Neurological Disorders and Stroke (NINDS). (2025). Stroke signs and symptoms. https://www.ninds.nih.gov/health-information/stroke/signs-and-symptoms
National Institute of Neurological Disorders and Stroke (NINDS). (2024a). Arteriovenous malformations (AVMs). https://www.ninds.nih.gov/Disorders/All-Disorders/Arteriovenous-Malformation-Information-Page
National Institute of Neurological Disorders and Stroke (NINDS). (2024b). Stroke recovery. https://www.ninds.nih.gov/health-information/stroke/recovery
National Institutes of Health (NIH). (2025). How many people are affected by/at risk for stroke? https://www.nichd.nih.gov/health/topics/stroke/conditioninfo/risk
Nehring S, Tadi P, & Tenny S. (2023). Cerebral edema. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK537272/
Neurolutions. (2025). A guide to rehabilitation after stroke. https://www.neurolutions.com/post/a-guide-to-rehabilitation-after-stroke
NeuroRestorative. (2025). What are the Brunnstrom stroke recovery stages? https://neurorestorative.com/blog-post/what-are-the-brunnstrom-stroke-recovery-stages/
North Dakota Health and Human Services (NDHHS). (n.d.). Transition to tenecteplase checklist. https://www.hhs.nd.gov/sites/www/files/documents/DOH%20Legacy/EMS/Stroke/2024/2024%20Revised%20PHAC%20Tenecteplase%20Resource.pdf
Nydahl P, Baumgarte F, Berg D, Bergjan M, Borzikowsky C, Franke C, Green D, et al. (2022). Delirium in stroke units: A prospective, multicentric quality-improvement project. Journal of Neurology, 169, 3735–44. https://doi.org/10.1007/s00415-022-11000-6
Oliveira-Filho J & Mullen MT. (2025). Initial assessment and management of acute stroke. UpToDate. https://www.uptodate.com/contents/initial-assessment-and-management-of-acute-stroke
Oliveira-Filho J & Samuels O. (2025). Approach to reperfusion therapy for acute ischemic stroke. UpToDate. https://www.uptodate.com/contents/approach-to-reperfusion-therapy-for-acute-ischemic-stroke
Panuganti K, Tadi P, & Lui F. (2023). Transient ischemic attack. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK459143/
Patrizz A, El Hamamy A, Maniskas M, Munshi Y, Atadja L, Ahnstedt H, Howe M, et al. (2023). Stroke-induced respiratory dysfunction is associated with cognitive decline. Stroke, 54(7). https://doi.org/10.1161/STROKEAHA.122.04123
Paz C, Perez-Mato M, Bellemain-Sagnard M, Gonzalez-Dominguez M, Marie P, Perez-Gayol L, Lopez-Arias E, et al. (2024). Pharmacological preclinical comparison of tenecteplase and alteplase for the treatment of acute stroke. Journal of Cerebral Blood Flow Metabolism, 44(8), 1306–18. https://doi.org/10.1177/0271678X241237427
Physiopedia. (2025a). Neurology treatment techniques. https://www.physio-pedia.com/Neurology_Treatment_Techniques
Physiopedia. (2025b). NIH stroke scale. https://www.physio-pedia.com/NIH_Stroke_Scale
Physiopedia. (2025c). Stroke medical management. https://www.physio-pedia.com/Stroke_Medical_Management
Physiopedia. (2025d). Stroke: Physiotherapy assessment. https://www.physio-pedia.com/Stroke:_Physiotherapy_Assessment
Physiopedia. (2025e). Stroke: Physiotherapy treatment approaches. https://www.physio-pedia.com/Stroke:_Physiotherapy_Treatment_Approaches
Picmonic. (2025). Histologic evolution of ischemic stroke. https://www.picmonic.com/api/v3/picmonics/50302/pdf
Pinto V & Adeyinka A. (2025). Increased intracranial pressure. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK482119/
Powers W, Rabinstein A, Ackerson T, Adeoye O, Bambakidis N, Becker K, Biller J, et al. (2019). Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 50, e344–418. https://www.ahajournals.org/doi/10.1161/STR.0000000000000211
Prabhakaran S & Ibeh C. (2025). Cryptogenic stroke and embolic stroke of undetermined source (ESUS). UpToDate. https://www.uptodate.com/contents/cryptogenic-stroke
Rahmati B. (2024). Ischemic penumbra: A critical window for stroke intervention. Journal of Experimental Stroke & Translational Medicine, 16(6), 289–90. https://www.openaccessjournals.com/articles/ischemic-penumbra-a-critical-window-for-stroke-intervention.pdf
Rajashekar D & Liang J. (2023). Intracerebral hemorrhage. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK553103/
Reeve N, Best V, Cannings-John R, Gillespie D, Hughes K, Lugg-Widger F, Torabi F, et al. (2025). Risk of myocardial infarction and stroke following microbiologically confirmed urinary tract infection: A self-controlled case series study using linked electronic health data. BMJ Open, 15(6), e097754. https://bmjopen.bmj.com/content/15/6/e097754
RNpedia. (2025). Cerebrovascular accident nursing care plan & management. https://www.rnpedia.com/nursing-notes/medical-surgical-nursing-notes/cerebrovascular-accident/
Rordorf G & McDonald C. (2025a). Spontaneous intracerebral hemorrhage: Acute treatment and prognosis. UpToDate. https://www.uptodate.com/contents/spontaneous-intracerebral-hemorrhage-acute-treatment-and-prognosis
Rordorf G & McDonald C. (2025b). Spontaneous intracerebral hemorrhage: Pathogenesis, clinical features, and diagnosis. UpToDate. https://www.uptodate.com/contents/spontaneous-intracerebral-hemorrhage-pathogenesis-clinical-features-and-diagnosis
Rose D, Cavalier A, Kam W, Cantrell S, Lusk J, Schrag M, Yaghi S, et al. (2023). Complications of intravenous tenecteplase versus alteplase for the treatment of acute ischemic stroke: A systematic review and meta-analysis. Stroke, 54(5), 1192–1204. https://www.ahajournals.org/doi/full/10.1161/STROKEAHA.122.042335
Rost N & Voetsch B. (2025). Definition, etiology, and clinical manifestations of transient ischemic attack. UpToDate. https://www.uptodate.com/contents/definition-etiology-and-clinical-manifestations-of-transient-ischemic-attack
Sabih A, Tadi P, & Kumar A. (2023). Stroke prevention. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK470234/
Sadan O & Akbik F. (2022). Treating delayed cerebral ischemia: Should we focus on blood pressure or vasodilation? Stroke, 53 (8), 2617–9. https://doi.org/10.1161/STROKEAHA.122.039800
Saver J. (2024). Thrombolytic therapy in stroke. Medscape. https://emedicine.medscape.com/article/1160840-overview
Shahid J, Kashif A, & Shahid M. (2023). A comprehensive review of physical therapy interventions for stroke rehabilitation: Impairment-based approaches and functional goals. Brain Scencei, 13(5), 717. https://www.mdpi.com/2076-3425/13/5/717
Sharma R. (2025a). Acute basilar artery occlusion. Radiopaedia. https://radiopaedia.org/articles/acute-basilar-artery-occlusion?lang=us
Sharma R. (2025b). Expanded treatment in cerebral infarction (eTICI) score. Radiopaedia. https://radiopaedia.org/articles/expanded-treatment-in-cerebral-infarction-etici-score?lang=us
Sharma R. (2025c). Ischemic stroke. Radiopaedia. https://radiopaedia.org/articles/ischaemic-stroke?lang=us
Sharma R. (2025d). Modified treatment in cerebral infarction (mTICI) score. Radiopaedia. https://radiopaedia.org/articles/modified-treatment-in-cerebral-infarction-mtici-score?lang=us
Sharma R. (2025e). Thrombolysis in cerebral infarction (TICI) scale. Radiopaedia. https://radiopaedia.org/articles/thrombolysis-in-cerebral-infarction-tici-scale?lang=us
Shmerling R. (2025). Thrombotic stroke. Harvard Health Publishing. https://www.health.harvard.edu/a_to_z/thrombotic-stroke-a-to-z
Silva G, de Andrade J, Martins E, Santo K, & Machline-Carrion M. (2024). Blood pressure management to prevent recurrent stroke: Current evidence and perspectives. npj Cardiovascular Health, 1(18). https://www.nature.com/articles/s44325-024-00021-x
Silver B. (2024). Stroke prevention. Medscape. https://emedicine.medscape.com/article/323662-overview#a4
Singer R, Ogilvy C, & Rordorf G. (2025). Unruptured intracranial aneurysms. UpToDate. https://www.uptodate.com/contents/unruptured-intracranial-aneurysms
Society of NeuroInterventional Surgery (SNIS). (2025). Stroke scales for EMS mobile app. https://getaheadofstroke.org/resource/stroke-scales-for-ems-mobile-application/
Soetanto A. (2025). Stroke: Supportive care. MedLink Neurology. https://www.medlink.com/articles/stroke-supportive-care-2
SpeechTherapy.org. (2025). Speech therapy after stroke: Best recovery exercises & strategies. https://speechtherapy.org/disorders/adults/stroke-recovery-exercises/
Stanford Medicine. (2025). Transesophageal echocardiogram (TEE). https://stanfordhealthcare.org/medical-tests/t/transesophageal-echocardiogram.html
Statista. (2025). Death rates for stroke in the United States in 2022, by state. https://www.statista.com/statistics/671114/death-rate-from-stroke-leading-us-states/
Statista. (2024). Percentage of adults in the United States who were told by a doctor they had a stroke in 2020–2022, by state. https://www.statista.com/statistics/1480188/prevalence-of-stroke-us-adults-by-state/
Steenblock A. (2025). The role of occupational therapy in stroke recovery. The Stroke Foundation. https://www.thestrokefoundation.org/news/the-role-of-occupational-therapy-in-stroke-recovery
Stieg P. (2024). Intracerebral hemorrhage. Weill Cornell Medicine. https://weillcornellbrainandspine.org/condition/intracerebral-hemorrhage/surgery-intracerebral-hemorrhage
Strokenetwork. (2025). Stroke school, Part 3: Syndromes. https://www.strokenetworkseo.ca/sites/default/files/files/stroke_school_brockville_part_3.pdf
Stromsdorfer S. (2025). Occupational therapy in the acute care setting. https://www.myotspot.com/occupational-therapy-acute-care-setting/
Sutter Health. (2024). Stroke before age 45. https://www.sutterhealth.org/health/heart/stroke-before-age-forty-five
Swieten J. (2024). Modified Rankin Scale for neurologic disability. MDCalc. https://www.mdcalc.com/calc/1890/modified-rankin-scale-neurologic-disability
TactusTherapy. (2025). What is a cognitive communication disorder? http://tactustherapy.com/whatiscogcomm/
Tadi P, Lui F, & Budd L. (2023). Acute stroke (nursing). StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK568693/
Tenny S, Das J, & Thorell W. (2024). Intracranial hemorrhage. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK470242/
The Joint Commission (TJC). (2025a). Stroke certification. https://www.jointcommission.org/en-us/certification/stroke
The Joint Commission (TJC). (2025b). The Joint Commission advanced stroke certification—Program concept comparison. https://digitalassets.jointcommission.org/api/public/content/1c589c49792946f7b11d7ad6903b38a1?v=1e0bc051
Thilak S, Brown P, Lawrence E, Gopal S, Mullhi R, Chacko C, Ahmed Z, et al. (2025). Delayed cerebral ischaemia after subarachnoid haemorrhage: Pathophysiology, diagnosis and neurotherapeutic opportunities. Discover Medicine, 2(103). https://link.springer.com/article/10.1007/s44337-025-00302-z
Tiwana M & Bordoni B. (2023). Occupational therapy assessment in long term care. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK565869/
Travers J & Bazaar C. (2024). Nursing management of acute ischemic stroke. Nursing Made Incredibly Easy!, 22(5), 5–13. https://journals.lww.com/nursingmadeincrediblyeasy/fulltext/2024/09000/nursing_management_of_acute_ischemic_stroke.2.aspx
Tumber E. (2024). Monitoring vital signs: Stroke patients’ care frequency. MedShun. https://medshun.com/article/how-often-to-take-vitals-for-stroke-patient
UMass Chan Medical School. (n.d.). Module 3—The blood supply of the brain. https://www.umassmed.edu/strokestop/modules/module-3-the-blood-supply-of-the-brain/brain-circulation/
Unnithan A, Das J, & Mehta P. (2023). Hemorrhagic stroke. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK559173/
Vanderpool E. (2025). Occupational therapy and stroke rehab. OT Potential. https://otpotential.com/treatment/occupational-therapy-and-stroke-rehab
Vera M. (2025). 5 anxiety and panic disorders nursing care plans. NurseLabs. https://nurseslabs.com/anxiety-panic-disorders-nursing-care-plans/
Wang L, Hao M, Wu N, Wu S, Fisher M, & Xiong Y. (2024). Comprehensive review of tenecteplase for thrombolysis in acute ischemic stroke. JAHA, 13(9). https://doi.org/10.1161/JAHA.123.031692
Wayne G. (2024). Impaired tissue/skin integrity (wound care) nursing diagnosis & care plans. NurseLabs. https://nurseslabs.com/risk-for-impaired-skin-integrity/
Wegener S. (2022). Triggers of stroke: Anger, emotional upset, and heavy physical exertion. New insights from the INTERSTROKE study. European Heart Journal, 43(3), 210–2. https://doi.org/10.1093/eurheartj/ehab755
Wen H & Wang M. (2022). Backward walking training impacts positive effect on improving walking capacity after stroke: A meta-analysis. International Journal of Environmental Research and Public Health, 19(6), 3370. https://doi.org/10.3390/ijerph19063370
Wenstrup J, Hestoy B, Sagar M, Blomberg S, Christensen H, Christensen H, & Kruuse C. (2024). Emergency medical services dispatcher recognition of stroke: A systematic review. European Stroke Journal, 9(2), 283–94. https://doi.org/10.1177/23969873231223339
World Stroke Organization (WSO). (2025). Impact of stroke. https://www.world-stroke.org/world-stroke-day-campaign/about-stroke/impact-of-stroke
Yartsev A. (2023). Classical stroke syndromes. Deranged Physiology. https://derangedphysiology.com/main/required-reading/neurology-and-neurosurgery/Chapter%201501/classical-stroke-syndromes
Zubair A & Sheth K. (2023). Hemorrhagic conversion of acute ischemic stroke. Neurotherapeutics, 20(3), 705–11. https://www.sciencedirect.com/science/article/pii/S1878747923000090






Customer Rating
4.9 / 318 ratings